Abstract
Background
The reproducibility of gastric emptying (GE) measured with scintigraphy in patients is poorly understood. Our aims were to assess the intra and inter-individual reproducibility of these parameters in patients with upper gastrointestinal symptoms.
Methods
Sixty patients (21 diabetics, 39 non-diabetics) with upper gastrointestinal symptoms underwent scintigraphic-assessment of GE of a solid meal (296kcal,30% fat) over 4 hours on 2 occasions at an average interval of 15 days. The concordance correlation coefficient (CCC), intra and inter-individual coefficients of variation (COV) of GE endpoints were analyzed.
Results
The GE t1/2 was 134 ± 8 minutes (Mean ± SEM) for the first and 128 ± 6 minutes for the second study. The mean (95% CI) CCC between the two studies was 0.79 (0.67, 0.87) for GE at 1h, 0.83 (0.75, 0.9) for GE at 2h, 0.54 (0.34, 0.7) for GE at 4h and 0.79 (0.68, 0.86) for GE t1/2. However, in 18 of 60 patients (30%), the characterization of GE as normal, delayed or rapid differed between the first and second studies. For gastric empting t1/2, the inter-individual coefficients of variation was 40%; the intra-individual COV was 20%, comparable in diabetics and non-diabetics, and greater in patients with rapid (28%) than delayed (18%) or normal gastric emptying (12%).
Conclusions & Inferences
Among patients with upper gastrointestinal symptoms, GE measured with scintigraphy is relatively reproducible. In 30% of cases, the interpretation was different between the two assessments. Hence, a diagnosis of gastroparesis based on a single study may occasionally be inaccurate.
Keywords: gastric emptying, scintigraphy, reproducibility, diabetes mellitus, gastroparesis
Graphical Abstract
Abbreviated abstract: Assessments of gastric emptying measured with scintigraphy are relatively reproducible in patients with upper gastrointestinal symptoms. In 30% of patients, the characterization of gastric emptying as normal, delayed and rapid differed between the two studies.
BACKGROUND
The American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine endorse the use of gastric emptying of solids by scintigraphy (1) to identify gastric motor function abnormalities in gastroparesis and dumping syndrome, to investigate pathophysiological mechanisms that may be associated with patients symptoms or syndromes, such as functional dyspepsia, and to evaluate the effects of treatment, such as prokinetic agents in the treatment of gastroparesis (2) or octreotide (3) for treating the dumping syndrome. The intra- and inter-individual reproducibility for gastric emptying measured with scintigraphy is well established in healthy subjects (4) but not in patients. Indeed, to our knowledge, only one study specifically evaluated the reproducibility of gastric emptying measured with scintigraphy in patients, i.e., in type 1 diabetes.(5) Our aims were to evaluate the day-to-day reproducibility of gastric emptying assessed by scintigraphy in patients with normal, delayed, and rapid gastric emptying.
MATERIAL AND METHODS
Participants
All subjects gave their written informed consent to participate in the protocol, which had been approved previously by the Institutional Review Board and Radiation Safety Committee of the Mayo Clinic. Among 60 patients [38 females, median age 48 yr, range 18–84 yr, BMI 26.81 ± 0.85 kg/m2 (Mean ± SEM)] with upper gastrointestinal symptoms who underwent a clinically-indicated assessment of gastric emptying with scintigraphy, symptoms and gastric emptying were re-evaluated with scintigraphy 15 ± 1 days after the first gastric emptying assessment. There was no change in treatment between the first and second gastric emptying studies. The exclusion criteria included severe nausea or vomiting, which may preclude study assessments, medications (e.g., narcotics, medications with significant anticholinergic effects, glucagon like peptide -1 (GLP-1) agonists, or prokinetic agents) that were not discontinued within 4 half-lives prior to scintigraphy, clinical evidence of severe systemic diseases that may interfere with the objectives of the study, prior gastric or major intestinal (i.e., resection of greater than 50 cm) or colonic surgery (i.e., hemi or subtotal colectomy), allergy to eggs or reluctance to consume milk, and abdominal radiation therapy.
