Abstract
Recent work on the pathogenesis of type 1 diabetes has led to an evolving recognition of the heterogeneity of this disease, both with regards to clinical phenotype and responses to therapies to prevent or revert diabetes. This heterogeneity not only limits efforts to accurately predict clinical disease but also is reflected in differing responses to immunomodulatory therapeutics. Thus, there is a need for robust biomarkers of beta cell health, which could provide insight into pathophysiological differences in disease course, improve disease prediction, increase the understanding of therapeutic responses to immunomodulatory interventions and identify individuals most likely to benefit from these therapies. In this review, we outline current literature, limitations and future directions for promising circulating markers of beta cell stress and death in type 1 diabetes, including markers indicating abnormal prohormone processing, circulating RNAs and circulating DNAs.
Keywords: Biomarker, Diabetes mellitus, Pancreatic beta cells, Pancreatic islets, Review, Type 1 diabetes
Introduction
In recent years, it has become increasingly apparent that the definition of type 1 diabetes as a purely autoimmune disease belies its strikingly heterogeneous pathophysiology. For example, postmortem studies show that in individuals with type 1 diabetes only about 24% of those aged <14 years exhibit evidence of islet inflammation (insulitis) and even fewer (only 10%) aged >15 years have detectable insulitis [1]. Likewise, loss of islet insulin positivity (once thought to be the uniform hallmark of type 1 diabetes) displays striking variability, with some individuals exhibiting insulin positivity in up to 50% of islets at type 1 diabetes clinical diagnosis [2]. This heterogeneous pathology is reflected in clinical trials of immune-modulating drugs, which have shown limited success in slowing destruction/dysfunction of beta cells in type 1 diabetes [3]. Taken together, these studies suggest that the institution of therapies that diminish both immune responses against beta cells and boost beta cell resistance to stress might be needed to prevent or reverse type 1 diabetes. Trials of such therapies, or their implementation at preclinical stages of disease, require high-confidence indices of beta cell health and disease. Currently, indices such as the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS), Index60, islet-derived autoantibody number and titres, first-phase insulin response to an intravenous glucose load and alterations in HbA11c are used to stratify risk of progression to overt type 1 diabetes [4, 5]. While these indices reflect prevailing autoimmunity, beta cell function or glycaemic control, none directly reflect the health or survival of beta cells. In this review, we summarise the status of research into biomarkers of beta cell stress and death in type 1 diabetes.
Beta cell stress and death: origins of biomarkers
The beta cell, like most cell types, has highly conserved molecular responses to cope with stressful signals (e.g. viral infections, proinflammatory cytokines, metabolic overload). These molecular responses have the common goal of stress ‘remediation’, whereby an attempt is made to mitigate the impact of the stressor on beta cell health. Failing stress mitigation, cell death pathways eventually prevail. In recent years, it has become apparent that the unfolded protein response (UPR) pathway is a focal point of extracellular stress signalling in type 1 diabetes and exemplifies remediation vs cell death balance. The UPR in beta cells is activated under conditions of inflammation, oxidative stress and insulin production/folding imbalance [6]. and results in rapid translational inhibition to alleviate the deleterious effects of accumulating misfolded proteins in the endoplasmic reticulum (ER). In the setting of persistent and severe stress, the UPR activates c-Jun N-terminal kinase (INK) and C/EBP-homologous protein (CHOP) cascades, leading to apoptosis. The UPR also exemplifies how intracellular biomolecules, such as proteins and nucleic acids, might escape extracellularly [7]. thereby providing circulating biomarkers that reflect the cellular state of emergency.
