Abstract
Uveitis is defined as intraocular inflammation. It is an extra-articular manifestation of many forms of joint disease which include spondyloarthritis, juvenile idiopathic arthritis, and Behcet’s disease. Rheumatologists may be asked to consult on patients with uveitis in order to identify an associated systemic illness. Diagnoses such as spndyloarthritis, sarcoidosis, and interstitial nephritis with uveitis are frequently overlooked by referring ophthalmologists. Alternatively rheumatologists may be asked to help manage the immunosuppression including biologics which can be required to treat a subset of patients with uveitis. This review is written to provide rheumatologists with the necessary information to facilitate collaboration in co-managing patients with uveitis.
Imagine a symphony orchestra in which the conductor cannot coordinate the play of the wind instruments with the strings or percussion. Imagine a basketball or soccer team that has not mastered the concept of passing the ball. Imagine an epidemiologic study in which the statisticians and those who conceptualized the study, each have different understandings of the study’s purpose. Many endeavors represent a gestalt for which the anticipated goal requires multiple parts; and the whole is greater than the sum of those parts. Since rheumatologists see patients with multi-system disease, they are well aware of this need for collaboration. Uveitis, also known as intraocular inflammation, is a prototypical illness that begs for collaboration. Uveitis is often best assessed and optimally treated by an inter-disciplinary team. In this review, we seek to prepare rheumatologists with information that can facilitate the success of this collaborative effort.
BACKGROUND
The term, uvea, derives from the Latin word for grape. The Roman anatomists felt that peeling away the outer layer of the eye, the cornea and sclera, left a grape-like structure: the iris, ciliary body, and choroid (see Figure 1). Any portion of the uvea can be inflamed and often the inflammation involves adjacent structures (see Figure 2). So anatomic subsets of uveitis include iritis (synonymous with anterior uveitis), iridocyclitis, intermediate uveitis, posterior uveitis (such as choroiditis, retinochoroiditis, chorioretinitis), and panuveitis. Just as increased leukocytes in synovial fluid are indicative of synovitis, so leukocytes in either the aqueous humor or vitreous humor are taken as evidence of uveitis, even though neither the anterior chamber that is filled with aqueous humor nor the vitreous cavity is technically a part of the uveal tract.
Figure 1.
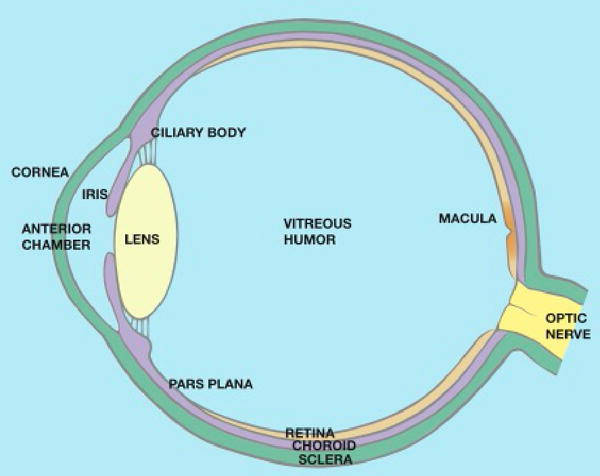
Anatomy of the eye. The uveal tract includes the iris, ciliary body, and choroid. It is shown in lavender.
Figure 2.
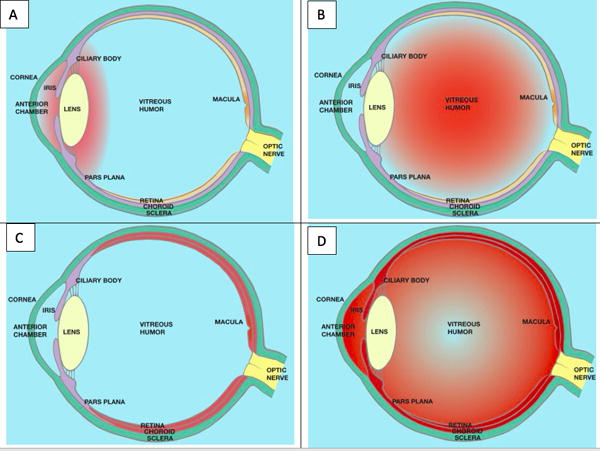
A. Anterior uveitis is defined as inflammation of the iris and/or ciliary body.
B. Intermediate uveitis is diagnosed when inflammatory cells in the vitreous humor are the predominant finding.
C. Posterior uveitis is diagnosed if the retina and/or choroid are inflamed.
D. Panuveitis is diagnosed if inflammation is present in the anterior uvea, the vitreous humor, and the retina or choroid. It is also possible to have anterior and intermediate uveitis if the retina and choroid are not involved or intermediate and posterior uveitis if inflammation in the anterior chamber is minimal or non-existent.