Assessments
Before the gastric emptying study, gastrointestinal symptoms were evaluated with symptom questionnaires based on the Rome III symptom criteria,(6, 7) the Gastrointestinal Cardinal Symptom Index (GCSI), which inquires about upper GI symptoms over the past 2 weeks,(8) Patient Assessment of Upper Gastrointestinal Disorders-Symptom Severity (PAGI-SYM) index,(9) and the Hospital Anxiety and Depression Questionnaire.(10) Females of child-bearing potential underwent a pregnancy test within 48 h of each study.
Gastric emptying study
After an overnight fast, subjects ingested a 99mTc-labeled meal consisting of two scrambled eggs, one slice of whole wheat bread, and one glass of skim milk.(4) Abdominal images of 2 minute duration were acquired with anterior and posterior gamma cameras immediately following ingestion of the radiolabeled meal and at specified time points during the subsequent 4 h period, typically every 15 min during the first 2 h and every 30 min during the subsequent 2 h. Gastric emptying was defined as normal, rapid, and delayed using data (5th to 95th percentile values) in 319 healthy people.(4) In females, the normal range for gastric emptying is 4% to 31% at 1 hour, 25% to 71% at 2 hours and 76% to 100% at 4 hours. In males, the corresponding values are 5% to 40% at 1 hour, 28% to 82% at 2 hours and 77% to 100% at 4 hours. Patients in whom emptying was less than these values at 2 or 4 hours were characterized as delayed. Conversely, patients in whom emptying was greater than these values at 1 or 2 hours were characterized as rapid.
Statistical Analysis
The concordance between gastric emptying measurements on 2 separate days was assessed by Lin’s concordance statistic (the concordance correlation coefficient [CCC]. (11) The paired t-test was used to compare within subject differences against zero. A Bland Altman assessment examined whether the magnitude of differences between 2 measurements was correlated with the magnitude of the measured responses (i.e., the average value for both studies) using Pearson’s correlation coefficient. (12) The inter-individual coefficient of variation (COV) was the SD divided by the mean, expressed as a percentage.(4) The intra-individual COV2 (%) for each participant was calculated by the formula [((Difference between 1st and 2nd studies)2/2)/(Average of 1st and 2nd studies)2]*100. This parameter was averaged across all participants; the square root of this average, expressed as a percentage provided the intra-individual COV.(13)
RESULTS
Demographics and Associated Conditions
The age, sex, and BMI were not significantly different among patients with normal, rapid and delayed gastric emptying (Table 1). Of 60 patients, 21 had diabetes mellitus. Eight additional patients had other conditions associated with dysmotility, i.e., postural orthostatic tachycardia syndrome (3 patients); one patient each had generalized autonomic dysfunction, chronic lymophocytic leukemia, fundoplication, Parkinson’s disease, scleroderma, and marijuana use.
Table 1.
Demographic and Baseline Clinical Features
| Characteristic | Normal GE (N=25) | Rapid GE (N=18) | Delayed GE (N=17) |
|---|---|---|---|
| Age, yrs | 48 ± 4 | 49 ± 4 | 41 ± 4 |
| Sex, number of women (%) | 18 (72%) | 8 (44%) | 12(71%) |
| BMI kg/m2 | 27.4 ± 1.4 | 28.6 ± 1.6 | 24 ± 1.4 |
| Diabetes mellitus, number of patients | 7 (28%) | 8 (44%) | 6 (35%) |
| HbA1c level, % | 7.7 ± 0.4 | 7.1 ± 0.5 | 8.2 ± 0.7 |
| FDA NVFP* composite score | 1.8 ± 0.2 | 2.4 ± 0.3 | 2.9 ± 0.3 |
| GCSI Total# | 0.9 ± 0.1 | 1.7 ± 0.2 | 2 ± 0.2 |
| Anxiety (HAD score ≥ 11), Number of patients | 7 | 7 | 1 |
| Depression (HAD score ≥ 11), Number of patients | 4 | 8 | 3 |
All values are Mean ± SEM unless stated otherwise
FDA NVFP score is the average of nausea, vomiting, fullness, and pain scores in PAGI-SYM questionnaire
GCSI Total is the average of satiety, nausea/vomiting/regurgitation and bloating sub-scores obtained from the PAGI-SYM questionnaire
Diabetes Mellitus
Among 21 diabetics, 6 had type 1 DM; one also had celiac disease. The duration of DM was 14 ± 2 years and the mean glycosylated hemoglobin (HbA1c) was 7.6 ± 0.3%. The fasting blood sugar level in diabetics was 149 ± 12 mg/dl before the first study and 168 ± 19 mg/dl before the second study. Three patients had significant hyperglycemia (> 275 mg/dl) before one of the scans. Two of these had delayed and 1 had rapid emptying. Fifteen DM patients had complications including peripheral and/or autonomic neuropathy (8 patients), retinopathy (4 patients), and nephropathy (5 patients). Among diabetics, 9 were treated oral hypoglycemic agents, 11 with insulin, and 1 with a combination of these agents.
