Abstract
Introduction
Neuromuscular electrical stimulation (NMES) for the treatment of swallowing disorders is delivered at a variety of stimulation frequencies. We examined the effects of stimulation frequency on tongue muscle plasticity in an aging rat model.
Methods
Eighty-six young, middle-aged, and old rats were assigned to either bilateral hypoglossal nerve stimulation at 10 or 100 Hz (5 days/wk, 8 wks), sham, or no implantation conditions. Muscle contractile properties and myosin heavy chain (MyHC) composition were determined for hyoglossus (HG) and styloglossus (SG) muscles.
Results
Eight-weeks of 100 Hz stimulation resulted in the greatest changes in muscle contractile function with significantly longer contraction and half-decay times, greatest reduction in fatigue, and a transition toward slowly contracting, fatigue-resistant MyHC isoforms.
Discussion
NMES at 100 Hz induced considerable changes in contractile and phenotypic profiles of HG and SG muscles, suggesting higher frequency NMES may yield a greater therapeutic effect.
Keywords: aging, swallowing disorders, neuromuscular electrical stimulation, stimulation frequency, tongue musculature, rodent model
Introduction
Swallowing disorders affect 20% of elderly people, and have substantial negative effects on quality of life, health, and rehabilitative potential. Age-related reductions in tongue strength, mass, and transformations or selective loss of specific muscle fiber types1–8 may underlie observed decrements in the aging oropharyngeal swallow.3,6,9–13 Treatments that include increased levels of muscle activity, such as neuromuscular electrical stimulation, have the potential of improving swallowing function.
Neuromuscular electrical stimulation (NMES) is used in the treatment of swallowing disorders.14–18 Clinical research findings regarding the effectiveness of NMES for improving swallowing outcomes have been mixed.19 While some studies have reported favorable effects, 15,20–24 other studies have not found benefits to using NMES in swallowing treatment over traditional therapeutic methods.25–27 One source of variability in the application of NMES to dysphagia may be that various stimulation parameters have been used in different implementations, such as variation in stimulation frequency, duration, and treatment length.
Stimulation frequency has not been standardized in clinical dysphagia treatment, ranging from 30 Hz to 80 Hz,28,29 nor has the stimulation duration (5 minutes to 1 hour) or treatment period (weeks to months).30 All of these factors contribute to the total stimulation dose,31 and alterations in muscle phenotype appear to be dose-dependent.32,33 For example, low frequency stimulation has been associated with muscle fiber transformations within rapidly contracting muscles to more slowly contracting, fatigue-resistant fibers,34–36 while high frequency stimulation has been shown to drive conversion of slowly contracting muscles to more rapidly contracting, fast-fatiguing muscle fiber types.35,37–39 Additionally, cross innervation studies have demonstrated muscle fiber type transformations consistent with this notion.35,36,40 Accordingly, NMES of the tongue muscles, which are primarily composed of rapidly-contracting myosin heavy chain (MyHC) type II fibers and isoforms,41,42 can be transformed, theoretically, to either more slowly or more rapidly contracting muscle phenotypes depending on stimulation frequency.35
In our prior study of NMES of the hypoglossal nerves over 8 weeks in an aging rat model, a 40 Hz stimulation frequency was used, which represented an approximate midpoint between the low (10 Hz) and high (100 Hz) frequencies used previously in the literature.34,35,37–39 With 40 Hz NMES, we found reduced muscle fatigue, increased contraction and half decay times, increased twitch and tetanic tension, and increased Type I MyHC in the extrinsic tongue muscles, suggesting conversion to a more slowly contracting, fatigue resistant muscle fiber type.43 However, the question remained whether conversion of muscle fiber characteristics could be further optimized using different stimulation frequencies.
The purpose of our study was to examine the effects of low (10 Hz) and high (100 Hz) frequency neuromuscular electrical stimulation (NMES) of the hypoglossal nerves on tongue muscle contractile properties and myosin heavy chain (MyHC) isoform composition in an aging rodent model. Following neuromuscular electrical stimulation (NMES), we hypothesized that in the retrusive, extrinsic tongue muscles (hyoglossus [HG] and styloglossus [SG]), high, 100 Hz frequency stimulation would result in conversion of the HG and SG muscles to a more rapidly contracting, fatigable muscle fiber phenotype, while low, 10 Hz frequency stimulation would result in phenotypic transformation to a more slowly contracting fatigue-resistant muscle fiber type.
Methods
This study was performed in accordance with the Public Health Service Policy on care and use of laboratory animals, the NIH Guide for the Care and Use of Laboratory Animals, 8th Edition, 2011. The animal care and use protocol was approved by the University of Wisconsin-Madison School of Medicine and Public Health Animal Care and Use Committee.
Male Fischer 344/Brown Norway rats in Young Adult (9 mo. old; n=32), Middle-Aged44 (24 mo. old; n=25) and Old44 (32 mo. old; n=29) groups were randomly assigned to the following groups: 1) bilateral hypoglossal nerve stimulation at 10 Hz (n=20); 2) bilateral hypoglossal nerve stimulation at 100 Hz (n=23); 3) implantation of the electrode array but no stimulation (sham, n=6); 4) no implantation, no stimulation control ( n=37). Eleven of the control animals were used to for comparisons to the sham group. Sham, 10 Hz, and 100 Hz groups underwent implantation of an electrode assembly designed and fabricated by Dr. David Zealear (Vanderbilt University School of Medicine),43,45 followed by 8 weeks of supramaximal bilateral electrical stimulation of the hypoglossal nerves (10 Hz and 100 Hz groups only). Briefly, rats were anesthetized with isoflurane, implanted with the electrode assembly immediately below the bifurcation of the hypoglossal nerve, allowed to recover, and then a Grass S88 stimulator was used to present bilateral, supramaximal stimulation at either 10 Hz or 100 Hz 5 days per week for a total of 8 weeks.
