Abstract
Background
Previous studies found that visceral sensitivity is increased in bowel obstruction (BO). We hypothesized that mechanical stress-induced expression of BDNF in smooth muscle cells (SMC) of the distended bowel plays a critical role in visceral hypersensitivity in BO by altering voltage-gated K+ channel (Kv) activity in sensory neurons.
Methods
Partial colon obstruction was maintained in rats for 7 days. Colon-projecting neurons in the dorsal root ganglia (DRG, T13 to L2) were isolated for electrophysiological and gene expression studies.
Key results
Compared to controls, membrane excitability of colon-projecting DRG neurons was markedly enhanced in BO. The densities of total Kv and transient A-type (IA) K+ currents, but not sustained delayed IK current, were significantly reduced in the neurons in BO. The mRNA expression of IA subtype Kv1.4 in colon neurons was down-regulated in BO. Expression of BDNF mRNA and protein was dramatically increased in colonic smooth muscle of the distended segment, but not in the non-distended aboral segment. Mechanical stretch of colon SMC in vitro increased BDNF expression. Treatment with anti-BDNF antibody restored total Kv and IA currents of neurons from BO rats. Administration of Trk B inhibitor ANA-12 blocked BO-associated changes of neuronal excitability, Kv activity and gene expression in obstruction.
Conclusions & Inferences
Mechanical stress-induced expression of BDNF in colon SMC plays a critical role in visceral hypersensitivity in BO by suppressing A-type K+ currents and gene expression in sensory nerve. These findings help to identify therapeutic targets for distention-associated abdominal pain in the gut.
Keywords: Visceral sensitivity, Abdominal pain, Smooth muscle cell, Mechanical stress, Neurotrophin
Graphical Abstract
We found that mechanical stress-induced expression of BDNF in colon SMC plays a critical role in visceral hypersensitivity in bowel obstruction by suppressing A-type K+ currents and gene expression in sensory nerve. These findings help to identify therapeutic targets for distention-associated abdominal pain in the gut.
INTRODUCTION
Bowel obstruction (BO) may be acute or chronic, mechanical or functional. It is a significant health concern in adults and children [1, 2]. Mechanical BO accounts for more than 300,000 hospital admissions per year in the United States alone, and the aggregate cost for hospital stay is more than $2.7 billion annually, topping all other gastrointestinal conditions such as appendicitis, peptic ulcer diseases, etc [3]. Distension-associated abdominal pain is a main symptom in BO, and severely compromises quality of life in the patients. In acute complete or complicated obstructions, surgical release may be the treatment of choice; distention-associated abdominal pain in these patients may disappear soon after surgery. However, pain is a major concern in the conservative management for chronic partial obstruction and in palliative treatments for inoperable and malignant BO [4–6]. In fact, mechanical BO occurs in up to 25% and 42% of patients with colon and ovarian cancers, respectively [4, 6]. Moreover, 92% of patients with malignant obstruction have distention-associated abdominal pain [4, 7]. In addition, distention-associated abdominal pain and discomfort are major symptoms in chronic functional obstruction, such as intestinal pseudo-obstruction, megacolon, and Hirschsprung’s disease [8–10]. As there is no specific analgesic for distention-associated pain in BO, high dose opioids are the primary anti-pain treatment for abdominal pain in BO [4–6, 11]. However, opiates are well known to further cause opioid-induced bowel dysfunction, i.e. constipation and narcotic bowel syndrome [12, 13]. It is thus imperative to uncover the mechanisms of distension-associated abdominal pain, so that specific therapeutic targets may be identified.
Visceral hypersensitivity (VHS) is a well-recognized mechanism for abdominal pain [14–18]. Abnormal hyper-excitability of primary sensory neurons plays an important role in the development of VHS [15–17]. Recent studies found that the cell excitability of dorsal root ganglia (DRG) neurons and visceral sensitivity were augmented significantly in BO [19, 20]. However, the mechanisms underlying neuronal hyper-excitability in BO remain incompletely understood.
Neuron cell excitability is determined in large part by voltage-gated ion channels (VGIC) in the cell membrane [15–17]. Recent studies found that activities of voltage-gated Na+ channels (Nav) especially TTX-resistant Nav are significantly increased in DRG neurons in BO [20]. However, it is not known if other ion channels are involved in sensory neuron hyper-excitability in BO. Voltage-gated potassium channels (Kv) represent the most diverse class of VGIC from functional and structural standpoints. Kv regulate cell excitability by affecting resting membrane potential (RP) and the duration and frequency of action potential (AP) in sensory neurons [21, 22]. Two major types of Kv currents are identified in primary sensory neurons: slowly inactivating ‘delayed’ currents (IK) and rapidly inactivating ‘transient’ A-type currents (IA) [21–23]. The IA group subtypes include Kv1.4 and Kv4.3, and the IK subtypes, Kv1.1 [21, 22]. It is not known whether Kv function and expression are altered in BO, and if so what accounts for the changes.
Brain-derived neurotrophic factor (BDNF) belongs to the neurotrophin (NT) family of growth factors [24, 25]. There is ample evidence suggesting that BDNF regulates pain processing [24, 26, 27]. BDNF may be released from peripheral tissues and DRG neurons, and acts on the low affinity p75 NT receptor and high affinity tyrosine receptor kinase B (Trk B) [24]. As a pain mediator, BDNF is well known for its role in central sensitization of pain processing [24, 26, 27]. However, recent studies suggest that BDNF may also be involved in peripheral sensitization of visceral pain pathways by acting on primary afferent neurons in conditions such as gut inflammation and irritable bowel syndrome [28, 29]. In these conditions, BDNF was reportedly released from inflammatory cells in the peripheral tissues [28, 29]. As it was reported recently, BDNF may modulate K+ channel expression and function in the primary sensory neurons [25, 30, 31].
Lumen distention-associated mechanical stretch is a cardinal feature in bowel obstruction [32–34]. In the present study, we tested the hypothesis that mechanical stress-induced expression of BDNF in the obstructed colon plays a crucial role in the development of sensory neuron hyper-excitability by altering Kv activity in colon-projecting sensory neurons (colon neurons), contributing to distention-associated abdominal pain in BO. We found that mechanical stretch in BO induced a robust expression of BDNF selectively in gut smooth muscle cells (SMC). We examined whether Kv currents are altered in the colon neurons, and determined if BDNF up-regulation in the colon contributes to the Kv current changes and abnormal hyper-excitability in the colon-projecting neurons. Furthermore, we sought to determine the molecular mechanisms underlying the changes of Kv activity in colon neurons in obstruction.
METHODS
Rat model of bowel obstruction
The Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch (UTMB) in Galveston approved all procedures performed on the animals in the study. Experiments were performed in accordance to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA. Sprague-Dawley rats (male) weighing 200–275 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). The rats were housed in a controlled environment (22°C, 12-h light-dark cycle) and allowed food and water ad libitum.
The rodent model of partial colon obstruction was prepared by following procedures described previously [33–35]. In brief, rats were anesthetized with 2% isoflurane inhalation by an E-Z Anesthesia vaporizer (Palmer, PA). After midline laparotomy, a distal colon segment 4 cm proximal to the end of colon was carefully exposed. A small mesenteric opening (5 × 5 mm2) was made next to the exposed colon segment. A 3-mm wide medical grade silicon ring was placed around the colon wall through the small mesenteric opening. The size of the silicon ring (21 mm in length) was ~1–2 mm greater than the outer circumference of the colon when the colon segment was filled with fecal pellets, allowing partial obstruction. The procedure to implement the silicon ring was completed within 2 min. The sham control rats underwent the same surgical procedure except that the ring was removed immediately after the 2-min procedure [34]. Rats were euthanized at different time points up to 7 days following the induction of partial obstruction. A 3-cm-long colon segment starting at 1 cm oral to the site of obstruction was collected as distended tissue, and a 2-cm-long colon segment starting at 0.5 cm aboral to the obstruction was taken as non-distended internal control. The mucosal/submucosal (M/SM) and muscularis externa (ME) layers were separated by microdissection as described previously [32, 38–40]. These tissues were used for histological, biochemical, and molecular studies.