Gastrointestinal Symptoms
Forty five patients satisfied Rome III criteria for upper GI symptoms, i.e., functional dyspepsia (26 patients), nausea/vomiting and/or rumination (10 patients), heartburn (8 patients), and functional abdominal pain (1 patient). Of the remaining 15 patients, 13 had a score of 2.5 or greater on at least one sub scale (i.e., satiety, nausea/vomiting, bloating, heartburn, or upper abdominal pain) of the PAGI questionnaire. The other 2 patients, who had dyspepsia (1 patient) and upper gastrointestinal symptoms (1 patient) with unexplained weight loss, had a symptom score < 2.5 on the PAGI questionnaire. Forty six patients also had functional lower GI symptoms. Many patients had overlapping symptoms (not shown). Among 55 patients who returned the GI symptom questionnaires, the mean GCSI total score was 1.47 ± 0.1 and the mean FDA composite score was 2.3 ± 0.1. Fifteen and 15 patients had significant anxiety and depression as evidenced by HAD scores ≥ 11 respectively.
Intra-individual Reproducibility
Among 60 patients, 25 had normal, 18 had rapid, and 17 had delayed gastric emptying during the first study. During the second study, 37 had normal, 11 had rapid, and 12 had delayed gastric emptying. In 28 of 29 studies with delayed gastric emptying, the diagnosis of delayed gastric emptying was based on delayed gastric emptying at 4 hours. The remaining patient had delayed gastric emptying at 2 hours only. In the first study, 17 participants had delayed GE, of which nine had delayed GE at 4 hours only, seven had delayed GE at 2 and 4 hours, and only one had delayed GE at 2 hours only. Of the 12 participants with delayed GE in the second study, 9 had delayed GE at 4 hours only and 3 had delayed GE at both 2 and 4 hours.