Supramaximal stimulation was performed using a rectangular waveform, duty cycle of 1 sec on – 1 sec off, and 0.2 sec pulse width at low (10 Hz) or high (100 Hz) frequencies reported previously in the literature.34,35,37–39 Representative tongue force signals are shown in Supplementary Figure 1. Supramaximal current amplitude was determined for each rat and was defined as 1.5 times the minimum current level needed to generate maximum peak tongue muscle twitch force, which varied between 300 – 500 μA for each rat. Sham rats did not receive any hypoglossal nerve stimulation and were included to allow us to discern effects of the presence of the electrode cuff on the nerve. Duration of daily treatment was 30 min for the 10 Hz group and 10 min for the 100 Hz group. Differences in treatment duration were employed to normalize the extent of muscle fatigue within a training session. Preliminary studies in our laboratory indicate a 40% reduction in the fatigue index was achieved at the end of a single 10 or 30 min training session at 100 or 10 Hz stimulation, respectively, prior to 8 weeks of NMES. All other stimulation parameters were held equal to allow for comparison of muscle outcomes following 10 Hz or 100 Hz NMES treatment. The Control group did not receive electrical stimulation.
Following the 8-week study period, tongue muscle contractile properties were recorded in vivo for all rats, using procedures described previously.43,46–49 Whole hypoglossal nerves were then stimulated bilaterally via the electrode cuffs surrounding the nerve. The following retrusive tongue muscle contractile properties were recorded in each rat: maximal twitch tension (peak tension [mN] generated following a single electrical stimulus ), twitch contraction time (interval [ms] between onset of stimulation and 50% peak twitch tension) and half-decay times (interval [ms] between onset of stimulation and 50% decay from peak twitch tension), maximal tetanic tension (maximal tension (mN) of each stimulated fused wave ), and a fatigue index (percent fused tetanic tension [mN] at the end of 2 minutes of stimulation as function of initial fused tetanic tension [mN]). Rats were then euthanized and the hyoglossus (HG) and styloglossus (SG) muscles were harvested, snap frozen with liquid nitrogen, and stored at −80° C for later analysis.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed and gels were silver-stained and analyzed to determine the MyHC isoform composition of the HG and SG. The HG and SG muscles were homogenized and protein concentration determined (Bradford Protein Assay; Bio-Rad). SDS-PAGE was performed with a 0.75 mm thick 6% acrylamide/30% glycerol separating gel (18 ×16 cm) and a 4% acrylamide/30% glycerol stacking gel and 0.4 μg of protein was added in triplicate to each gel channel. The gel was stained using a silver staining kit (SliverQuest Silver Staining Kit; Invitrogen) to visualize protein bands, digitally imaged, and the density of each band determined using available software (UN-Scan-IT gel Version 6.1; Silk Scientific, Inc). Percent of each MyHC isoform in each column of the gel was calculated using the density of a particular MyHC band over the total density for all MyHC bands in that column. Accordingly, relative MyHC composition was calculated. The reliability and validity of these methods are high and were established previously.50
Data Analysis
Age, stimulation treatment, and interactions were examined using a two-way Analysis of Variance (ANOVA) for contraction and half-decay times, fatigue, and MyHC isoform composition. Because differences were found across groups in animal weight (F4,78 = 2.52, p=.05), a two-way Analysis of Covariance (ANCOVA) with a weight covariate was used for twitch and tetanic tension analyses. Pair-wise comparisons were made between groups using Fisher’s protected least significant difference tests (LSD). The critical value for obtaining statistical significance was set at α=.05 level.
Results
Sham versus Control Groups
Presence of the electrode cuff on the hypoglossal nerve had no effect on muscle force production, but did alter temporal properties of muscle contraction, with significantly increased contraction and half-decay times in the sham group compared with the control (no electrode cuff) group (Table 1).
Table 1.
Effect of hypoglossal nerve cuff on muscle force production and temporal properties of muscle contraction
| Maximum twitch tension (mN) | Contraction time (ms)* | Half-decay time (ms)* | Maximum tetanic tension (mN) | Fatigue Index (%) | |
|---|---|---|---|---|---|
| Sham Group | 264.8 (8.53) | 9.96 (0.14) | 37.52 (0.84) | 810.2 (48.17) | 0.89 (0.01) |
| Control Group | 290.1 (8.85) | 9.15 (0.13) | 35.15 (0.62) | 875.4 (41.94) | 0.85 (0.03) |
Data expressed as averages (standard error).
p<.05 = significant differences between groups.