Uveitis has a prevalence of roughly one per thousand1. Several forms of uveitis are episodic and the prevalence is usually stated as a point prevalence meaning that active disease was present at the time of the survey. Despite its relative rarity, uveitis accounts for approximately the same amount of visual morbidity as either macular degeneration or diabetic retinopathy2, although the latter two diseases are generally more commonly recognized by the public as major causes of visual loss. The duration of some subsets of uveitis probably accounts for this paradox. Both macular degeneration and diabetic retinopathy are diseases that occur toward the end of life. In contrast, uveitis might start in childhood or early adulthood and persist through decades.
THE UVEITIS-RHEUMATOLOGY INTERFACE
Patients with uveitis often do need a rheumatologist. As shown in Table I, many systemic, rheumatic diseases can involve both the joints and the uveal tract. Furthermore, most forms of uveitis are immune-mediated and respond to immune suppression, a type of therapy that is outside the expertise of most ophthalmologists.
Table 1.
The differential diagnosis of uveitis in association with arthritis. Although this table includes rare causes of arthritis and uveitis such as Blau syndrome or Whipple’s disease, it does not include everything within the differential diagnosis. For example, tuberculosis could cause a uveitis and an arthritis simultaneously and some patients with arthritis of unknown cause also develop uveitis.
| Diagnosis | Comments |
|---|---|
| Adverse reaction to medication | Drugs include TNF inhibitors, intravenous bisphosphonates, checkpoint inhibitors |
| Ankylosing spondylitis | Affects about 40% of patients with AS; usually unilateral, sudden onset, anterior, and recurrent |
| Behcet’s syndrome | Affects about 60 to 80% of patients with Behcet’s; usually bilateral, recurrent, often severe with associated retinal vasculitis |
| Blau syndrome and other auto-inflammatory syndromes | For Blau, uveitis is a common manifestation of an uncommon disease, usually bilateral with chorioretinitis; optic nerve edema or uveitis are also reported in NOMID or other auto-inflammatory syndromes |
| Crohn’s disease | Uveitis and scleritis can be associated, often along with skin and joint disease; phenotype of uveitis resembles PsA-uveitis phenotype |
| Juvenile idiopathic arthritis | In the pauci-articular, early onset, ANA + subset, usually a bilateral, insidious onset, chronic anterior uveitis. Uveitis also associated with juvenile AS or juvenile onset PsA |
| Kawasaki’s disease and rarely other forms of vasculitis | Bilateral, mild anterior uveitis in association with conjunctivitis. PAN, GPA, LCV, or GCA very rarely cause uveitis. |
| Lyme disease | Rare but reported cause of uveitis. |
| Psoriatic arthritis | Affects about 7% of patients with PsA; can be bilateral, chronic, anterior and intermediate, and insidious in onset |
| Reactive arthritis | Conjunctivitis is classical eye manifestation but sudden onset, unilateral, anterior uveitis is well described |
| Relapsing polychondritis | Can cause iritis, episcleritis, or scleritis |
| Rheumatic fever | Very rarely associated with uveitis |
| Sweet’s syndrome | Rarely reported with uveitis |
| Systemic lupus erythematosus | Rarely causes uveitis or optic neuritis; often causes lacrimal gland disease; can cause cotton wool spots or choroidal vasculopathy |
| Ulcerative colitis | Uveitis or scleritis less common than with Crohn’s disease but clearly associated. |
| Whipple’s disease | Vitreous humor inflammation can be associated |
Abbreviations: AS=ankylosing spondylitis; NOMID=neonatal onset multi-system inflammatory disease; PsA=psoriatic arthritis; ANA=anti-nuclear antibody; PAN=polyarteritis nodosa; GPA=granulomatosis with polyangiitis; LCV=leukocytoclastic vasculitis; GCA=giant cell arteritis.
Just as arthritis has multiple causes, so uveitis has multiple etiologies. Broad etiologic categories are provided in Table 2. The majority of patients with uveitis have an immune-mediated process. This is supported by observation of leukocytes in the eye and by the known ability to produce uveitis in laboratory animals by stimulating the immune response. But just as systemic lupus and rheumatoid arthritis differ in the preferred approach to therapy even though both are immune-mediated, so it is likely that the preferred treatment for a form of uveitis, such as that secondary to juvenile idiopathic arthritis, will differ from the optimal therapy for another form of uveitis such as that secondary to sarcoidosis. Currently, however, most therapy for non-infectious uveitis is determined more by the severity and the anatomic location rather than the etiology, although some notable exceptions are slowly emerging.