Twenty four of 29 rapid GE studies were diagnosed by rapid GE at 1 hour, with or without rapid GE at 2 hours. Only 5 of 29 studies with rapid GE were diagnosed based on rapid GE at 2h only. In the second GE study in these patients, all 5 patients satisfied criteria for rapid GE at 1 and 2 hours (4 patients) or at 1 hour alone (1 patient).
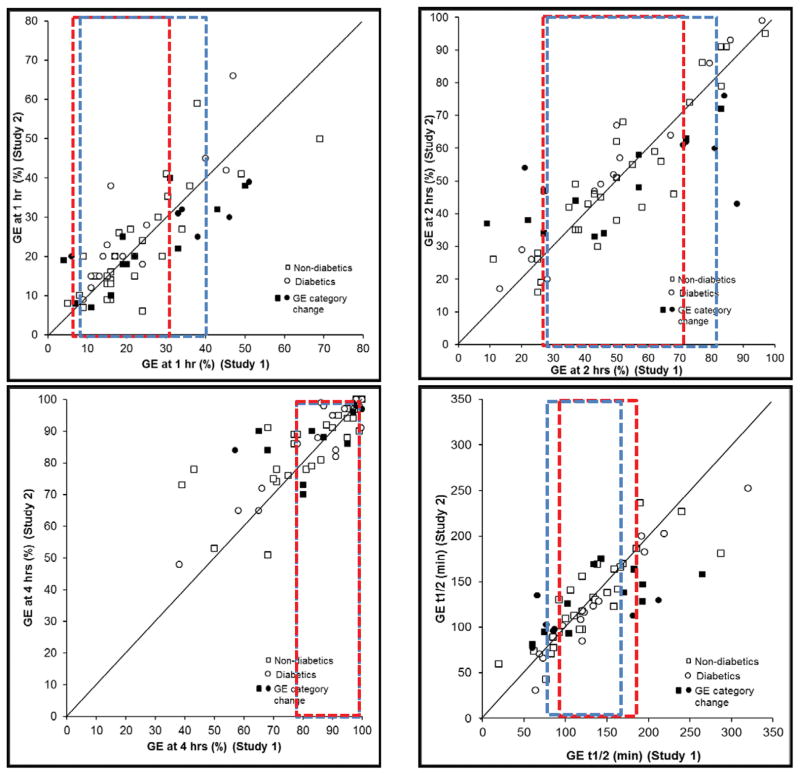
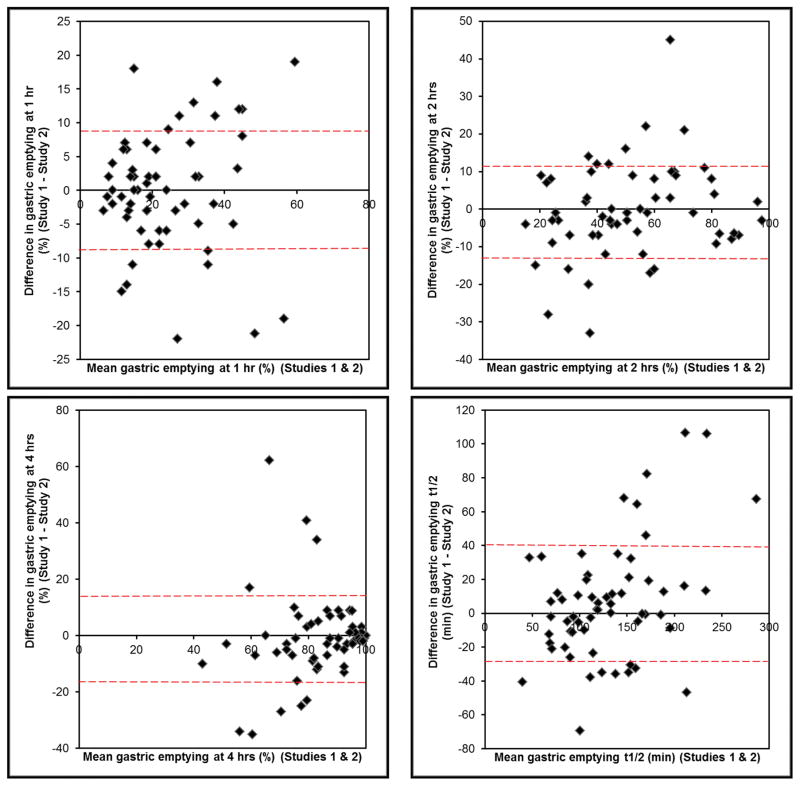
The average absolute difference in gastric emptying between the two studies was 7% at 1, 9% at 2, and 7% at 4 hours and 25 minutes for GE t1/2. This difference was not different among patients with normal, rapid, and delayed emptying. Overall, gastric emptying measurements at 1 hour [CCC (95% CI) = 0.79 (0.67, 0.87)] and 2 hours (0.83; 95% CI, 0.75, 0.9) and to a lesser extent at 4 hours (0.54; 95% CI, 0.34, 0.7) were significantly correlated between the first and second studies. (Table 2, Figure 1) Values for first and second assessments at corresponding time were different (p=0.01) at 4 hours but not at 1 or 2 hours. For t1/2, but not the other parameters, the Bland Altman test was significant, i.e., differences between the first and second studies were related to the average gastric emptying, being greater in patients with delayed gastric emptying (Figure 2).
Table 2.
Intra-individual Day-to-Day Reproducibility of Gastric Emptying Measurements with Scintigraphy
| Measurements | First study | Second study | CCC (95% CI) | p-value for Bland Altman Assessment # |
|---|---|---|---|---|
| Gastric emptying at 1 hour, % | 24 ± 2 | 24 ± 2 | 0.79 (0.67,0.87) | 0.52 |
| Gastric emptying at 2 hours, % | 51 ± 3 | 52 ± 3 | 0.83 (0.75, 0.9) | 0.13 |
| Gastric emptying at 4 hours, % | 83 ± 2 | 85 ± 2 a | 0.54 (0.34, 0.7) | 0.32 |
| Gastric emptying t ½, minutes | 134 ± 8 | 128 ± 6 | 0.79 (0.68, 0.86) | .001 |
All values are Mean ± SEM unless stated otherwise
Test for (Pearson) correlation of differences versus overall mean
p = 0.01 versus first study
Figure 1.