Muscle Contractile Properties
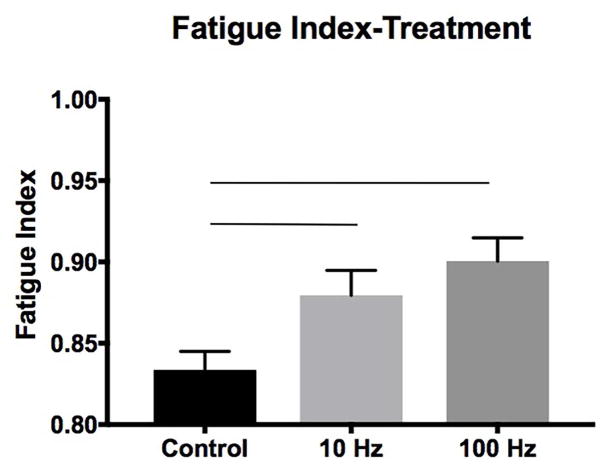
Descriptive data for contractile property measures by age and condition are shown in shown in Table 2. The middle-aged and old groups had longer contraction times than the young adult group (F2,78 = 6.23, p=.003; Figure 1A). Main effects for condition were found for contraction time, half-decay time, and fatigue ratio. The 100 Hz group had longer contraction times than the control group (F2,78 = 5.51, p=.006; Figure 1B). Longer half-decay times were found in the 10 Hz and 100 Hz conditions than in the control group (F2,78 = 5.34, p=.007; Figure 1C). For fatigue ratio, both 10 Hz and 100 Hz stimulation groups evidenced greater fatigue resistance than the control group (F2,78 = 7.32, p=.001; Figure 2) with the 100 Hz stimulation treatment group having the highest resistance to fatigue. No significant age, condition, or interaction effects were found for twitch or tetanic tension.
Table 2.
Effect of hypoglossal nerve stimulation treatment on retrusive tongue muscle contractile properties
| Maximum twitch tension(mN) | Contraction time (ms)* | Half-decay time (ms)* | Maximum tetanic tension (mN) | Fatigue Index (%)* | ||
|---|---|---|---|---|---|---|
| Young Adult | Control | 302.80 (14.24) | 9.15 (0.23) | 35.15 (0.98) | 930.05 (48.06) | 0.84 (0.02) |
| 10 Hz | 303.96 (15.40) | 9.75 (0.29) | 38.41 (1.24) | 937.18 (51.97) | 0.88 (0.03) | |
| 100Hz | 303.24 (14.50) | 9.79 (0.28) | 38.45 (1.16) | 901.92 (48.93) | 0.92 (0.02) | |
| Middle Aged | Control | 285.97 (12.70) | 9.73 (0.22) | 37.04 (0.95) | 874.68 (42.86) | 0.82 (0.02) |
| 10 Hz | 312.23 (15.68) | 9.94 (0.31) | 37.98 (1.34) | 951.46 (52.91) | 0.87 (0.03) | |
| 100Hz | 310.91 (14.74) | 10.84 (0.29) | 40.32 (1.24) | 909.75 (49.72) | 0.90 (.03) | |
| Old | Control | 321.10 (11.46) | 10.09 (0.21) | 37.14 (0.91) | 889.86 (38.66) | 0.83 (0.02) |
| 10 Hz | 300.29 (14.35) | 10.34 (0.29) | 38.99 (1.24) | 851.10 (48.43) | 0.89 (0.03) | |
| 100Hz | 319.59 (13.42) | 10.38 (0.27) | 38.61 (1.66) | 938.50 (45.30) | 0.88 (0.02) |
Data are expressed as averages (standard error).
p<.05 = significant differences among treatment groups.
Figure 1.
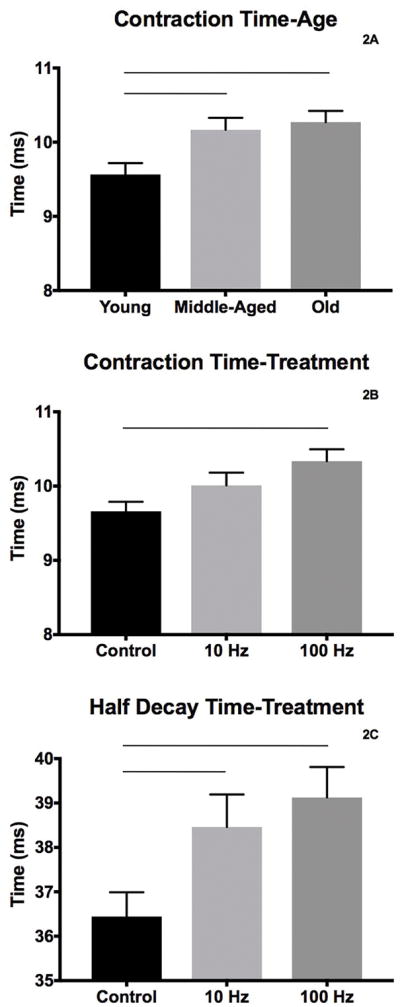
Temporal properties of muscle contraction with significantly altered by age (A, contraction time) and stimulation treatment (B, contraction time; C, half decay time). Bars denote significance, p<.05.
Figure 2.
Reductions in fatigue were observed following the 8-week treatment period in both 10 Hz and 100 Hz stimulation groups versus the control group. Bars denote significance, p<.05.