Three categories of potential conduits for cellular escape of biomolecules are shown in Fig. 1: (1) The ER–Golgi secretory network; (2) extracellular vesicle (EV) pathways and (3) apoptotic bodies/cellular necrosis. Specifically, the ER–Golgi network is the physiological pathway through which the processing, folding and secretion of proteins (e.g. insulin, amylin) is routinely achieved in beta cells. Stress-induced activation of the UPR has a profound impact on the amount and structure of the proteins released through this network. The EV pathways, by contrast, are conduits through which a multitude of biomolecules, including nucleic acids, proteins, lipids and metabolites, are released under physiological and pathological conditions [8]. In this pathway, biomolecules enter EVs (e.g. exosomes, microvesicles; ranging in size from 50 to 1000 nm) either through endosomes or plasma membrane outcroppings (for a review, see [8]). The content of EVs changes dynamically according to the physiological state of the cell. Importantly, EVs may carry and present antigens to the immune system or communicate apoptotic signals between beta cells [9, 10]. Last, release of biomolecules within cellular fragments (apoptotic bodies) or directly into circulation (through spillage of cellular contents) is observed following apoptosis or necrosis, respectively [11]; biomarkers released in such fashion represent an end-stage fate of beta cells.
Fig. 1.
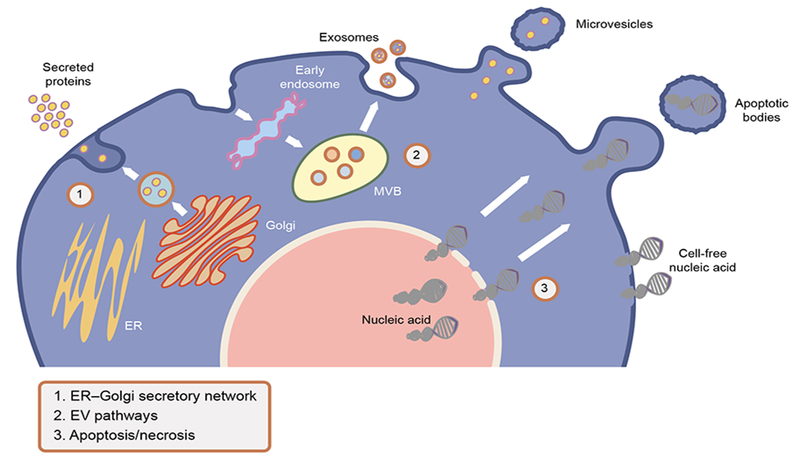
Potential conduits for cellular escape of biomolecules: (1) the ER–Golgi secretory network is the physiological pathway through which the processing, folding and secretion of proteins (e.g. insulin, amylin) is routinely achieved in beta cells; (2) the EV pathways are recognised as conduits through which a multitude of biomolecules, including nucleic acids, proteins, lipids and metabolites are released, either through endosomes (exosomes) or plasma membrane outcroppings into EVs (microvesicles); (3) apoptosis/cellular necrosis results in the release of biomolecules within cellular fragments (apoptotic bodies) or directly into circulation (through necrosis and spillage of cellular contents) and represents an end-stage fate of beta cells. MVB, multivesicular body. This figure is available as a downloadable slide
Biomarkers of beta cell stress and death
Circulating proteins
Because beta cell plasma membranes are disrupted upon cell necrosis, quantification of changes in circulating beta cell proteins released via this mechanism could serve as a marker of beta cell death. Along these lines, plasma glutamate decarboxylase 65 kDa (GAD65), which is specific to islets and neural and reproductive tissues, was acutely increased in a small group of humans receiving islet transplants [12]. Analysis of GAD65 in at-risk or recently diagnosed individuals is needed to understand the potential of this marker in the context of type 1 diabetes.
Multiple studies have explored the possibility of using abnormalities in prohormone processing as markers of beta cell stress. The hallmark of a normally functioning beta cell is production and release of insulin in response to nutrients, and abnormalities in insulin production and processing are among the earliest markers of beta cell dysfunction. Under conditions of beta cell stress (e.g. autoimmune or inflammatory stress), hormone processing capabilities become overwhelmed and incompletely-processed intracellular proinsulin is released extracellularly either through the ER–Golgi pathway or in EVs [10, 13]. Circulating proinsulin molecules can be compared with circulating mature insulin or C-peptide, with increases in relative circulating proinsulin reflecting beta cell dysfunction [7]. ProinsulimC-peptide (PI:C) ratios outperform proinsulin:insulin ratios in predicting incident diabetes in populations with insulin resistance, as circulating insulin values can reflect altered hepatic insulin clearance [14].