Day-to-day reproducibility of gastric emptying at 1 hour (top left), 2 (top right), 4 hours (bottom left) and gastric emptying t1/2 (bottom right). Observe excellent agreement at all time points. Participants in which the overall interpretation (i.e., as normal, rapid, or delayed GE) was different between the first and second studies are depicted with filled markers. The range of normal values in the first study are depicted by the rectangular areas outlined by dotted red (in women) and blue lines (in men). During the first study, GE1 was accelerated in 14 participants, GE2 was accelerated in 13 and delayed in 8 participants, and GE4 was delayed in 16 participants
Figure 2.
Bland-Altman plots for gastric emptying reproducibility at 1 hour (top left panel), 2 hours (top right panel), 4 hours (bottom left panel) and gastric emptying t1/2 (bottom right panel). The red interrupted lines show the limits of agreement for 1 standard deviation.
The intra-individual COV for gastric emptying at 1, 2, 4 hours and the t1/2 was 28%, 23%, 11%, and 20% respectively (Table 3). At 1 (p<0.0001), 2 (p<0.0001) and 4 hours (p<0.0001); the intra-individual COV was different among patients with normal, delayed and gastric emptying (Table 3). At 1 hour, the intra-individual COV was 30%, 20% and 34% in patients with normal, rapid, and delayed gastric emptying respectively. At 2 hours, this intra-individual COV was greater in patients with delayed (36%) than normal (14%) or rapid gastric emptying (14%). Likewise, at 4 hours, the intra-individual COV was greater in patients with delayed (19%) than normal (6%) or rapid (4%) gastric emptying.
Table 3.
Intra- and Inter-Individual Coefficient of Variation (%) of Gastric Emptying
| All subgroups (n=60) |
Normal emptying (n=25) |
Rapid Emptying (n=18) |
Delayed Emptying (n=17) |
|||||
|---|---|---|---|---|---|---|---|---|
| Inter- individual COV (%) |
Intra- individu al COV (%) |
Inter- individu al COV (%) |
Intra- individual COV (%) |
Inter- individual COV (%) |
Intra- individual COV (%) |
Inter- individual COV (%) |
Intra-individual COV (%) |
|
| GE 1 hr | 56 | 28 | 39 | 30 | 24 | 20 | 38 | 34 |
| GE 2 hrs | 43 | 23 | 22 | 14 | 15 | 14 | 31 | 36 |
| GE 4 hrs | 17 | 11 | 8 | 6 | 4 | 3 | 18 | 19 |
| GE t1/2 | 40 | 20 | 18 | 12 | 26 | 28 | 19 | 18 |
Among 25 patients with normal gastric emptying in the first study, 22 had normal, 1 had rapid, and 2 had delayed gastric emptying during the second study. Of 18 patients who had rapid emptying in the first study, 8 had normal and 10 had rapid emptying in the second study. Among the 17 patients who had delayed emptying during the first study, 7 had normal and 10 had delayed emptying in the repeat study. In no patients did the diagnosis change from delayed to rapid gastric emptying or vice versa between the first and second studies.
The intra-individual COV at 1, 2, and 4 hours was respectively 29%, 23%, and 12% in non-diabetics and 26%, 23%, and 9% in diabetics. The intra-individual COV for gastric emptying t1/2 in non-diabetics and diabetics respectively was 20% and 21%.
Inter-individual variability
Among all participants, the inter-individual COV was 56% at 1, 43% at 2, and 17% at 4 hours (Table 3). For gastric emptying t1/2, the inter-individual COV was 40%. Similar to the intra-individual COV, the inter-individual COV for gastric emptying at 1 hour was similar among patients with normal, rapid, and delayed gastric emptying. At 2 and 4 hours, the inter-individual COV was greater in patients with delayed than normal or rapid gastric emptying.
The inter-individual COV for gastric emptying at 1, 2, and 4 hours and t1/2 was respectively 59%, 42%, 17%, and 37% in non-diabetics and 52%, 43%, 19%, and 47% in diabetics.