Myosin Heavy Chain Isoform Composition
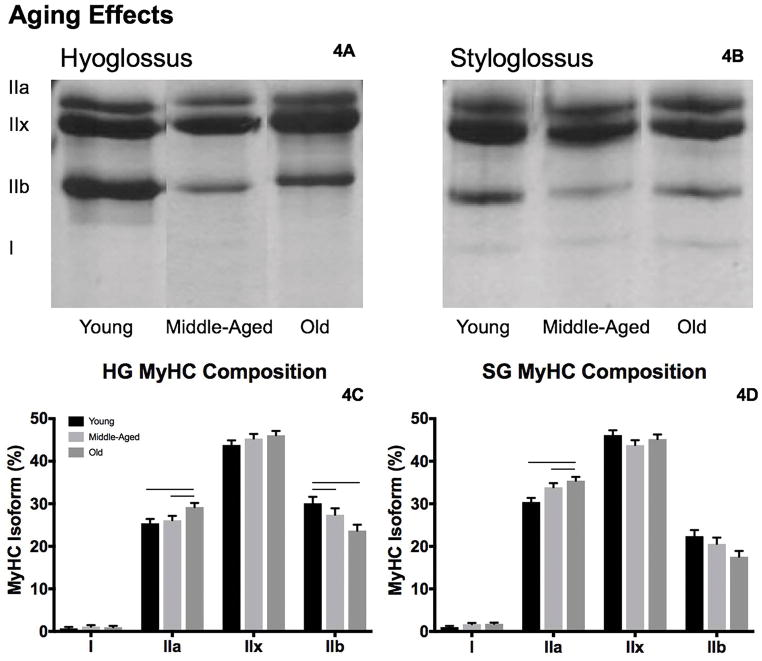
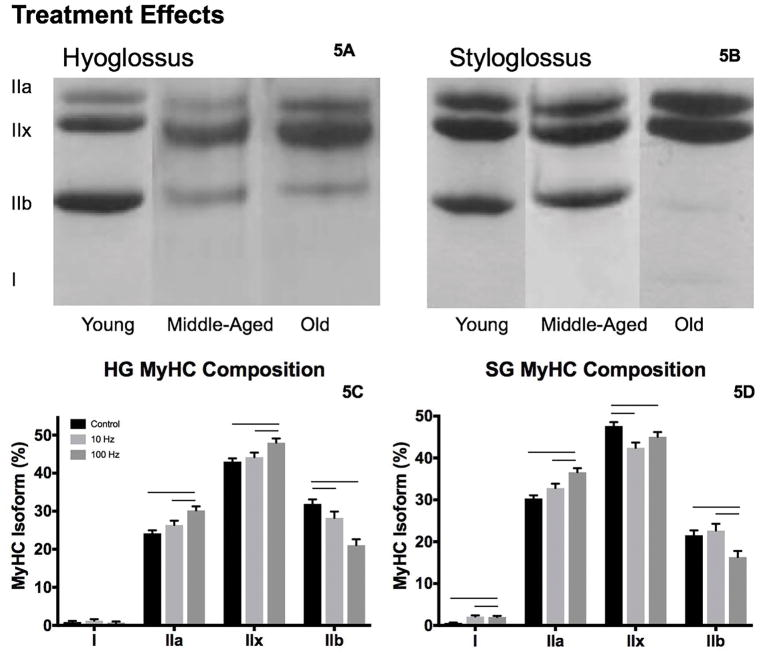
Representative gels are shown in Figures 3A, 3B and 4A, 4B for each age by treatment condition, with MyHC isoform proportion data found in Table 3.
Figure 3.
Representative gels from the hyoglossus (HG, A) and styloglossus (SG, B) muscles. With age alterations in MyHC IIa and MyHC IIb were observed in the HG (C), and in MyHC IIa in the SG (D). Bars denote significance, p<.05.
Figure 4.
A. Representative SDS-PAGE gels from the HG and SG muscles following l0 and 100 Hz stimulation treatments (A, B, respectively). Following the 8-week stimulation treatment period, alterations in MyHC isoform composition of the HG (C) and SG (D) muscles were observed. Bars denote significance, p<.05.
Table 3.
Effect of stimulation treatment on the isoform composition of hyoglossus and styloglossus muscles
| HG MyHC I | HG MyHC IIa* | HG MyHC IIx* | HG MyHC IIb* | ||
|---|---|---|---|---|---|
| Young Adult | Control | 0.72 (0.51) | 24.01 (1.48) | 41.24 (1.56) | 34.03 (2.15) |
| 10 Hz | 0.93 (0.70) | 23.89 (2.00) | 43.88 (2.11) | 31.30 (2.92) | |
| 100Hz | 0.37 (0.64) | 28.29 (1.85) | 46.29 (1.95) | 25.05 (2.70) | |
| Middle Aged | Control | 1.50 (0.49) | 22.34 (1.41) | 43.45 (1.49) | 32.71 (2.06) |
| 10 Hz | 0.77 (0.70) | 25.16 (2.00) | 43.51 (2.11) | 30.56 2.92) | |
| 100Hz | 1.11 (0.64) | 30.88 (1.85) | 49.00 (1.95) | 19.01 (2.70) | |
| Old | Control | 0.45 (0.47) | 26.15 (1.36) | 44.38 (1.43) | 29.02 (1.98) |
| 10 Hz | 1.96 (0.64) | 30.02 (1.85) | 45.19 (1.95) | 22.84 (2.70) | |
| 100Hz | 0.52 (0.60) | 31.51 (1.73) | 48.75 (1.82) | 19.21 (2.53) |
| SG MyHC I* | SG MyHC IIa* | SG MyHC IIx* | SG MyHC IIb* | ||
|---|---|---|---|---|---|
| Young Adult | Control | 0.24 (0.40) | 28.69 (1.37) | 47.44 (1.64) | 23.63 (2.10) |
| 10 Hz | 1.10 (0.54) | 28.70 (1.86) | 44.44 (2.22) | 25.75 (2.84) | |
| 100Hz | 1.76 (0.46) | 33.92 (1.61) | 46.55 (1.93) | 17.77 (2.46) | |
| Middle Aged | Control | 0.13 (0.38) | 29.11 (1.31) | 47.25 (1.57) | 23.51 (2.01) |
| 10 Hz | 2.45 (0.54) | 33.20 (1.86) | 40.50 (2.22) | 23.84 (2.84) | |
| 100Hz | 2.59 (0.50) | 39.32 (1.72) | 43.62 (2.06) | 14.46 (2.63) | |
| Old | Control | 1.05 (0.36) | 33.17 (1.26) | 48.29 (1.51) | 17.50 (1.93) |
| 10 Hz | 2.72 (0.50) | 36.55 (1.72) | 42.33 (2.06) | 18.40 (2.63) | |
| 100Hz | 1.68 (0.46) | 36.55 (1.61) | 45.00 (1.93) | 16.77 (2.46) |
HG = hyoglossus; SG = styloglossus. Data are expressed as mean % isoform (standard error).
p<.05 = significant differences among treatment groups.