Analyses of relatives at risk for type 1 diabetes indicate that elevated PI:C ratios are predictive of progression to diabetes and can augment the performance of other traditional markers of diabetes risk, such as autoantibodies [15–17]. A comparison of fasting PI:C ratio vs first-phase insulin secretion (measured using hyperglycaemic clamp studies) in autoantibody-positive relatives suggested that fasting PI:C ratio, adjusted for differences in insulin sensitivity, is as informative of impending type 1 diabetes as the more invasive clamp studies [16]. Further analyses of at-risk groups suggest that this marker performs best in pre-adolescent individuals, in whom differences in ratios between those who progressed and those who did not progress to type 1 diabetes were the most pronounced [17]. Several groups have reported that PI:C ratios may also be increased in euglycaemic relatives of individuals with type 1 diabetes, even those who are autoantibody-negative or do not have high-risk HLA genotypes [18–20].
At the time of diagnosis of type 1 diabetes, circulating PI:C ratios have been found to be elevated relative to those in control groups without diabetes [21, 22]. However, there is no clear consensus on PI:C ratios at the time of clinical remission/the ‘honeymoon’ period. Studies in older individuals suggest a reduction in PI:C ratios during the honeymoon period of type 1 diabetes compared with levels at diagnosis, whereas a report in paediatric participants suggested continued elevations in PI:C ratios during this period, suggestive of persistent beta cell stress despite improved C-peptide production [21–23].
Of note, PI:C ratios may allow identification of individuals most likely to benefit from immunomodulatory therapies, as elevations in ratios at type 1 diabetes diagnosis were associated with subsequent response to ciclosporin treatment [21]. A recent analysis of donor pancreases from individuals with longstanding type 1 diabetes suggested that the majority of individuals retain islet proinsulin immunostaining, despite very low or absent islet C-peptide immunostaining [24]. Other reports have identified circulating proinsulin in C-peptide-negative individuals with longstanding type 1 diabetes, raising the possibility that circulating proinsulin may be more useful as a biomarker of persistent or remaining beta cells as compared with insulin or C-peptide [25, 26]. Larger-scale longitudinal studies with sensitive C-peptide assays and stimulated analyses of beta cell function are required to fully elucidate this possibility.
The altered processing of other prohormones in association with beta cell dysfunction may also represent other promising novel stress-related biomarkers. A recent report identified elevations in plasma pro-islet amyloid polypeptide (pro-IAPP) relative to total IAPP in a cross-section of children with longstanding type 1 diabetes and islet transplant recipients with type 1 diabetes [27]. Unexpectedly, pro-IAPP levels were not elevated in samples from two cross-sections of individuals with type 2 diabetes, despite elevations in circulating proinsulin [27]. Additional longitudinal studies are needed to better understand the efficacy of circulating pro-IAPP as a marker of beta cell stress in at-risk populations.
One drawback of measuring prohormone ratios is that there may be overlap between some individuals with type 1 diabetes and control individuals without diabetes, emphasising the heterogeneity in beta cell prohormone processing dysfunction among groups with or at risk for type 1 diabetes. Additionally, the following questions regarding the pathophysiology surrounding these markers in type 1 diabetes remain to be answered: (1) what are the underlying mechanisms of prohormone processing dysfunction in type 1 diabetes (i.e. ER stress vs alterations in expression of processing enzymes and/or genetic predisposition to altered prohormone processing)?; (2) will differences in prohormone ratios predict heterogeneity in clinical disease course or responses to different immunomodulatory therapies?; (3) which will perform more effectively as biomarkers of beta cell stress in type 1 diabetes: intact or total (inclusive of all partially processed split products) prohormones?