DISCUSSION
By virtue of the distribution of patients with and without diabetes mellitus, the typical upper gastrointestinal symptoms, and the range of gastric emptying disturbances, this cohort is representative of patients seen in clinical practice. Indeed, the initial GE test was performed as part of the clinical assessment. While gastric emptying measured with scintigraphy was relatively reproducible over 2 weeks, the characterization of gastric emptying as normal, delayed or rapid was different between the first and second studies in 18 of 60 patients (30%). Among these patients, the characterization switched from normal to rapid or delayed gastric emptying, or vice versa, but not from rapid to delayed emptying or vice versa. In part, these differences probably reflect regression to the mean and the limitations of summarizing continuous metrics as dichotomous (ie, normal or abnormal) variables such that relatively small differences in the amount emptied may affect the interpretation of the final result.(14) Four of 6 patients with <65% emptying at 4 hours also had delayed gastric emptying during the second study. Hence, in patients with markedly delayed emptying, a single assessment is probably sufficient. When the delay is less severe, it may be prudent to consider documenting delayed gastric emptying on 2 occasions before assigning the diagnosis of gastroparesis, particularly given the overlap between gastroparesis and dyspepsia with delayed gastric emptying.(15, 16)
The intra-individual variability of gastric emptying at 1, 2, and 4 hours and the gastric emptying t1/2 was respectively 28%, 23%, 11%, and 20%. Between 1 and 4 hours, the variability declines as more food empties from the stomach, reflecting a ceiling effect. For the gastric emptying t1/2, the intra-individual COV of 20% is comparable to the corresponding value of 24% in healthy people,(4) 29% in fourteen patients with DM,(5) and 24% in ten DM patients randomized to placebo in a randomized controlled trial with relamorelin.(17) These values and all data in this manuscript express gastric emptying as the amount emptied. Alternatively, gastric emptying can be expressed as the amount retained, for example at 1, 2, and 4 hours. At any time, the values for intra-individual COV expressed as the amount emptied or retained may be different. For example, for a woman in whom emptying at 4 hours is 78% in the first and 98% during the replicate study, the COV of 3%. However, the COV for the amount remaining (i.e., 22% during the first and 2% during the second study) is 139%. While the latter value is high, it is noteworthy that gastric emptying at 4 hours is normal in both studies.
In this study, the inter-individual COV (40%) was greater than the intra-individual COV (20%) for the gastric emptying t1/2. In the phase 2 trial of relamorelin for gastroparesis, a 10% reduction in gastric emptying measured with the gastric emptying breath test was clinically significant.(18) With an inter-individual COV of 40%, it is estimated that 231 subjects will be necessary to detect an effect size of 10% between two treatments with 80% power and a 2-sided α level of 0.05. By comparison, among healthy subjects, the inter- and intra-individual reproducibility for scintigraphic t1/2 were approximately 24 %.(4) Similarly, Lartigue et al observed that the inter-individual COV was greater in diabetics (76%) than healthy people (20%).(5)
The intra-individual COV for GE measured with scintigraphy in this study is lower than that measured with the SmartPill capsule in 10 healthy male subjects, i.e., 40% at 2 weeks and 24% at 4 weeks.(19) The intra-individual COV for measured with the SmartPill capsule in patients is unknown. The intra- (20%) and inter-individual (40%) COV for gastric emptying t1/2 measured with scintigraphy are greater than corresponding values, respectively 21% and 15% for MRI measurements of gastric volumes, which are used to calculate gastric emptying, in patients with dyspepsia.(20)
In summary, gastric emptying measured with scintigraphy is relatively reproducible in patients with upper GI symptoms. However, in 30% of patients, the interpretation of gastric emptying as normal, rapid, or delayed was different between the studies. For gastric emptying t1/2, the inter-individual COV (40%) was greater than the intra-individual COV (20%). These observations are useful for estimating the sample size in therapeutic trials that include gastric emptying as an endpoint.
Key Points.
Assessments of gastric emptying measured with scintigraphy are relatively reproducible in patients with upper gastrointestinal symptoms.
The inter-individual coefficient of variation for gastric emptying t1/2 (40%) was greater than the intra-individual COV (20%).