In the HG, there was greater proportion of MyHC IIa (F2,76 = 4.27, p=.018) and less MyHC IIb (F2,76 = 4.98, p=.01) in old versus young adult and middle-aged (Figure 3C). In the 100 Hz stimulation condition versus control and 10 Hz stimulation groups, we observed increased MyHC IIa (F2,76 = 4.27, p=.0001) and MyHC IIx (F2,76 = 6.5, p=.003), and less MyHC IIb (F2,76 = 15.64, p<.0001; Figure 4C). No differences in MyHC I or interaction effects were found.
For the SG muscle, the proportion of MyHC IIa increased in the middle-aged and old groups relative to young adult (F2,77 = 7.68, p=.001; Figure 3D). With 10 Hz and 100 Hz stimulation treatment, the proportion of the MyHC IIa (F2,77 = 13.29, p<.0001) and MyHC I (F2,77 = 13.94, p<.0001) isoforms increased and MyHC IIx decreased (F2,77 = 5.88, p=.004; Figure 4D) versus the control condition. We also observed less MyHC IIb with 100Hz stimulation treatment relative to 10 Hz stimulation and control conditions (F2,77 = 5.39, p=.007).
Discussion
The results of our study demonstrate that NMES may be a promising treatment for swallowing disorders where improvements in fatigue resistance are a therapeutic target. The primary finding of our study was the transition toward more slowly contracting, fatigue resistant contractile and phenotypic profiles in the HG and SG muscles, regardless of the stimulation frequency implemented over the 8-week treatment period. Both 10 Hz and 100 Hz stimulation parameters appeared to increase the MyHC I composition in the SG muscles and reduce fatigue during evoked tongue retrusion. We observed that chronic low frequency stimulation at 10 Hz has the capacity to drive contractile and phenotypic profiles of the rat tongue, which is predominantly composed of MyHC Type II fibers,41–43 toward a more slowly contracting fatigue-resistant profile. However, the greatest degree of contractile and phenotypic adaptability occurred following 100 Hz, high frequency stimulation. The 100 Hz group exhibited decreased MyHC IIb and increased MyHC I, IIa, and IIx, and increased contraction and half decay times, and the greatest reduction in fatigue compared to the control group. Surprisingly, stimulation frequency did not alter the directionality of phenotypic adaptation35 in the 100 Hz stimulation group from a slow to fast fiber type transition. NMES training programs, that normalize to the extent of evoked muscle fatigue in a single training session, may mimic the muscular plastic effects of voluntary exercise, to achieve a fast to slow muscle fiber transformation and reduced muscular fatigue. Perhaps to combat the fatigue evoked by our stimulation parameters during each daily stimulation session over the course of 8 weeks, the phenotypic profile of the SG and HG muscles in young adult, middle-aged, and old rats transitioned to more slowly contracting, fatigue-resistant MyHC isoforms. These changes in MyHC isoform composition were also consistent with observed alterations in muscle contractile properties: muscle fatigue was significantly reduced and longer contraction times observed following 8 weeks of NMES.
Our previous results using 40 Hz NMES in rat tongue showed that the proportion of MyHC I increased following stimulation treatment.43 Another study in rabbit tongue had similar findings with chronic low frequency stimulation, demonstrating increased MyHC I and decreased MyHC II in the normally fast-contracting genioglossus muscle.34,35 These results are consistent with phenotype transitions in fast-twitch limb muscles stimulated at low frequencies.34–36 Although, an atypical transition in the contractile and phenotypic profile of HG and SG muscles of the rat tongue was observed following 100 Hz NMES, this is not the first report of a fast to slow MyHC fiber transition following high frequency stimulation.53 This atypical phenotypic adaption has been attributed to the effects that NMES has on the cellular, molecular, biochemical, neuromuscular, and functional properties of muscle, due to differences in motor unit recruitment of muscle fibers and enhanced muscle fatigue.54–59 Because we normalized each training session to induce a 40% reduction in the fatigue index, it is likely that we exhausted all energetic and metabolic reserves in the muscle, further enhancing fatigue onset in the predominantly MyHC Type II HG and SG muscles. In the future, it would be beneficial to study motor nerve conduction, ATP depletion, oxidative stress, and glycolytic enzymes following stimulation to better understand underlying mechanisms that are contributing to increased muscle fatigue at high stimulation intensities and frequencies.