In some in vivo experiments, specific Trk B inhibitor ANA-12 (1 mg/kg in 200 microliter 10% DMSO, i.p.) (Sigma-Aldrich, St. Louis, MO] [36, 37] was administered to sham and BO rats once a day on day 2, 4, and 6. Rats were euthanized on day 7. Colon tissue and DRG were isolated for further molecular and electrophysiological studies.
Primary culture of RCCSMC and in vitro stretch of RCCSMC in culture
Rat colonic circular smooth muscle cells (RCCSMC) were isolated as described previously [34, 35, 41]. In brief, the circular muscle tissue pieces in 0.5 × 0.5 cm2 size were incubated in sterile HEPES buffer (in mmol/l: 120 NaCl, 2.6 KH2SO4, 4 KCl, 2 CaCl2, 0.6 MgCl2, 25 HEPES, 14 glucose, and 2.1% essential amino acid mixture, pH 7.4) with 1.5 mg/ml collagenase (type II, 319 U/mg; Worthington, Freehold, NJ) and 1.0 mg/ml soybean trypsin inhibitor (Sigma-Aldrich) for 45 min at 31°C. At the end of digestion, tissue pieces were incubated in fresh buffer without digestion enzymes. The spontaneously dispersed cells were collected and cultured in DMEM supplemented with 10% fetal bovine serum in the presence of 100 U/ml of penicillin G, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B (Invitrogen). The culture medium was changed every 3 days. Immunofluorescence staining showed that more than 95% of the cultured cells stained positive for smooth muscle-specific α-actin [34, 35, 41, 42].
Primary culture of RCCSMC was allowed to grow for 8–10 days until it was confluent. The cells were then seeded at 8 × 104 cells/well in six-well BioFlex culture plates coated with type I collagen (Flexcell, Hillsborough, NC), grown to ~80% confluence, and then subjected to DMEM/1% FBS for 24 h prior to stretch. Cells were subjected to stretch via a FX-4000 Flexercell Tension Plus System (Flexcell). This computer-regulated bioreactor applies multiaxial strain to cultured cells [34, 35, 39]. Through vacuum pressure, cultured cells are deformed on flexible membrane plates. To mimic tonic lumen distention as in BO, cells were subjected to static, rather than cyclic, stretch at 5, 10, or 18% elongation in the experiments of this study. Cells incubated in parallel under identical conditions but without exposure to stretch served as controls.
Measurement of referred visceral sensitivity
Direct assessment of visceral sensitivity by measuring visceromotor response (VMR) to colorectal distension with a balloon [23, 38, 43, 44] is not feasible during colon obstruction. However, as visceral pain has the unique feature that a painful sensation is found in a referred somatic region, we measured referred visceral sensitivity in our model with Von Frey filament (VFF) test as described elsewhere [15, 19, 20]. Rats were shaved in the abdomen, and a 3 × 3 cm2 area of the lower abdomen along the midline was marked for VFF test. Rats were kept in a translucent cage (3.5 in × 7.0 in × 3.5 in) for 30 min for pre-test adaptation and during VFF test. The Von Frey filaments for each of the forces (0.1, 0.2, 0.5, 1.0, 2.0, 4.0 and 8.0 g) were applied to the marked lower abdomen 10 times (each for 2 seconds at 10-second interval) in an ascending order of forces. The numbers of withdrawal responses including sharp abdominal retraction, licking or scratching of the filament, or immediate movement or jumping were recorded.
Labeling of colon specific sensory neurons in DRG for patch clamp study and for mRNA detection
Colon specific neurons in DRG were labeled for patch clamp recordings as described previously [20, 28, 34] by injecting 1,1′-dioleyl-3,3,3′,3-tetramethylindocarbocyanine methane sulfonate (DiI, Invitrogen, Carlsbad, CA) into the colon wall during the same operation to induce obstruction. Five microliter (5 μl) of DiI (50 mg/ml in methanol) was injected into the muscle layer of the gut wall at 6~8 sites of the mid colon segment (~4 cm in length) oral to obstruction in BO rats or the counterpart mid colon in sham controls. Animals were returned to normal housing until euthanasia for patch clamp recordings 7 days after DiI injection.
To determine gene expression in colon specific neurons, cholera toxin B subunit (CTB, 40 μg in 20 μL PBS per rat) (Invitrogen) was injected to the wall of mid colon (6 sites). After 7 days, rats were euthanized, and DRGs were isolated, immediately embedded in OCT, frozen in dry ice and stored at −80 °C until further processing. Then, serial sections (10 or 20 μm thick) of DRGs were cut at −20 °C using a cryostat (Thermo Scientific). The sections were mounted on glass slides, dehydrated, and cleared. The CTB labeled colon specific DRG neurons were identified under fluorescence microscope (488 nm) and harvested by laser capture micro-dissection [20, 34] for qPCR detection of mRNA expression.
DRG neuron dispersion and patch-clamp study
DRG neurons from sham and obstruction rats were isolated as described previously [20, 34, 38, 44]. Briefly, rats were euthanized by decapitation. The spinal cord was removed and transferred to ice-cold, oxygenated fresh dissecting solution containing (in mmol/l) 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES, pH 7.2 (osmolarity = 305 mosM). Thoracolumbar DRG (T13–L2) were obtained bilaterally. The ganglia were digested in dissecting solution containing collagenase D (~1.5 mg/ml; Roche, Indianapolis, IN) and trypsin (~1.2 mg/ml; Sigma, St. Louis, MO) at 34.5°C for 90 min. The samples were washed in enzyme-free solution containing DNase (0.5 mg/mL, Sigma) and triturated repetitively with glass pipettes to obtain single cell suspension. The cell suspension was then plated onto culture dishes containing acid-cleaned coverslips and incubated in 5% CO2 at 37°C before they were used for electrophysiological recordings. In some experiments, neurons cells were treated for 3 to 4 hours with recombinant BDNF (Sigma-Aldrich, St. Louise, MS), anti-BDNF antibody (Millipore, Temecula, CA), or vehicle control, before they were recorded.
Cells were plated onto acid-cleaned glass coverslips and perfused with normal external solution containing (in mmol/L) 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH (295–300 mOsM). Recording pipettes, pulled from borosilicate glass tubing, had resistance of 4–7 MΩ and filled with pipette solution containing (in mM): 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, with pH adjusted to 7.4 with NaOH (osmolarity: 305 mOsM). DiI-labeled small- to medium-sized DRG neurons (< 35 μm in diameter) were accepted for analysis if they had a stable resting membrane potential (> −45 mV) and displayed overshooting action potentials. The small- to medium-sized neurons are chosen, as they are involved in the transmission of nociception in rat colon [17, 20, 45]. DiI-labeled colon-specific DRG neurons were identified by using a fluorescence microscope (Olympus, Tokyo, Japan) with a rhodamine filter (excitation 546 mm, barrier filter at 580 mm). Whole-cell current and voltage were recorded by a Dagan 3911 patch-clamp amplifier (Dagan, Minneapolis, MN) as described previously [20, 34, 38]. Capacitive transients were corrected by using capacitive cancellation circuitry on the amplifier that yielded the whole-cell capacitance and access resistance. Up to 90% of the series resistance was compensated electronically. The currents were filtered at ~2–5 kHz and sampled at 50 or 100 μs per point. Data were acquired and stored on a Dell computer for later analysis by using pCLAMP 9.2 (Axon Instruments, Sunnyvale, CA). All experiments were performed at 22°C.