In 30% of patients, the characterization of gastric emptying as normal, delayed and rapid differed between the two studies.
Acknowledgments
Funding. This study was supported in part by USPHS NIH Grant R01 DK068055 to Dr. Bharucha.
Abbreviations
- COV
coefficient of variation
- CCC
concordance correlation coefficient
- GE
gastric emptying
- t1/2
time taken for 50% emptying
- DM
diabetes mellitus
- CI
confidence interval
- GCSI
Gastrointestinal Cardinal Symptom Index
- PAGI-SYM
Patient Assessment of Upper Gastrointestinal Disorders-Symptom Severity index
- HAD
Hospital Anxiety and Depression
Footnotes
Competing Interests. The authors have no competing interests.
Author Contributions. AD analyzed the data and wrote the manuscript; MOC helped design the study; BN was the study coordinator; KD helped conduct the study; AZ did the statistical analysis; AEB designed the study, analyzed the data, and wrote the manuscript. MC was involved in writing the paper. All authors reviewed the final version of the manuscript.
References
- 1.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine.[reprint in J Nucl Med Technol. 2008 Mar;36(1):44–54; PMID: 18287197] Am J Gastroenterol. 2008;103:753–763. doi: 10.2967/jnmt.107.048116. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Malagelada JR, Abell TL, Brown ML, Hench V, Zinsmeister AR. Effect of six weeks of treatment with cisapride in gastroparesis and intestinal pseudoobstruction. Gastroenterology. 1989;96:704–712. [PubMed] [Google Scholar]
- 3.Foxx-Orenstein A, Camilleri M, Stephens D, Burton D. Effect of a somatostatin analogue on gastric motor and sensory functions in healthy humans. [see comment] Gut. 2003;52:1555–1561. doi: 10.1136/gut.52.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012;24:1076–e1562. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lartigue S, Bizais Y, Des Varannes SB, Murat A, Pouliquen B, Galmiche JP. Inter- and intrasubject variability of solid and liquid gastric emptying parameters. A scintigraphic study in healthy subjects and diabetic patients. Dig Dis Sci. 1994;39:109–115. doi: 10.1007/BF02090069. [DOI] [PubMed] [Google Scholar]
- 6.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders.[erratum appears in Gastroenterology. 2006 Jul;131(1):336] Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–150. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 9.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–1749. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 10.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 11.Carrasco JL, Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics. 2003;59:849–858. doi: 10.1111/j.0006-341x.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Comparing two methods of clinical measurement: a personal history. Int J Epidemiol. 1995;24:S7–14. doi: 10.1093/ije/24.supplement_1.s7. [DOI] [PubMed] [Google Scholar]
- 13.Bland M. How should I calculate a within-subject coefficient of variation? 2006 https://www-users.york.ac.uk/~mb55/meas/cv.htm.
- 14.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacy BE. Functional dyspepsia and gastroparesis: one disease or two? Am J Gastroenterol. 2012;107:1615–1620. doi: 10.1038/ajg.2012.104. [DOI] [PubMed] [Google Scholar]
- 16.Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972–1978. doi: 10.1136/gutjnl-2013-306084. [DOI] [PubMed] [Google Scholar]
- 17.Shin A, Camilleri M, Busciglio I, et al. Randomized Controlled Phase Ib Study of Ghrelin Agonist, RM-131, in Type 2 Diabetic Women With Delayed Gastric Emptying. Pharmacokinetics and pharmacodynamics. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, McCallum RW, Tack J, Spence SC, Gottesdiener K, Fiedorek FT. Efficacy and Safety of Relamorelin in Diabetics With Symptoms of Gastroparesis: A Randomized, Placebo-Controlled Study. Gastroenterology. 2017;153:1240–1250. e1242. doi: 10.1053/j.gastro.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz Tartera HO, Webb DL, Al-Saffar AK, et al. Validation of SmartPill wireless motility capsule for gastrointestinal transit time: Intra-subject variability, software accuracy and comparison with video capsule endoscopy. Neurogastroenterol Motil. 2017;29:1–9. doi: 10.1111/nmo.13107. [DOI] [PubMed] [Google Scholar]
- 20.Fidler J, Bharucha AE, Camilleri M, et al. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil. 2009;21:42–51. doi: 10.1111/j.1365-2982.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]