We did not observe any treatment by age interaction effects. With increasing age, retrusive actions of the tongue in middle-aged and old rats showed increased contraction times, which indicates a slower time to peak muscle contraction. In addition, there was a corresponding transition to more slowly contracting and fatigue-resistant MyHC isoforms in both the HG and SG muscles. Evidence for this transition in the HG muscle is the reduction of MyHC IIb (rapidly contracting and highly fatigable) and increase in the MyHC IIa (slower contracting, fatigue-resistant) in the old rat group. Similarly, in the SG muscles of the middle-aged and old group, there was an increase in proportion of the MyHC IIa isoform. These results are consistent with aging lingual muscle literature.43,48,50,60
There are a few limitations that must be considered before translating the findings of our study. First, we did not examine swallowing outcomes. In the future, it would be advantageous to include videofluoroscopic swallowing measures to determine whether contractile and phenotypic changes induced by NMES also affect the functional swallow. It would also be important to know whether following a single stimulation treatment, an increase in muscular fatigue translates into a fatigued swallow. Second, because increased contraction and half-decay times were observed in the sham group, the alterations in temporal properties of muscle contraction that were also observed following 8 weeks of 10 Hz and 100 Hz hypoglossal stimulation must be examined carefully. The presence of the nerve cuff alone may have contributed to increased contraction and half decay times following the NMES treatment, however these changes in muscle contraction are also consistent with the transition to a slowly contracting, fatigue resistant phenotype that occurred in the HG and SG muscles in both stimulation groups. Third, it is possible that our stimulation parameters induced muscle damage, potentially due to the metabolic and mechanical stresses imposed on the muscle during NMES treatment.61 Because we are not able assess symptoms of muscle damage, such as muscle soreness, weakness, or pain, in a rat model it would be beneficial to analyze blood protein markers associated with muscle damage, specifically myoglobin and creatine kinase62.
The results of our study suggest that direct NMES of the hypoglossal nerves may be a potential and beneficial clinical treatment modality that should be tested in randomized clinical trials for swallowing disorders. This study contributes to our understanding of stimulation parameter optimization and suggests that higher stimulation frequencies may have greater benefit in tongue muscle adaptation than lower frequencies where the goals of treatment are reductions in fatigue and gains in endurance. NMES may drive beneficial muscular adaptations leading to improved swallowing function where increased fatigue resistance is the therapeutic target and/or in populations where conventional exercise therapies have been unsuccessful due to tongue muscle immobility issues.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Deafness and Other Communication Disorders at the National Institute of Health, grants R01DC005935, R01DC008149, R01DC014358, by the National Cancer Institute R01CA225608, and T32DC009401 and by the National Institute on Aging, grant F31AG054315. The authors gratefully acknowledge the assistance of Dr. Allison J Schaser and Benjamin J Becker for their support and critical analysis of the study.
Abbreviations
- ANOVA
analysis of variance
- HG
hyoglossus
- Hz
frequency
- LSD
least significance difference
- mA
milli-amp
- mN
milli-newton
- MyHC
myosin heavy chain
- NMES
neuromuscular electrical stimulation
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Sec
second
- SG
styloglossus
Footnotes
Part of the material contained within this manuscript was presented at the 24th Annual Meeting of the Dysphagia Research Society in Tucson, AZ February 25–27, 2016.
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
References
- 1.Nakayama M. Histological study on aging changes in the human tongue. Nihon Jibiinkoka Gakkai kaiho. 1991;94(4):541–555. doi: 10.3950/jibiinkoka.94.541. [DOI] [PubMed] [Google Scholar]
- 2.Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American journal of speech-language pathology/American Speech-Language-Hearing Association. 2003;12(1):40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- 3.Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, Pierce M. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. Journal of Speech Language and Hearing Research. 2000;43(4):1011–1023. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatrica Et Logopaedica. 2003;55(4):199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 5.Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ. The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation. 2007;88(2):150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Baum BJ, Bodner L. Aging and Oral Motor Function - Evidence for Altered Performance among Older Persons. Journal of dental research. 1983;62(1):2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- 7.Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2000;55(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 8.Mortimore IL, Fiddes P, Stephens S, Douglas NJ. Tongue protrusion force and fatiguability in male and female subjects. European Respiratory Journal. 1999;14(1):191–195. doi: 10.1034/j.1399-3003.1999.14a32.x. [DOI] [PubMed] [Google Scholar]