Kv currents were recorded as previously described [23, 46]. Na+ in control external solution was replaced with equimolar choline and the Ca2+ concentration was reduced to 0.03 mM to suppress Ca2+ currents and to prevent Ca2+ channels becoming Na+ conducting [47]. The reduced external Ca2+ would also be expected to suppress Ca2+-activated K+ current. The two kinetically distinct Kv currents, IA and IK, were isolated by the biophysical analysis and pharmacological approaches described in previous studies [48–50]. IA and IK were separated biophysically by manipulating the holding potentials. The total outward currents (Itotal) were recorded in response to voltage steps from −100 to +30 mV in 5-mV increments with duration of 400 ms. IK was isolated when the membrane potential was held at −50 mV. Subtraction of IK from Itotal represents IA. To control for changes in cell size, the current density was measured by dividing the current amplitude by whole cell membrane capacitance (pA/pF), which was obtained by reading the value for whole cell input capacitance cancellation directly from the patch-clamp amplifier.
RNA preparation and quantitative RT-PCR
Total RNA was extracted from colon ME and M/SM tissues, and from CTB-labeled colon specific DRG neurons by using the Qiagen RNeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was reverse-transcribed with the SuperScript III First-Strand Synthesis System (Invitrogen) for quantitative RT-PCR with the Applied Biosystems 7000 real-time PCR system (Foster City, CA) [20, 32–34, 39].
The assay IDs for TaqMan detection of rat Trk A, Trk B, Kv1.4, Kv4.3, mRNAs are Rn00572130, Rn01441749, Rn02532059 and Rn01534234, respectively (Applied Biosystems). For relative quantification of mRNA expression, real-time qPCR was performed with 40 ng of cDNA for the target gene and for the endogenous control 18S rRNA (Part no. 4352930E, Applied Biosystems).
Western blot
Colonic muscularis externae (ME) and mucosa/submucosa (M/S) were homogenized on ice in lysis buffer supplemented with protease inhibitor cocktails (Sigma-Aldrich, St. Louis, MO) as described previously [34, 35, 40, 51]. After spinning at 12,000 g at 4°C for 15 min, the supernatant proteins were collected and resolved by a standard immunoblotting method [34, 35, 40, 51]. Equal quantities of total protein were run on premade 4–12% Bis-Tris SDS-PAGE (Invitrogen, Carlsbad, CA). The primary antibody to BDNF (1:200) was purchased from Santa Cruz (Santa Cruz, CA). β-actin antibody (1:5,000, Sigma) was used as loading control. The protein detection was performed using ODYSSEY Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Immunohistochemistry study
Immunohistochemical staining of BDNF protein was performed on formalin-fixed, paraffin-embedded mid colon segments (5–6 cm from the anus) isolated from rats in sham control and with obstruction, as described previously [20, 34]. Sections at 4-μm thickness were blocked with 5% normal goat serum in PBS for 20 min at room temperature, and incubated with the rabbit anti-BDNF antibody (1:200, Santa Cruz Biotech, CA) and a biotin-conjugated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA). After being incubated with avidin-biotin complex (Vector kit, Vector Laboratories), the sections were stained in diaminobenzidine tetrahydrochloride with 0.03% hydrogen peroxide. As a negative control, sections of the same specimens were processed by the same method but omitting anti-NGF primary antibody.
Statistical analysis
All data points are expressed as means ± SEM. Statistical analysis was performed by analysis of variance with non-repeated measures (by Student-Newman-Keuls test) for comparisons of multiple groups and Student’s t-test for comparisons of two groups. A p value of ≤0.05 was considered statistically significant.
RESULTS
1. Mechanical stress-induced expression of BDNF mRNA and protein in colon smooth muscle cells in bowel obstruction
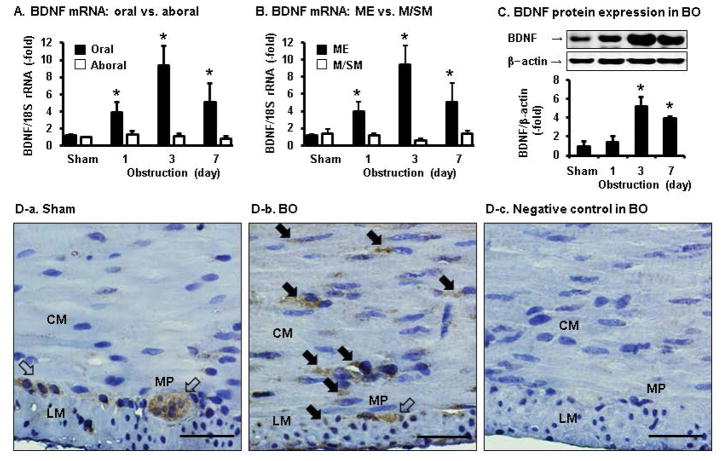
We first determined if the expression levels of BDNF mRNA and protein were altered in the colon tissue in obstruction. Quantitative RT-PCR revealed that BDNF mRNA expression was dramatically increased in a time-dependent manner (day 1 to day 7) in the colonic muscularis externae (ME) of the dilated segment oral to obstruction, but not in the non-dilated aboral segment (Fig. 1A). The maximal increase of BDNF mRNA expression was 8.0(+/−1.9) –fold on day 3 in BO (p < 0.01 vs. sham), compared to sham controls. Interestingly, the BDNF mRNA expression was not increased in the mucosa/submucosa (M/SM) layer (Fig. 1B). Western blot results showed that BDNF protein expression was significantly increased in the ME on day 3 and day 7 of obstruction (Fig. 1C).
Fig. 1.
Expression of BDNF mRNA and protein in the colon in BO detected by qPCR (A, B), Western blot (C), and immunohistochemistry (D). Note that BDNF gene expression was up-regulated in the distended oral segment, but not in the non-distended aboral segment (A). The up-regulation of BDNF mRNA expression in the distended colon occurred in the muscularis externae (ME), but not mucosa/submucosa layer (M/SM). Shown in panel C was Western blot detection of BDNF in the colonic ME samples of the oral segment in sham and BO rats (day 1 to 7). N = 5 or 6 in each group or time point, * p < 0.05 vs sham. Immunohistochemical staining of BDNF (in brown) in the muscularis externae (ME) of mid colon specimens is shown in sham (D–a) and BO (day 3, D–b). Note that BDNF expression was detected only in the myenteric plexus (MP) (white arrow) in sham control. However, BDNF expression was detected also in SMC (solid black arrow) in obstruction. Image D–c is the negative control result where no anti-BDNF antibody was added into the reaction. CM, circular muscle; LM, longitudinal muscle; MP, myenteric plexus. Bar, 50 μm. Images are representatives of 4 independent experiments.
Further immunohistochemistry study of the ME revealed that BDNF was detectable only in the myenteric plexus of the colon in sham control rats (Fig. 1D–a). However, in the obstructed colon, BDNF expression was detected also in the SMC (Fig. 1D–b). When no anti-BDNF antibody was added into the reaction, we detected no staining in either myenteric plexus or smooth muscle cells (Fig. 1D–c).