Circulating RNAs
A variety of RNA types have been measured in the circulation as cell-free species that have the potential to indicate beta cell health. To date, the greatest emphasis has been on non-coding RNAs, which include microRNAs (miRNAs), long non-coding RNAs, small nucleolar RNAs and circular RNAs. These function post-transcriptionally to alter cellular identity and function. Circulating miRNAs have generated perhaps the most significant interest as biomarkers of disease. Although these small RNAs (21–23 nucleotides) largely function intracellularly, emerging data suggest that they can be shed extracellularly from apoptotic or necrotic cells, in association with lipoprotein particles or Argonaute-2 protein complexes, or as molecular cargo within EVs (reviewed in [28]), possibly as a means of intercellular communication. Notably, although groups have studied miRNAs in the circulation or in isolated human islets [29]. none have yet described beta cell miRNAs in the context of human pancreatic tissue in situ. Because miRNAs are important regulators of gene expression within the cell, circulating miRNAs can provide a ‘liquid biopsy’ of changes in gene expression in response to different diseases, including those occurring in the beta cell in diabetes [8]. In this context, miR-375-5p (hereafter referred to as miR-375) has been studied most extensively as a putative biomarker of beta cell death [30]. Relative expression of miR-375 is enriched in mouse islets compared with other tissues and miR-375 is released extracellularly as mouse islets die [30]. In studies of mice under non-stressed conditions, beta cells only contribute ~1% of the total miR-375 signal in plasma [31]. However, acute beta cell death caused by streptozotocin is associated with increases in plasma miR-375 and plasma levels are also increased in NOD mice models of diabetes prior to diabetes onset [30, 31]. Nevertheless, data in human type 1 diabetes are inconclusive, with some reports showing increased circulating levels of miR-375 in those with type 1 diabetes and others showing unchanged or decreased levels compared with control individuals without diabetes [30–35].
Unbiased approaches have been used in an attempt to identify other miRNAs that may be associated with individuals with or at risk for diabetes. Datasets arising from such approaches in type 1 diabetes-related populations have been reported, but these data have so far failed to identify consistently differentially expressed miRNAs in such cohorts [32, 35–39]. These outcomes are likely related to miRNA release from multiple organs, leading to relatively nonspecific signatures in the circulation. An alternative approach to analysis of global circulating miRNAs is characterisation of EV-associated miRNAs. The miRNA cargo within EVs is dynamically modulated under different physiological conditions and disease states [8] and, as such, treatment of beta cells in vitro with inflammatory cytokines induces the differential expression of miRNAs within EVs compared with control cells [9]. Differences in circulating EV miRNA cargo are present when comparing people with type 1 diabetes with control individuals without diabetes [33, 40]. Importantly, these EV-associated miRNAs can be distinct from total serum or plasma miRNA levels and may represent different modes through which miRNAs are released or the nature of the EVs in which they are released (e.g. exosomes vs microvesicles vs apoptotic bodies) [33]. For example, while miR-375 was found to be increased in both serum EVs and total serum from a cross-section of children with new-onset type 1 diabetes, serum EV and total serum miR-21-5p levels were discordant in individuals with diabetes (increased in serum EVs and decreased in total serum samples) [33]. Although work in circulating EV-associated miRNAs is limited by specificity issues (similar to those encountered in global circulating miRNA analyses), the use of EVs has the potential for future analyses enriching for beta cell or islet-derived EVs [41].
Another area that requires further development is the identification of other types of RNAs that are released by beta cells under diabetogenic stress conditions. These could include mRNAs, mRNA spliced variants and other non-coding RNAs that play important roles in the regulation of islet function and may be altered in the circulation in type 1 diabetes. Identification of other classes of cell-free RNAs emanating predominantly from beta cells should allow for identification of more specific beta cell-stress signatures in type 1 diabetes. To date, reports are lacking on extracellular RNAs that are truly beta cell-specific or are specifically released in response to beta cell death.