- 9.Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: Preliminary evidence of prevalence, risk factors, and socioemotional effects. Annals of Otology Rhinology and Laryngology. 2007;116(11):858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- 10.Ekberg O, Feinberg MJ. Altered Swallowing Function in Elderly Patients without Dysphagia - Radiologic Findings in 56 Cases. American Journal of Roentgenology. 1991;156(6):1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- 11.Shaw DW, Cook IJ, Gabb M, Holloway RH, Simula ME, Panagopoulos V, Dent J. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1995;268(3):G389–G396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 12.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal Swallowing in Normal Adults of Different Ages. Gastroenterology. 1992;103(3):823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 13.McKee GJ, Johnston BT, McBride GB, Primrose WJ. Does age or sex affect pharyngeal swallowing? Clinical otolaryngology and allied sciences. 1998;23(2):100–106. doi: 10.1046/j.1365-2273.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- 14.Sheffler LR, Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle & Nerve. 2007;35(5):562–590. doi: 10.1002/mus.20758. [DOI] [PubMed] [Google Scholar]
- 15.Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respiratory care. 2001;46(5):466–474. [PubMed] [Google Scholar]
- 16.Huckabee ML, Doeltgen S. Emerging modalities in dysphagia rehabilitation: neuromuscular electrical stimulation. The New Zealand medical journal. 2007;120(1263):U2744. [PubMed] [Google Scholar]
- 17.Braid V, Barber M, Mitchell SL, Martin BJ, Granat M, Stott DJ. Randomised controlled trial of electrical stimulation of the quadriceps after proximal femoral fracture. Aging Clinical and Experimental Research. 2008;20(1):62–66. doi: 10.1007/BF03324749. [DOI] [PubMed] [Google Scholar]
- 18.Ferrante S, Schauer T, Ferrigno G, Raisch J, Molteni F. The effect of using variable frequency trains during functional electrical stimulation cycling. Neuromodulation. 2008;11(3):216–226. doi: 10.1111/j.1525-1403.2008.00169.x. [DOI] [PubMed] [Google Scholar]
- 19.Humbert IA, Michou E, MacRae PR, Crujido L. Electrical Stimulation and Swallowing: How Much Do We Know? Seminars in Speech and Language. 2012;33(3):203–216. doi: 10.1055/s-0032-1320040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogaardt H, van Dam D, Wever NM, Bruggeman CE, Koops J, Fokkens WJ. Use of Neuromuscular Electrostimulation in the Treatment of Dysphagia in Patients With Multiple Sclerosis. Annals of Otology Rhinology and Laryngology. 2009;118(4):241–246. doi: 10.1177/000348940911800401. [DOI] [PubMed] [Google Scholar]
- 21.Carnaby-Mann GD, Crary MA. Adjunctive neuromuscular electrical stimulation for treatment-refractory dysphagia. Annals of Otology Rhinology and Laryngology. 2008;117(4):279–287. doi: 10.1177/000348940811700407. [DOI] [PubMed] [Google Scholar]
- 22.Lim KB, Lee HJ, Lim SS, Choi YI. Neuromuscular electrical and thermal-tactile stimulation for dysphagia caused by stroke: a randomized controlled tril. Journal of Rehabilitation Medicine. 2009;41(3):174–178. doi: 10.2340/16501977-0317. [DOI] [PubMed] [Google Scholar]
- 23.Oh BM, Kim DY, Paik NJ. Recovery of swallowing function is accompanied by the expansion of the cortical map. International Journal of Neuroscience. 2007;117(9):1215–1227. doi: 10.1080/00207450600936254. [DOI] [PubMed] [Google Scholar]
- 24.Shaw GY, Sechtem PR, Searl J, Keller K, Rawi TA, Dowdy E. Transcutaneous neuromuscular electrical stimulation (VitalStim) curative therapy for severe dysphagia: Myth or reality? Annals of Otology Rhinology and Laryngology. 2007;116(1):36–44. doi: 10.1177/000348940711600107. [DOI] [PubMed] [Google Scholar]
- 25.Bulow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23(3):302–309. doi: 10.1007/s00455-007-9145-9. [DOI] [PubMed] [Google Scholar]
- 26.Kiger M, Brown CS, Watkins L. Dysphagia management: An analysis of patient outcomes using VitalStim (TM) therapy compared to traditional swallow therapy. Dysphagia. 2006;21(4):243–253. doi: 10.1007/s00455-006-9056-1. [DOI] [PubMed] [Google Scholar]
- 27.Suiter DM, Leder SB, Ruark JL. Effects of neuromuscular electrical stimulation on submental muscle activity. Dysphagia. 2006;21(1):56–60. doi: 10.1007/s00455-005-9010-7. [DOI] [PubMed] [Google Scholar]
- 28.Sun SF, Hsu CW, Lin HS, Sun HP, Chang PH, Hsieh WL, Wang JL. Combined Neuromuscular Electrical Stimulation (NMES) with Fiberoptic Endoscopic Evaluation of Swallowing (FEES) and Traditional Swallowing Rehabilitation in the Treatment of Stroke-Related Dysphagia. Dysphagia. 2013;28(4):557–566. doi: 10.1007/s00455-013-9466-9. [DOI] [PubMed] [Google Scholar]
- 29.Park JW, Kim Y, Oh JC, Lee HJ. Effortful Swallowing Training Combined with Electrical Stimulation in Post-Stroke Dysphagia: A Randomized Controlled Study. Dysphagia. 2012;27(4):521–527. doi: 10.1007/s00455-012-9403-3. [DOI] [PubMed] [Google Scholar]
- 30.Pownall S, Enderby P, Sproson L. Electrical Stimulation for the Treatment of Dysphagia. In: Majid A, editor. Electroceuticals: Advances in Electrostimulation Therapies. Cham: Springer International Publishing; 2017. pp. 137–156. [Google Scholar]
- 31.Lake DA. Neuromuscular electrical-stimiulation- an overview and its application in the treatment of sports injuries. Sports Medicine. 1992;13(5):320–336. doi: 10.2165/00007256-199213050-00003. [DOI] [PubMed] [Google Scholar]