The above in vivo studies indicate that mechanical stress may be the driving force for the up-regulated expression of BDNF in colon SMC in BO. We further determined in vitro in the primary culture of rat colonic SMC whether mechanical stretch with a Flexcell system [34, 35] alters gene expression of BDNF in the cells. When the cells were stretched at 5% elongation for 1 hour and samples harvested 3 hours later, we found no significant change of BDNF mRNA in the stretched samples compared to no-stretch controls. However, 10% and 18% elongation of the cells significantly increased BDNF mRNA expression by 1.6 (+/− 0.3)- and 1.8 (+/− 0.3)-fold, respectively (p < 0.05 vs. no-stretch controls. N = 5 or 6 independent experiments for each group) (Fig. 2).
Fig. 2.
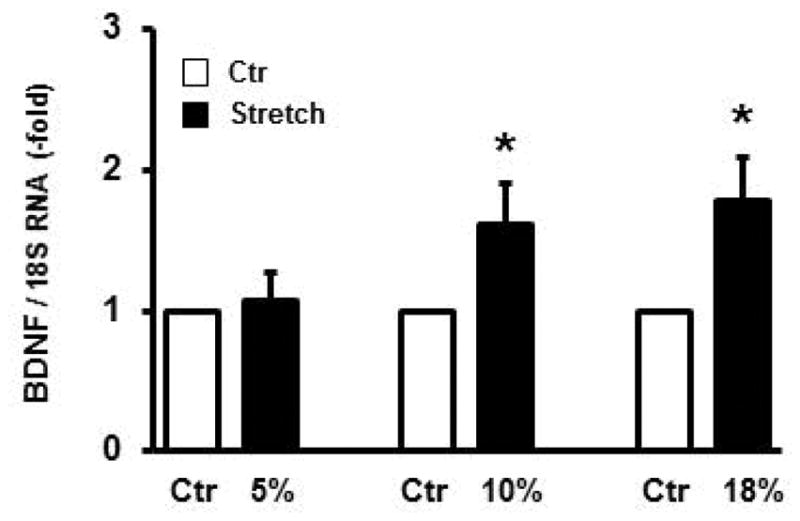
Effect of mechanical stretch on expression of BDNF mRNA in rat colonic circular SMC in vitro. Primary culture of rat colonic circular SMCs were treated without (empty bar) or with static stretch (filled bar) at different elongation (5%, 10%, and 18%) for 1 hr, and cells were harvested 3 hours after stretch ended. Total RNA was extracted for qPCR quantification of BDNF mRNA expression. N = 5 or 6 independent experiments, * p < 0.05 vs no-stretch (N.S.) control.
2. Abnormal hyper-excitability of primary sensory neurons in bowel obstruction
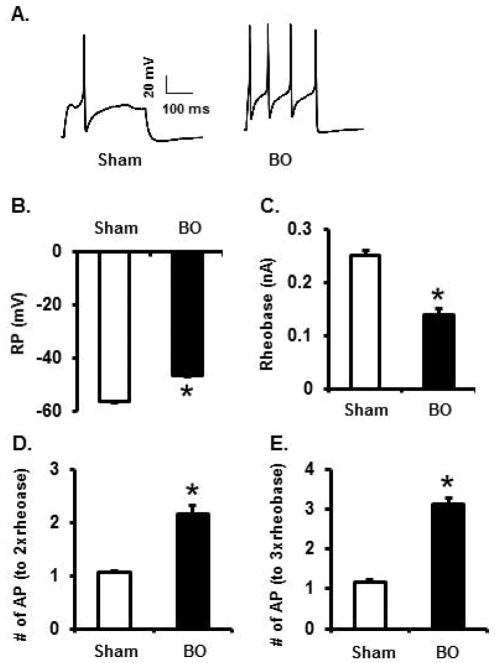
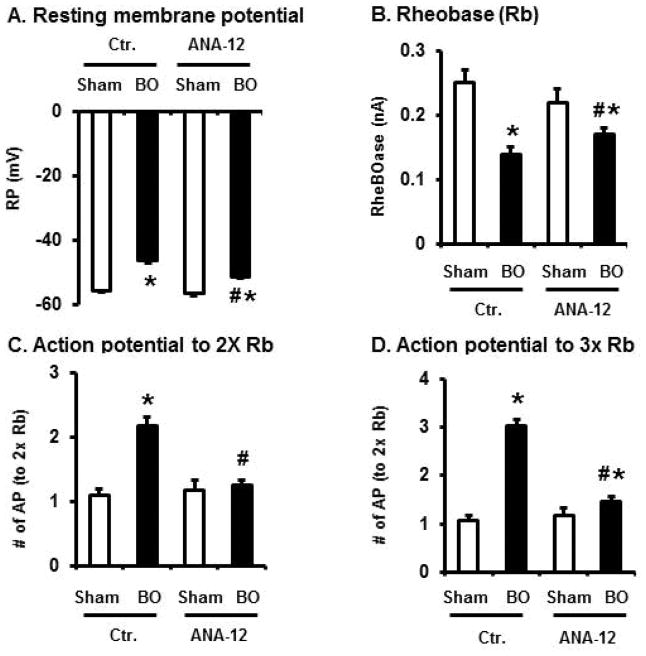
Abnormal hyper-excitability of primary sensory neurons contributes to peripheral sensitization and visceral pain [15–17]. We found that membrane excitability of colon specific DRG neurons was markedly augmented in BO (day 7) (Fig. 3A–E). The resting membrane potential (RP) became less hyperpolarized from −55.9 (+/− 0.47) mV in sham to −46.2(+/− 0.75) mV in BO (p < 0.01, N = 5 sham rats with 31 neurons recorded, and 5 BO rats with 25 neurons recorded). The rheobase was reduced from 0.25 (+/− 0.01) nA in sham to 0.14 (+/− 0.01) nA in BO (p < 0.01). Moreover, 2× and 3× rheobase stimulations induced significantly more action potentials (AP) in BO, compared to sham. This data suggest that visceral sensitivity was heightened in the BO rats, compared to sham.
Fig. 3.
Patch clamp study of cell excitability on colon projecting DRG neurons in rats with sham surgery and bowel obstruction (BO) for 7 days. The colon-specific DRG neurons were highly excited in BO rats (representative trace in A) with decreased resting membrane potential (B) and rheobase (C), and increased number of action potentials in response to stimulation of 2 times and 3 times of rheobase (D and E, respectively). The tracings in panel A represent recordings of neurons from sham and BO rats in response to stimulation of 3 times rheobase. N = 31 neurons (5 rats) in sham and 25 neurons in BO (5 rats). *p < 0.05 vs. sham.