Circulating DNA
The appearance of cell-free DNA in the circulation is thought to arise primarily from apoptotic or necrotic cells, since DNA does not undergo routine turnover in living, quiescent cells. Although the DNA sequence of every non-tumorous cell in an organism is identical, the epigenetic modification of DNA (e.g. cytosine methylation) can vary from cell type to cell type. In this respect, modifications of DNA that are unique to beta cells could allow for the attribution of circulating DNA fragments bearing that modification to dying beta cells. To date, all studies involving circulating DNA biomarkers of beta cell death have relied on the notion that specific genes that are repressed bear cytosine methylation marks, whereas genes that are expressed are devoid of this modification. As such, the beta cell-specific gene encoding preproinsulin (INS) has been the major focus of investigations into biomarkers of beta cell death, and studies have shown the absence of cytosine methylation at this gene to be a characteristic feature of beta cells [42].
Discrimination of methylated vs unmethylated INS is achieved by the bisulphite reaction, which converts unmethylated cytosines to uracil (equivalent to thymidine) and can be differentially detected by PCR. Using different PCR methodologies that targeted different cytosine residues in the INS gene, several studies have demonstrated elevated levels of unmethylated INS DNA in the circulation of mice acutely treated with streptozotocin or in NOD mice just prior to diabetes development. These findings are consistent with the notion that dying beta cells give rise to increasing levels of circulating unmethylated INS DNA [43, 44]. These findings were subsequently verified in individuals before progression to or with new- or recent-onset type 1 diabetes [45–47]. subpopulations of individuals with ketosis-prone diabetes [48] and in individuals post-islet transplant [49–51]. all of whom are credibly in states where beta cells are dying. Other beta cell-enriched genes have also been investigated (GCK, IAPP) [52, 53] but the use of these genes for stratifying populations with or at risk for type 1 diabetes remains untested.
Whereas the sensitivity of these DNA-based biomarkers seems to be of little concern (since they are detectable using sensitive PCR techniques), a major limitation of the availability of DNA-based biomarkers is their specificity. Bisulphite-based sequencing of different human tissues [51] showed that some tissues exhibit evidence of unmethylated INS DNA, albeit at low levels relative to the levels of methylated INS (<20%). Nevertheless, given the difference in mass between beta cells (very low) and other cell types in the body, it is conceivable that an unmethylated INS signal could arise from one of these other tissues. A recent study showed that many beta cell-specific gene promoters also demonstrate comparable rates of methylation/unmethylation in alpha cells [54]. This finding reflects the common origin of all islet cell types but emphasises that the DNA biomarkers identified to date probably at best reflect islet cell death and not beta cell death. To address the specificity concern, two approaches are necessary: (1) determination of unmethylated DNA levels by different laboratories, using samples from individuals with type 1 diabetes that have been provided blindly by a central laboratory (as done in the original validation of autoantibodies; these tests are presently ongoing); and (2) genome-wide approaches to screen cytosines that exhibit differential methylation in human beta cells to obtain unbiased identification of genes (irrespective of their expression pattern) that might exhibit better beta cell-type specificity.
Conclusions and perspectives
The clinical heterogeneity of type 1 diabetes limits the accuracy of current risk prediction tools, as well as the effectiveness of current prevention and treatment strategies. These limitations have led to mounting recognition of a need for improved tools to monitor evolving beta cell stress and death and their contributions to diabetes development. To date, significant advances have been made, but these have been limited by sensitivity, specificity and reproducibility of individual markers. Further identification and validation of highly specific beta cell markers will facilitate their implementation in diabetes prediction and clinical use. These limitations may also arise in part because of the cross-sectional nature of many biomarker analyses, whereas beta cell stress and death in evolving type 1 diabetes are most likely waxing and waning processes. Longitudinal analyses (based on blinded samples) using promising beta cell biomarkers in at-risk populations are necessary to understand better the accumulating changes in beta cell health as disease develops over time. Additionally, results to date suggest that, as with the heterogeneity in the course of clinical diabetes, biomarkers of beta cell stress and death are variably altered in at-risk individuals. Thus, long-term success will likely require the use of a combination of multiple beta cell and other non-beta cell biomarkers to provide a comprehensive panel of markers of beta cell health in the context of evolving autoimmunity. Such a panel would allow for a more personalised approach to diabetes prevention and care, permitting identification of individuals at highest risk for diabetes development and a better understanding of individual responses to therapies.