- 32.Demirel HA, Powers SK, Naito H, Hughes M, Coombes JS. Exercise-induced alterations in skeletal muscle myosin heavy chain phenotype: dose-response relationship. Journal of Applied Physiology. 1999;86(3):1002–1008. doi: 10.1152/jappl.1999.86.3.1002. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland H, Jarvis JC, Kwende MMN, Gilroy SJ, Salmons S. The dose-related response of rabbit fast muscle to long-term low-frequency stimulation. Muscle & Nerve. 1998;21(12):1632–1646. doi: 10.1002/(sici)1097-4598(199812)21:12<1632::aid-mus3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Pae EK, Hyatt JPK, Wu J, Chien P. Short-term electrical stimulation alters tongue muscle fibre type composition. Archives of oral biology. 2007;52(6):544–551. doi: 10.1016/j.archoralbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochemistry and cell biology. 2001;115(5):359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 36.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microscopy research and technique. 2000;50(6):500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Gorza L, Gundersen K, Lomo T, Schiaffino S, Westgaard RH. Slow-to-fast transformation of devervated soleus muscles by chronic high-frequency stimulation in the rat. Journal of Physiology-London. 1988;402:627–649. doi: 10.1113/jphysiol.1988.sp017226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomo T, Westgaard RH, Dahl HA. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proceedings of the Royal Society of London Series B, Biological sciences. 1974;187(1086):99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- 39.Westgaard RH, Lomo T. Control of contractile properties within adaptive ranges by patterns of impulse activity in the rat. Journal of Neuroscience. 1988;8(12):4415–4426. doi: 10.1523/JNEUROSCI.08-12-04415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. The Journal of physiology. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato I, Suzuki M, Sato M, Sato T, Inokuchi S. A histochemical study of lingual muscle fibers in rat. Okajimas folia anatomica Japonica. 1990;66(6):405–415. doi: 10.2535/ofaj1936.66.6_405. [DOI] [PubMed] [Google Scholar]
- 42.Smith JC, Goldberg SJ, Shall MS. Phenotype and contractile properties of mammalian tongue muscles innervated by the hypoglossal nerve. Respiratory Physiology & Neurobiology. 2005;147(2–3):253–262. doi: 10.1016/j.resp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 43.Connor NP, Russell JA, Jackson MA, Kletzien H, Wang H, Schaser AJ, Leverson GE, Zealear DL. Muscle & nerve. 2012. Tongue muscle plasticity following hypoglossal nerve stimulation in aged rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 45.Widick MH, Tanabe T, Fortune S, Zealear DL. Awake evoked electromyography recording from the chronically implanted rat. Laryngoscope. 1994;104(4):420–425. doi: 10.1288/00005537-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Connor NP, Ota F, Nagai H, Russell JA, Leverson G. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. Journal of Speech Language and Hearing Research. 2008;51(4):818–827. doi: 10.1044/1092-4388(2008/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuller D, Mateika JH, Fregosi RE. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. Journal of Physiology-London. 1998;507(1):265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. Journal of Applied Physiology. 2013;114(4):472–481. doi: 10.1152/japplphysiol.01370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ota F, Connor NP, Konopacki R. Alterations in contractile properties of tongue muscles in old rats. Annals of Otology Rhinology and Laryngology. 2005;114(10):799–803. doi: 10.1177/000348940511401010. [DOI] [PubMed] [Google Scholar]
- 50.Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the Anterior, Medial, and Posterior Genioglossus in the Aged Rat. Dysphagia. 2011;26(3):256–263. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of Tongue Exercise on Protrusive Force and Muscle Fiber Area in Aging Rats. Journal of Speech Language and Hearing Research. 2009;52(3):732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagai H, Russell JA, Jackson MA, Connor NP. Effect of aging on tongue protrusion forces in rats. Dysphagia. 2008;23(2):116–121. doi: 10.1007/s00455-007-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gondin J, Brocca L, Bellinzona E, D’Antona G, Maffiuletti NA, Miotti D, Pellegrino MA, Bottinelli R. Neuromuscular electrical stimulation training induces atypical adaptations of the human skeletal muscle phenotype: a functional and proteomic analysis. Journal of Applied Physiology. 2011;110(2):433–450. doi: 10.1152/japplphysiol.00914.2010. [DOI] [PubMed] [Google Scholar]
- 54.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Physical Therapy. 2005;85(4):358–364. [PubMed] [Google Scholar]
- 55.Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. European Journal of Applied Physiology. 2011;111(10):2399–2407. doi: 10.1007/s00421-011-2128-4. [DOI] [PubMed] [Google Scholar]
- 56.Gorgey AS, Black CD, Elder CP, Dudley GA. Effects of Electrical Stimulation Parameters on Fatigue in Skeletal Muscle. Journal of Orthopaedic & Sports Physical Therapy. 2009;39(9):684–692. doi: 10.2519/jospt.2009.3045. [DOI] [PubMed] [Google Scholar]
- 57.Adams GR, Harris RT, Woodard D, Dudley GA. Mapping of electrical muscle stimulation using MRI. Journal of Applied Physiology. 1993;74(2):532–537. doi: 10.1152/jappl.1993.74.2.532. [DOI] [PubMed] [Google Scholar]
- 58.Bindermacleod SA, Guerin T. Preservation of force output through progressive reduction of stimulation frequency in human quadriceps-femoris muscle. Physical Therapy. 1990;70(10):619–625. doi: 10.1093/ptj/70.10.619. [DOI] [PubMed] [Google Scholar]
- 59.Kesar T, Chou LW, Binder-Macleod SA. Effects of stimulation frequency versus pulse duration modulation on muscle fatigue. Journal of Electromyography and Kinesiology. 2008;18(4):662–671. doi: 10.1016/j.jelekin.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsson L, Ansved T. Effects of Aging on the Motor Unit. Progress in neurobiology. 1995;45(5):397. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 61.Nosaka K, Aldayel A, Jubeau M, Chen TC. Muscle damage induced by electrical stimulation. European Journal of Applied Physiology. 2011;111(10):2427–2437. doi: 10.1007/s00421-011-2086-x. [DOI] [PubMed] [Google Scholar]
- 62.Paddon-Jones D, Muthalib M, Jenkins D. The effects of a repeated bout of eccentric exercise on indices of muscle damage and delayed onset muscle soreness. Journal of science and medicine in sport. 2000;3(1):35–43. doi: 10.1016/s1440-2440(00)80046-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.