3. Reduction of voltage gated K+ channel activity and in primary sensory neurons in bowel obstruction
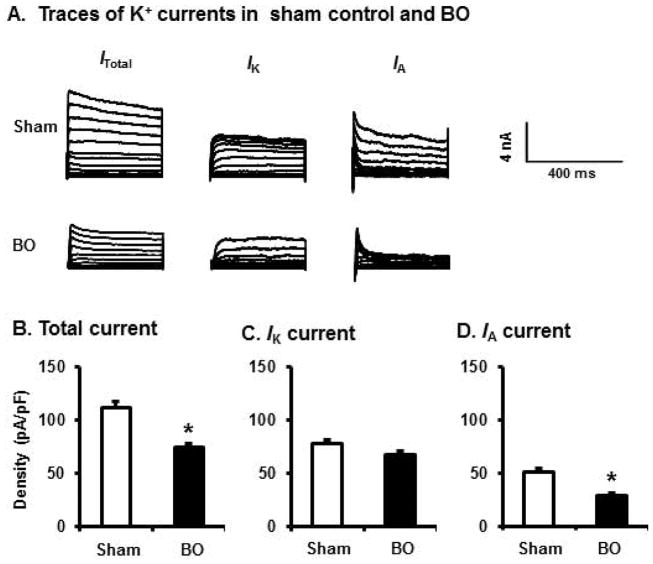
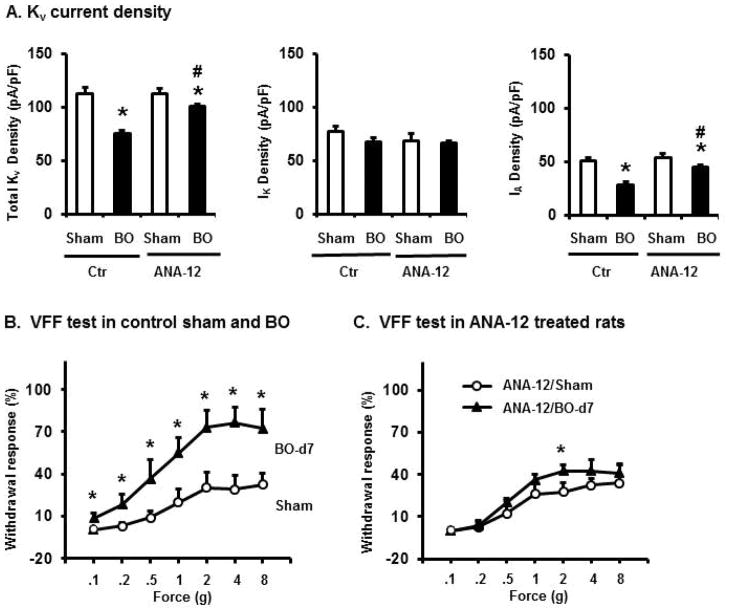
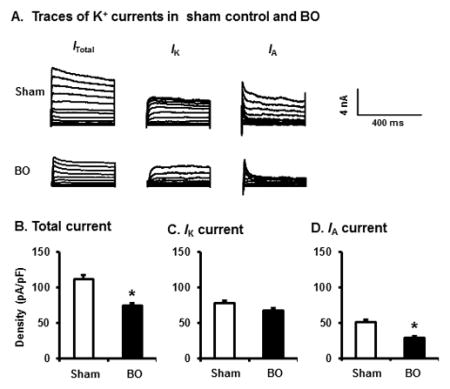
Voltage gated K+ channels (Kv) play essential roles in establishing membrane excitability [21, 22]. To determine if altered Kv channel activity is involved in the abnormal hyper-excitability of DRG neurons in obstruction, we recorded Kv currents in the colon-projecting neurons from sham and BO rats on day 7 after operation. Under voltage-clamp condition, neurons from both sham and BO rats exhibited transient A-type (IA) and sustained outward rectifier K+ currents (IK) (Fig. 4A–D). Compared with sham controls, colon neurons of the BO rats demonstrated significantly reduced densities of total K+ current (ITotal) and IA current. The mean peak density of ITotal is 121.9+/−9.6 pA/pF in sham vs 79.9+/−5.1 pA/pF in BO (p < 0.01). The mean peak density of IA is 57.8+/−3.8 pA/pF in sham, and 33.2+/−5.0 pA/pF in BO (p < 0.01). However, the average IK density was not significantly altered in BO.
Fig. 4.
Voltage-gated K+ current (Kv) in colon-projecting DRG neurons in sham and BO (day 7) rats. (A) Representative traces of Kv in sham and BO rats, with the Itotal in the left, the IK in the center of the panel, and the IA in the right. For total KV current (Itotal), the membrane potential was held at −100 mV and voltage steps were from −40 to +30 mV with 5-mV increments and 400-ms duration. For sustained Kv current (IK), the membrane potential was held at −50 mV and the voltage steps were the same as above. Currents generated by these two protocols were subtracted to produce IA. Bar graphs of the mean peak Kv density of DRG neurons from sham and BO rats were displayed in B for Itotal, in C for IK, and in D for IA currents. The current density (in pA/pF) was calculated by dividing the current amplitude by cell membrane capacitance. N= 30 neurons (5 rats) in sham and 31 neurons (6 rats) in BO. *p < 0.05 vs. sham.
5. Effects of anti-BDNF treatment
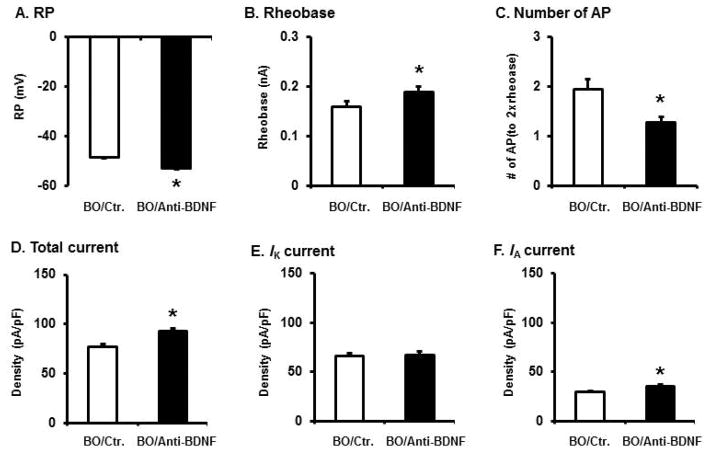
Previous in vitro studies showed that incubation of naïve DRG neurons with recombinant BDNF reduced total Kv and IA currents, and increased cell excitability [21]. To determine if up-regulation of BDNF in the colon plays a role in DRG neurons hyper-excitability and reduction of Kv activity in BO, we incubated DRG neurons isolated from BO rats with control medium and anti-BDNF antibody (0.1 μg/mL) for 24 hr. The colon-specific DRG neurons (labeled with DiI) were identified and recorded. As shown in Fig. 5, treatment of anti-BDNF antibody significantly attenuated BO-associated hyper-excitability of colon neurons (Fig. 5A–C) and increased the total and IA currents in these neurons (Fig. 5D–F). The IK current was not significantly changed by anti-BDNF treatment.
Fig. 5.
Effects of anti-BDNF antibody on membrane excitability (A–C) and Kv (D–F) in colon-projecting DRG neurons of BO rats. DRG neurons were isolated from T13 to L2 of rats with BO (7 days), and were incubated at 37°C for 24 hr in the absence and presence of anti-BDNF (0.1 μg/mL). DiI labelled colon projecting neurons were identified for patch clamp recording. N = 18 neurons recorded in each group (4 independent experiments). *p < 0.05 vs BO control.
5. Role of BDNF and Trk B in visceral hypersensitivity in BO: effects of Trk B inhibitor ANA-12
As BDNF acts on Trk B receptor in afferent nerve, we examined the in vivo effect of specific Trk B inhibitor ANA-12 on cell excitability and Kv function in the colon-projecting DRG neurons in obstruction. Our results revealed that ANA-12 significantly attenuated hyper-excitability (Fig. 6A–D) and blocked the reductions of Itotal and IA currents in OB (Fig. 7A).
Fig. 6.