Supplementary Material
Proposed biomarkers of beta cell stress and death.
Circulating proteins
GAD65
PI:C ratio
Pro-IAPP:total IAPP ratio
Circulating RNA species
miR-375
EV miR-21-5p
Circulating differentially-methylated DNA species
INS
GCK
IAPP
Moving forward in the field.
Improved tools to monitor beta cell stress and death are required to improve type 1 diabetes prediction, prevention and treatment. The following steps are required to improve the current landscape of biomarkers of beta cell health in type 1 diabetes:
Identification and validation of more specific beta cell biomarkers
More rigorous analyses of prospective biomarkers using samples from longitudinal studies
Generation of a comprehensive multiple-biomarker panel that reflects the state of beta cell health at different stages throughout the disease course
Acknowledgements
We would like to apologise to investigators whose work we were unable to include in this review due to space limitations.
Funding
The authors are supported by funding from National Institutes of Health grants: K08 DK103983 (EKS), R01 DK093954 (CEM), R01 DK60881(RGM), R01 DK105588 (RGM), UC4 DK 104166 (CEM, DLE, RGM) and P30 DK097512 (RGM). EKS is supported by a JDRF grant: 2-SRA-2017-498-M-B and DLE is supported by an Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 115797 (INNODIA) (this Joint Undertaking receives support from the Union’s Horizon 2020 research and innovation programme and ‘EFPIA’, ‘JDRF’ and ‘The Leona M. and Harry B. Helmsley Charitable Trust’).
Abbreviations
- ER
Endoplasmic reticulum
- EV
Extracellular vesicle
- IAPP
Islet amyloid polypeptide
- PI:C
Proinsulin-to-C-peptide
- UPR
Unfolded protein response
- miRNA
MicroRNA
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
References
- [1].In’t Veld P (2014) Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin Immunopathol 36: 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Campbell-Thompson M, Fu A, Kaddis JS, et al. (2016) Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 65: 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M, the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group (2010) Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol 160: 176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sosenko JM, Skyler JS, Mahon J, et al. (2012) The application of the diabetes prevention trial-type 1 risk score for identifying a preclinical state of type 1 diabetes. Diabetes Care 35: 1552–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sosenko JM, Skyler JS, DiMeglio LA, et al. (2015) A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes care 38: 271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brozzi F, Eizirik DL (2016) ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups J Med Sci 121: 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tersey SA, Nishiki Y, Templin AT, et al. (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61: 818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lakhter AJ, Sims EK (2015) Minireview: emerging roles for extracellular vesicles in diabetes and related metabolic disorders. Mol Endocrinol 29: 1535–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guay C, Menoud V, Rome S, Regazzi R (2015) Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Communication and Signaling 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cianciaruso C, Phelps EA, Pasquier M, et al. (2017) Primary human and rat beta-cells release the intracellular autoantigens gad65, ia-2, and proinsulin in exosomes together with cytokine-induced enhancers of immunity. Diabetes 66: 460–473 [DOI] [PubMed] [Google Scholar]
- [11].Jiang L, Paone S, Caruso S, et al. (2017) Determining the contents and cell origins of apoptotic bodies by flow cytometry. Sci Rep 7: 14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Costa OR, Stange G, Verhaeghen K, et al. (2015) Development of an enhanced sensitivity bead-based immunoassay for real-time in vivo detection of pancreatic β-cell death. Endocrinology 156: 4755–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eizirik DL, Miani M, Cardozo AK (2013) Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia 56: 234–241 [DOI] [PubMed] [Google Scholar]