Effect of ANA-12 treatment on sensory neurons’ excitability in BO. Sham and BO rats were treated without or with Trk B inhibitor ANA-12 (1 mg/kg in 200 microliter 10% DMSO, i.p.) once a day on day 2, 4, and 6. Rats were euthanized on day 7 for DRG neuron isolation and patch clamp. N = 20~31 neurons in each group (4 or 5 rats). * p<0.05 vs sham. # p<0.05 vs BO.
Fig. 7.
Effect of ANA-12 administration on Kv activity (panel A) and on withdrawal response (B and C) in sham and BO rats. Rats were euthanized on day 7 for DRG neuron isolation for patch clamp recording of Kv activity. ANA-12 treatment prevented reduction of total Kv and IA activity. N = 20~31 neurons (4 or 5 rats) in each group. * p<0.05 vs sham. # p<0.05 vs BO. The VFF test showed that the referred sensitivity to mechanical stimulation to abdominal wall was significantly increased in BO (day 7) (B). However, ANA-12 treatment partially attenuated obstruction-associated refereed hypersensitivity (C). N= 6 rats in each group. *p < 0.05 vs. sham control of the group.
Consistent with earlier reports [20], bowel obstruction significantly increased the withdrawal response of the rats to mechanical stimulation with von Frey filaments to the abdominal wall, indicating a heightened referred visceral sensitivity in BO. However, ANA-12 treatment significantly improved the referred hypersensitivity in obstruction (Fig. 7B, 7C).
6 Gene expression of neurotrophin receptors and Kv subunits in colon projecting DRG neurons
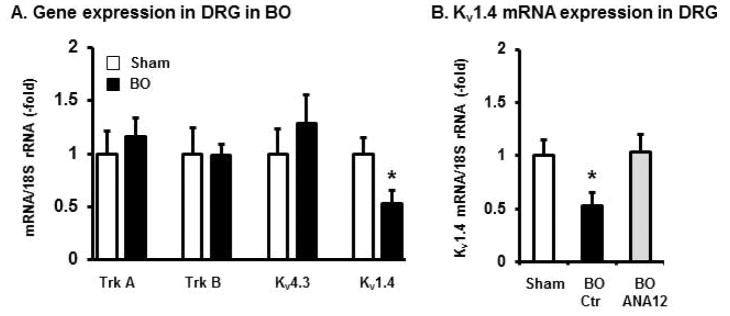
In an attempt to understand the mechanisms of altered Kv activity and abnormal hyper-excitability in the sensory neurons in BO, we determined mRNA expression of Trk A, Trk B, and select Kv subtypes in the colon-specific DRG neurons isolated by laser capture microdissection [20, 38]. As patch clamp showed that IA, but not IK, activity is selectively altered in BO, we chose to determine the expression of IA subunits Kv1.4 and Kv4.3 mRNAs. Our qPCR results showed that mRNA expression of Trk A, Trk B, and Kv4.3 was not significantly altered in obstruction (Fig. 8A). However, the Kv1.4 mRNA was significantly down-regulated in BO. In a separate experiment, we found that the mRNA expression of Trk B in the peripheral tissue (colon muscularis externae) was not altered in obstruction (98±36% in BO vs. 100±9% in sham, p > 0.05. N = 5 each group).
Fig. 8.
Expression of Trk A, Trk B, Kv4.3 and Kv1.4 mRNA in colon-specific DRG neurons. (A) Colon-specific DRG neurons were labeled retrogradely by injecting CTB to the mid colon wall of the sham and BO (day 7) rats, and were isolated with laser capture microdissection. Total RNA of the colon-specific DRG neurons was extracted for qPCR quantification of mRNA expression of Trk A, Trk B, Kv4.3 (KCND3), and Kv1.4 (KCNA4) in sham (empty bar) and BO (solid bar) rats. (B) Kv1.4 (KCNA4) mRNA quantification in sham, BO, and BO treated with ANA administration. N = 4 or 5 for each group, * p ≤ 0.05 vs. sham control.
Moreover, ANA-12 treatment significantly blocked the change of Kv1.4 gene expression (Fig. 8B). This data indicate that BDNF through Trk B receptor may alter Kv function (i.e. IA current) in BO via modulating specific Kv gene expression in afferent nerve.
DISCUSSION
In the present study, we confirmed that colon neuron excitability and visceral sensitivity were significantly augmented in a rodent model of BO. The abnormal hyper-excitability is due to the changes of lumen distension, as the dye used to label colon-projecting DRG neurons was injected to the distended colon segment (mid colon) in the BO rats and the mid colon of sham control rats. Our study showed that peripheral up-regulation of BDNF expression may play a critical role in the abnormal hyper-excitability of primary sensory neurons in BO, as intra-peritoneal administration with ANA-12, a Trk B inhibitor, significantly blocked hyper-excitability of the colon neurons in BO. This was associated with attenuation of referred visceral hypersensitivity. Moreover, anti-BDNF antibody treatment significantly attenuated membrane hyper-excitability of the colon neurons isolated from BO rats, while previous studies showed that exogenous BDNF increased cell excitability of naïve DRG neurons [21].
We determined the source of increased BDNF in obstruction. Quantitative RT-PCR detected up-regulation of BDNF mRNA expression in the colon segment oral to the site of obstruction, but not in the non-distended aboral segment. This data suggest that the increased BDNF expression is due to mechanical stress, as the oral segment is mechanically distended, whereas the aboral segment remained not distended. The increased BDNF production in the colon is unlikely due to any possible inflammatory changes associated with surgical operation, as there was no increase in BDNF expression in sham rats, which went through the similar surgical process except that in the sham controls the obstruction band was released immediately after it was implanted. Furthermore, if there is any inflammation-associated increase of BDNF in the site of obstruction, BDNF expression would be increased in both segments oral and aboral to the site of obstruction. We found that BO induced expression of BDNF selectively in the colonic SMC, as the BDNF mRNA expression remained not changed in the mucosa/submucosa in obstruction, compared to sham. Immunohistochemical study detected BDNF in colonic SMC in obstruction, but not in sham control. Further studies in vitro confirmed that direct stretch of colon SMC induced mechano-transcription of BDNF.
Previous studies found that circumferential mechanical stretch as encountered in BO initiates mechano-transcription of COX-2 [34] and NGF [20] as well in gut SMC. The mechanical stress-induced gene expression of COX-2, NGF, and BDNF in gut SMC plays critical roles in motility dysfunction and abdominal pain in obstruction [34, 20, 52]. Taken together, these studies indicate that gut SMC, via the mechano-transcription mechanism [52], has a unique role in the pathogenesis of gut dysfunction in obstructive bowel disorders. It is noteworthy that increased expression of COX-2 peaked on day 1 to day 3 of BO [34]. Other inflammatory mediators such as MCP-1 and iNOS were found increased only in the first 1 to 3 days of obstruction [39]. However, obstruction induces long-lasting expression of neurotrophins NGF and BDNF. By day 7, the increase of NGF and BDNF in the distended bowel is still several fold higher than in sham controls [20]. These data indicate that inflammatory mediators may contribute to abdominal pain only in acute phase of lumen distention [38], whereas neurotrophins may account for long-lasting chronic pain in BO, especially with their profound genomic effects (i.e. altered expression of Nav1.8 and Kv1.4) on sensory neurons [20].
BDNF is well known for its role in central sensitization [24, 26, 27]. Normally, BDNF exists in DRG neurons, and serves as a modulator at the first synapse of the pain transmission pathway in the spinal dorsal horn [24, 27]. Several recent studies reported that BDNF produced from inflammatory cells in the gut may contribute to peripheral mechanisms of visceral hypersensitivity [28, 29]. In the present study, we found that obstruction-associated mechanical stress induced expression of BDNF in colon SMC of the distended colon. More importantly, systemic administration of Trk B inhibitor ANA-12 significantly attenuated neuronal hyper-excitability in BO. These data thus suggest that BDNF produced in gut smooth muscle of the obstructed bowel plays a critical role in peripheral sensitization of pain pathway in obstructive conditions.
As cell excitability was augmented remarkably in the colon-projecting DRG neurons in BO, we further studied the cellular mechanisms and ionic basis responsible for the hyper-excitability in the neurons. Voltage-gated K+ channels regulate neuronal excitability by affecting resting membrane potential and the duration and frequency of action potential [21–23]. A reduction of Kv activity can increase neuronal excitability [22, 23]. Among the two types of Kv currents in primary sensory neurons, IA is particularly important in the control of the spikes’ onset, the threshold of action potential firing, and the firing frequency. The IK is also involved in the control of the threshold of action potential firing. There are many different types of IK and IA subunits. Among the IA subunits are Kv1.4 and Kv4.3 [21, 22]. Our study showed that Kv activity, particularly the IA current, was suppressed significantly in the colon-projecting neurons in BO. The decreased Kv activity may well contribute to the sensory neuron hyper-excitability in BO.
BDNF has been reported to mediate Kv expression and activity in DRG neurons [21]. To determine if BDNF is responsible for the reduction of Kv activity in BO, we took a comprehensive approach. First, anti-BDNF antibody treatment significantly increased Kv, especially IA currents, and partially restored the membrane excitability of the colon neurons isolated from BO rats. Further in vivo studies showed that inhibition of BDNF receptor Trk B with ANA-12 administration blocked BO-associated reduction of Kv activity, especially IA activity. This is associated with improved sensory neuron excitability and withdrawal response to mechanical stimulation to the abdominal wall.
We further investigated the molecular mechanisms involved in the reduction of Kv activity. As IA, but not IK, activity was profoundly decreased in BO, we sought to determine if the expression of any specific IA subunits was altered. Among the IA subunits, we found that the mRNA of Kv1.4, but not Kv4.3, was significantly down-regulated in BO. Moreover, inhibition of BDNF receptor almost completely blocked the down-regulation of Kv1.4 in the DRG neurons. Our study thus suggests that increased BDNF in the colon may act on primary sensory nerve endings, and lead to down-regulation of specific IA subunit, i.e. Kv1.4 and reduction of Kv activity in the DRG neurons. The mechanisms underlying BDNF-mediated changes of Kv1.4 expression in colon-projecting neurons is not known. However, it is well recognized that retrograde transport of neurotrophic factors and their receptors is required for their signal transduction [24, 53]. It is suspected that binding of colon SMC-derived BDNF to Trk B receptor on the sensory nerve will initiate endocytosis of Trk B at the nerve terminals. The BDNF-Trk B complex associated with adaptor proteins and signaling effectors will be retrogradely transported along the long processes to the cell body of DRG neurons [53]. It is in the cell body that BDNF may exert its activities to regulate Kv gene expression and activity of the DRG neurons.
In summary, we found that expression of BDNF mRNA and protein was dramatically increased by mechanical stress in the colon SMC in BO. The cell excitability of colon specific DRG neurons was markedly enhanced in BO, and the densities of total Kv and IA currents were significantly reduced. BO also led to down-regulation of IA subtype Kv1.4 gene expression in colon neurons in BO. Treatment with anti-BDNF antibody or TrK B inhibitor partially but significantly prevented BO-associated changes of colon neuron excitability, Kv activity and gene expression. This is associated with improved withdrawal response to the von Frey filament stimulation to the abdominal wall. Thus, mechanical stress-induced BDNF in colon SMC plays a critical role in visceral hypersensitivity in BO mainly by reducing A-type Kv activity and gene expression in sensory nerve. These novel findings may help to develop therapeutic approaches towards distention-associated abdominal pain in obstructive conditions.
Key points.
Distention-associated abdominal pain is a main symptom in bowel obstruction (BO). Previous studies found that visceral sensitivity is increased in BO. However, the mechanisms of visceral hypersensitivity in BO are incompletely understood.
We now found that mechanical stress-induced BDNF in gut SMC plays a critical role in visceral hypersensitivity in BO via suppression of transient A-type (IA) K+ channel activity and gene expression in primary sensory nerve.
These findings may help to identify therapeutic targets towards to distention-associated abdominal pain.
Acknowledgments
This study was supported in part by National Institute of Health (R01 DK102811 to XZS).
This study was funded in part by a grant from NIH/NIDDK (R01DK102811).
Footnotes
The authors report no any conflict of interest that might be perceived to influence the results or discussion of this study.
Author contributions: YF, research performance, data analysis, and manuscript draft; YML, animal model, research performance, data analysis; JHW, experiment protocol, research performance; RR, experiment protocol, data analysis; LYH, study design, protocol, and data analysis; XZS, study design and performance, data analysis, and manuscript draft. All proved the final version of the manuscript.
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Summers RW. Textbook of Gastroenterology. In: Yamada, Alpers, Laine, Owyang, Powell, editors. Approach to the patient with ileus and obstruction. Vol. 1. Lippincott Williams & Wilkins; Philadelphia, PA: 1999. pp. 842–858. Chapter 39. [Google Scholar]
- 2.Welch JP. Bowel obstruction. In: Welch JP, editor. General considerations and mortality. Chapter 3 Saunders; Philadelphia, PA: 1990. pp. 59–95. [Google Scholar]
- 3.Milenkovic M, Russo CA, Elixhauser A. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Health Care Research and Quality; Rockville, MD, USA: Sep, 2006. Hospital Stays for Gastrointestinal Diseases, 2004: Statistical Brief 12. [PubMed] [Google Scholar]
- 4.Ripamonti C, Mercadante S. Pathophysiology and management of malignant bowel obstruction. In: Doyle D, Hanks G, et al., editors. Oxford Textbook of Palliative Medicine. 3. New York Oxford University Press; NY: 2004. pp. 496–507. [Google Scholar]
- 5.Ripamonti C, Twycross R, Baines M, Bozzetti F, Capri S, De Conno F, et al. Clinical-practice recommendations for the management of bowel obstruction with end-stage cancer. Support Care Cancer. 2001;9:223–233. doi: 10.1007/s005200000198. [DOI] [PubMed] [Google Scholar]
- 6.Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep. 2009;11:298–303. doi: 10.1007/s11912-009-0042-2. [DOI] [PubMed] [Google Scholar]
- 7.Baines M, Oliver DJ, Carter RL. Medical management of intestinal obstruction in patients with advanced malignant disease: A clinical and pathological study. Lancet. 1985;326(8462):990–993. doi: 10.1016/s0140-6736(85)90534-3. [DOI] [PubMed] [Google Scholar]
- 8.De Giorgio R, Cogliandro RF, Barbara G, Corinaldesi R, Stanghellini V. Chronic intestinal pseudo-obstruction: Clinical features, diagnosis, and therapy. Gastroenterol Clin North Am. 2011;40(4):787–807. doi: 10.1016/j.gtc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Hanauer SB, Wald A. Acute and chronic megacolon. Curr Treat Options Gastroenterol. 2007;10(3):237–47. doi: 10.1007/s11938-007-0017-z. [DOI] [PubMed] [Google Scholar]