- [14].Loopstra-Masters RC, Haffner SM, Lorenzo C, Wagenknecht LE, Hanley AJ (2011) Proinsulin-to-C-peptide ratio versus proinsulin-to-insulin ratio in the prediction of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetologia 54: 3047–3054 [DOI] [PubMed] [Google Scholar]
- [15].Truyen I, De Pauw P, Jorgensen PN, et al. (2005) Proinsulin levels and the proinsulin:c-peptide ratio complement autoantibody measurement for predicting type 1 diabetes. Diabetologia 48: 2322–2329 [DOI] [PubMed] [Google Scholar]
- [16].Van Dalem A, Demeester S, Balti EV, et al. (2016) Prediction of impending type 1 diabetes through automated dual-label measurement of proinsulin:C-peptide ratio. PLoS One 11: eO166702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sims ΕΚ, Chaudhry Z, Watkins R, et al. (2016) Elevations in the Fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes care 39: 1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heaton DA, Millward BA, Gray P, et al. (1987) Evidence of beta cell dysfunction which does not lead on to diabetes: a study of identical twins of insulin dependent diabetics. Br Med J 294: 145–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Spinas GA, Snorgaard O, Hartling SG, Oberholzer M, Berger W (1992) Elevated proinsulin levels related to islet cell antibodies in first-degree relatives of IDDM patients. Diabetes care 15: 632–637 [DOI] [PubMed] [Google Scholar]
- [20].Lindgren FA, Hartling SG, Dahlquist GG, Binder C, Efendic S, Persson BE (1991) Glucose-induced insulin response is reduced and proinsulin response increased in healthy siblings of type 1 diabetic patients. DiabetMed 8: 638–643 [DOI] [PubMed] [Google Scholar]
- [21].Snorgaard O, Hartling SG, Binder C (1990) Proinsulin and C-peptide at onset and during 12 months cyclosporin treatment of type 1 (insulin-dependent) diabetes mellitus. Diabetologia 33: 36–42 [DOI] [PubMed] [Google Scholar]
- [22].Watkins RA, Evans-Molina C, Terrell JK, et al. (2016) Proinsulin and heat shock protein 90 as biomarkers of beta-cell stress in the early period after onset of type 1 diabetes. Transl Res 168: 96–106;el [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scholin A, Nystrom L, Arnqvist H, et al. (2011) Proinsulin/C-peptide ratio, glucagon and remission in new-onset Type 1 diabetes mellitus in young adults. Diabet Med 28: 156–161 [DOI] [PubMed] [Google Scholar]
- [24].Wasserfall C, Nick HS, Campbell-Thompson M, et al. (2017) Persistence of pancreatic insulin mrna expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab 26: 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heding LG, Ludvigsson J (1977) Human proinsulin in insulin-treated juvenile diabetics. Acta Paediatr Scand Suppl: 48–52 [DOI] [PubMed] [Google Scholar]
- [26].Steenkamp DW, Cacicedo JM, Sahin-Efe A, Sullivan C, Stemthal E (2017) Preserved proinsulin secretion in long-standing type 1 diabetes. Endocr Pract 23: 1387–1393 [DOI] [PubMed] [Google Scholar]
- [27].Courtade JA, Klimek-Abercrombie AM, Chen YC, et al. (2017) Measurement of pro-islet amyloid polypeptide (1–48) in diabetes and islet transplants. J Clin Endocrinol Metab 102: 2595–2603 [DOI] [PubMed] [Google Scholar]
- [28].Guay C, Regazzi R (2013) Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 9: 513–521 [DOI] [PubMed] [Google Scholar]
- [29].Klein D, Misawa R, Bravo-Egana V, et al. (2013) MicroRNA expression in alpha and beta cells of human pancreatic islets. PLoS One 8: e55064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ (2013) Circulating miR-375 as a biomarker of β-cell death and diabetes in mice. Endocrinology 154: 603–608 [DOI] [PubMed] [Google Scholar]
- [31].Latreille M, Herrmanns K, Renwick N, et al. (2015) miR-375 gene dosage in pancreatic β-cells: implications for regulation of β-cell mass and biomarker development. J Mol Med 93: 1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Erener S, Marwaha A, Tan R, Panagiotopoulos C, Kieffer TJ (2017) Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI Insight 2: e89656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lakhter AJ, Pratt RE, Moore RE, et al. (2018) Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia 61:1124–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marchand L, Jalabert A, Meugnier E, et al. (2016) miRNA-375 a sensor of glucotoxicity is altered in the serum of children with newly diagnosed type 1 diabetes. J Diabetes Res 2016: 1869082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Seyhan