- 10.Nunez R, Blesa E, Cabrera R. Hirschsprung’s Disease: Clinical features. In: Nunez RN, Lopez-Alonso M, editors. Hirschsprung’s Disease: Diagnosis and treatment. Chapter 8. Nova Science Publishers; New York: 2009. pp. 125–136. [Google Scholar]
- 11.Jatoi A, Podratz KC, Gill P, Hartmann LC. Pathophysiology and palliation of inoperable bowel obstruction in patients with ovarian cancer. J Support Oncol. 2004;2(4):323–34. discussion 334–7. [PubMed] [Google Scholar]
- 12.Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep. 2013;15(9):344. doi: 10.1007/s11894-013-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5(10):1126–39. doi: 10.1016/j.cgh.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(Suppl):62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 15.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol gastrointest Liver Physiol. 2000;278(6):G834–38. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 16.Gold MS. Overview of pain and sensitization. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain. Informa Healthcare; 2006. pp. 17–32. [Google Scholar]
- 17.Bielefeldt K. Neurochemical and molecular basis of peripheral sensitization. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain. Informa Healthcare; 2006. pp. 67–83. [Google Scholar]
- 18.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107(1):271–93. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 19.Huang TY, Hanani M. Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G670–78. doi: 10.1152/ajpgi.00028.2005. [DOI] [PubMed] [Google Scholar]
- 20.Lin YM, Fu Y, Winston J, Radhakrishnan R, Sarna SK, Huang LM, et al. Pathogenesis of abdominal pain in bowel obstruction: role of mechanical stress-induced upregulation of nerve growth factor in gut smooth muscle cells. Pain. 2017;158(4):583–592. doi: 10.1097/j.pain.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem. 2010;114(5):1460–75. doi: 10.1111/j.1471-4159.2010.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vydyanathan A, Wu ZZ, Chen SR, Pan HL. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J Neurophysiol. 2005;93(6):3401–9. doi: 10.1152/jn.01267.2004. [DOI] [PubMed] [Google Scholar]
- 23.Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G424–31. doi: 10.1152/ajpgi.00560.2005. [DOI] [PubMed] [Google Scholar]
- 24.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 25.Cao XH, Chen SR, Li L, Pan HL. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J Neurochem. 2012;121(6):944–53. doi: 10.1111/j.1471-4159.2012.07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, Mcmahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A. 1999;96:7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delafoy L, Gelot A, Ardid D, Eschalier A, Bertrand C, Doherty AM, et al. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut. 2006;55(7):940–5. doi: 10.1136/gut.2005.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, et al. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61(5):685–94. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]
- 30.Kim DS, Choi JO, Rim HD, Cho HJ. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res Mol Brain Res. 2002;105(1–2):146–52. doi: 10.1016/s0169-328x(02)00388-1. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Choi JY, Kim RU, Lee YS, Cho HJ, Kim DS. Downregulation of voltage-gated potassium channel alpha gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol Cells. 2003;16(2):256–9. [PubMed] [Google Scholar]
- 32.Lin YM, Li F, Shi XZ. Mechano-transcription of COX-2 is a common response to lumen dilation of the rat gastrointestinal tract. Neurogastroenterol Motil. 2012;24(7):670–679. doi: 10.1111/j.1365-2982.2012.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YM, Sarna SK, Shi XZ. Prophylactic and therapeutic benefits of COX-2 inhibitor on motility dysfunction in bowel obstruction: roles of PGE2 and EP receptors. Am J Physiol Gastrointest Liver Physiol. 2012;302(2):G267–75. doi: 10.1152/ajpgi.00326.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi XZ, Lin YM, Powell DW, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol. 2011;300(1):G99–G108. doi: 10.1152/ajpgi.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li F, Lin YM, Sarna SK, Shi XZ. Cellular mechanism of mechano-transcription in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G670–679. doi: 10.1152/ajpgi.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cazorla M, Premont J, Man A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao XP, Zhang H, Wong-Riley M. Role of brain-derived neurotrophic factor in the excitatory-inhibitory imbalance during the critical period of postnatal respiratory development in the rat. Physiol Rep. 2015;3(11) doi: 10.14814/phy2.12631. pii: e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin YM, Fu Y, Wu CC, Xu GY, Huang LY, Shi XZ. Colon distention induces persistent visceral hypersensitivity by mechano-transcription of pain mediators in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2015;308(5):G434–41. doi: 10.1152/ajpgi.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YM, Li F, Shi XZ. Mechanical stress is a pro-inflammatory stimulus in the gut: in vitro, in vivo and ex vivo evidence. PLoS One. 2014;9(9):e106242. doi: 10.1371/journal.pone.0106242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi XZ, Sarna SK. Gene therapy of Cav1.2 channel with VIP and VIP receptor agonists and antagonists: a novel approach to designing promotility and antimotility agents. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G187–G196. doi: 10.1152/ajpgi.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi XZ, Sarna SK. Cell culture retains contractile phenotype but epigenetically modulates cell-signaling proteins of excitation-contraction coupling in colon smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2013;304(4):G337–45. doi: 10.1152/ajpgi.00369.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarna SK, Shi XZ. Function and regulation of colonic contractions in health and disease. In: Johnson LR, editor. Physiology of the gastrointestinal tract. Vol. 1. Amsterdam: Elsevier Academic Press; 2006. pp. 965–993. [Google Scholar]
- 43.Chen J, Winston JH, Fu Y, Guptarak J, Jensen KL, Shi XZ, Green TA, Sarna SK. Genesis of anxiety, depression, and ongoing abdominal discomfort in ulcerative colitis-like colon inflammation. Am J Physiol Regul Integr Comp Physiol. 2015;308(1):R18–27. doi: 10.1152/ajpregu.00298.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou YY, Wanner NJ, Xiao Y, Shi XZ, Jiang XH, Gu JG, et al. Electroacupuncture alleviates stress-induced visceral hypersensitivity through an opioid system in rats. World J Gastroenterol. 2012;18:7201–7211. doi: 10.3748/wjg.v18.i48.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brierley SM, Blackshaw . The neurobiology of visceral nociceptors. In: Pasricha PJ, Willis WD, Gebhart GF, editors. Chronic Abdominal and Visceral Pain. Informa Healthcare; 2006. pp. 45–66. [Google Scholar]
- 46.Fu Y, Xu GY, Chen JD, Shi XZ. Suppressed A-type K+ current accounts for enhanced excitability of colon specific sensory neurons in a rat model of irritable bowel syndrome: Therapeutic improvements by electroacupuncture (Abs) Gastroenterol. 2012;142(5, Suppl):S701. [Google Scholar]
- 47.Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akins PT, McCleskey EW. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993;56:759–769. doi: 10.1016/0306-4522(93)90372-m. [DOI] [PubMed] [Google Scholar]
- 49.Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- 50.McFarlane S, Cooper E. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol. 1991;66:1380–1391. doi: 10.1152/jn.1991.66.4.1380. [DOI] [PubMed] [Google Scholar]
- 51.Shi XZ, Sarna SK. G protein-mediated dysfunction of excitation-contraction coupling in ileal inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G899–905. doi: 10.1152/ajpgi.00408.2003. [DOI] [PubMed] [Google Scholar]
- 52.Shi XZ. Mechanical regulation of gene expression in gut smooth muscle cells. Front Physiol. 2017;8:1000. doi: 10.3389/fphys.2017.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito K, Enomoto H. Retrograde transport of neurotrophic factor signaling: implications in neuronal development and pathogenesis. J Biochem. 2016;160:77–85. doi: 10.1093/jb/mvw037. [DOI] [PubMed] [Google Scholar]