AA, Nunez Lopez YO, Xie H, et al. (2016) Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: a pilot cross-sectional study. Scientific Reports 6: 31479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nielsen LB, Wang C, Sorensen K, et al. (2012) Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual β-cell function and glycaemic control during disease progression. Experimental diabetes research 2012: 896362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Osipova J, Fischer D- C, Dangwal S, et al. (2014) Diabetes-associated micrornas in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. The Journal of Clinical Endocrinology & Metabolism 99: E1661–E1665 [DOI] [PubMed] [Google Scholar]
- [38].Snowhite IV, Allende G, Sosenko J, Pastori RL, Messinger Cayetano S, Pugliese A (2017) Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia 60: 1409–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Akerman L, Casas R, Ludvigsson J, Tavira B, Skoglund C (2018) Serum miRNA levels are related to glucose homeostasis and islet autoantibodies in children with high risk for type 1 diabetes. PLoS One 13: e0191067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Garcia-Contreras M, Shah SH, Tamayo A, et al. (2017) Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep 7: 5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vallabhajosyula P, Korutla L, Habertheuer A, et al. (2017) Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue. J Clin Invest 127: 1375–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kuroda A, Rauch TA, Todorov I, et al. (2009) Insulin gene expression is regulated by DNA methylation. PLoS One 4: e6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Akirav EM, Lebastchi J, Galvan EM, et al. (2011) Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A 108: 19018–19023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fisher MM, Perez Chumbiauca CN, Mather KJ, Mirmira RG, Tersey SA (2013) Detection of islet β-cell death in vivo by multiplex PCR analysis of differentially methylated DNA. Endocrinology 154: 3476–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fisher MM, Watkins RA, Blum J, et al. (2015) Elevations in circulating methylated and unmethylated preproinsulin dna in new-onset type 1 diabetes. Diabetes 64: 3867–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Herold KC, Usmani-Brown S, Ghazi T, et al. (2015) beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest 125: 1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lehmann-Werman R, Neiman D, Zemmour H, et al. (2016) Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci U S A 113: E1826–E1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mulukutla SN, Tersey SA, Hampe CS, Mirmira RG, Balasubramanyam A (2018) Elevated unmethylated and methylated insulin DNA are unique markers of A+β+ ketosis prone diabetes. J Diabetes Complications 32: 193–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Beilin MD, Clark P, Usmani-Brown S, et al. (2017) Unmethylated insulin DNA is elevated after total pancreatectomy with islet autotransplantation: assessment of a novel beta cell marker. Am J Transplant 17:1112–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gala-Lopez BL, Neiman D, Kin T, et al. (2018) Beta cell death by cell-free DNA and outcome after clinical islet transplantation Transplantation [DOI] [PubMed] [Google Scholar]
- [51].Husseiny MI, Kaye A, Zebadua E, Kandeel F, Ferreri K (2014) Tissue-specific methylation of human insulin gene and PCR assay for monitoring beta cell death. PLoS One 9: e94591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Olsen JA, Kenna LA, Spelios MG, Hessner MJ, Akirav EM (2016) Circulating differentially methylated amylin DNA as a biomarker of β-cell loss in type 1 diabetes. PLoS One 11: eO152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sklenarova J, Petruzelkova L, Kolouskova S, Lebl J, Sumnik Z, Cinek O (2017) Glucokinase gene may be a more suitable target than the insulin gene for detection of β cell death. Endocrinology 158: 2058–2065 [DOI] [PubMed] [Google Scholar]
- [54].Neiman D, Moss J, Hecht M, et al. (2017) Islet cells share promoter hypomethylation independently of expression, but exhibit cell-type-specific methylation in enhancers. Proc Natl Acad Sci U S A 114: 13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