Table 2.
Abbreviations: HSV=herpes simplex virus; VZV=varicella virus; CMV=cytomegalovirus
| Broad categories of disease associated with uveitis | Comments |
|---|---|
| Infections | Includes viruses such as HSV, VZV, and CMV; syphilis, toxoplasmosis, and tuberculosis among other infections |
| Masquerade syndromes | Includes B cell lymphoma, leukemia, and retinal detachments |
| Medication reactions | See Table 1 |
| Ocular syndromes | Includes birdshot retinochoroidopathy, pars planitis, acute multifocal placoid pigmentary epitheliopathy, and multifocal choroiditis with panuveitis |
| Systemic immune-mediated disease | See Table 1; in addition includes multiple sclerosis, tubulo-interstitial nephritis with uveitis, and Vogt-Koyanagi-Harada syndrome |
| Trauma | Penetrating trauma can cause sympathetic ophthalmia; surgical trauma as in cataract surgery usually causes some self-limited inflammation |
| “idiopathic” | Also called primary or undifferentiated uveitis; probably the most common diagnosis in a uveitis clinic |
Although case reports rarely elucidate pathogenesis, the course of a physician who survived Ebola infection may reveal clues about the intersection between uveitis and rheumatic diseases3. The patient contracted Ebola in Africa and was flown to Atlanta for care. Although he experienced multi-organ failure, he survived. Fourteen weeks after presentation, he had a severe uveitis and the Ebola virus could be recovered from his eye, presumably because the eye is an immune-privileged site. The virus was no longer detectable in blood. At the time that the uveitis developed, he also had low back pain and enthesitis, symptoms suggestive of a spondyloarthropathy, although the uveitis was intermediate and posterior, locations which are not typically inflamed with ankylosing spondylitis.
For joint swelling, we narrow the differential diagnosis based on parameters such as which joints are involved, whether the disease is symmetric or asymmetric, whether the onset was acute or insidious, and the patient’s age and gender. Similarly, uveitis subsets are recognized by such variables as the anatomic portion of the uveal tract which is inflamed, by whether the disease is unilateral or bilateral, by whether the inflammation is chronic or episodic, by whether the onset began suddenly or insidiously, and of course, by the patient’s age and gender. Both Behcet’s disease and ankylosing spondylitis, for example, are associated with uveitis. Both Behcet’s disease and ankylosing spondylitis might be associated with low back pain, peripheral arthritis and diarrhea. Oral sores are often present in HLA B27-associated reactive arthritis, just as they are present in Behcet’s disease. But the uveitis associated with ankylosing spondylitis almost always affects only one eye at a time, lasts no longer than 3 months, predominantly affects the anterior uveal tract, may cause hypopyon (pus in the anterior chamber of the eye; see Table 3 for a glossary of relevant terms), and tends to be recurrent4. The uveitis associated with Behcet’s disease is also recurrent but rarely is the eye completely uninflamed between attacks. The uveitis associated with Behcet’s disease is usually bilateral. It is usually both an anterior and intermediate uveitis (intermediate uveitis is recognized by leukocytes in the vitreous humor) or a panuveitis, often with retinal vasculitis. While both diseases cause hypopyon, the eye of a patient with ankylosing spondylitis and hypopyon is red, tender, and sensitive to light; fibrin is frequently present in the anterior chamber. In contrast, hypopyon in association with Behcet’s disease need not cause pain or redness. Fibrin is rarely present. In some instances, history alone can help distinguish the two entities since history will usually help determine if one or both eyes are affected or the duration of the eye inflammation. But other subtleties, such as the presence of retinal vasculitis, require a specialist eye examination to detect. Thus, the ophthalmologist potentially can help the rheumatologist make a diagnosis and the rheumatologist can assist the ophthalmologist in differential diagnosis of the systemic disease and collaborate with the management of immunosuppression.
Table 3.
A Glossary of Terms Often Used by A Uveitis Specialist
| Anterior uveitis: inflammation predominantly anterior to the lens of the eye. |
| Band keratopathy: deposition of calcium in the corneal epithelium. It is a common finding in the uveitis associated with JIA. |
| Cystoid macular edema: a common complication of uveitis that affects central vision and is often treated by a local injection of corticosteroid. |
| Flare: the diffraction of the slit lamp beam caused by the increased protein in the anterior chamber that results when the blood aqueous barrier is disrupted as in anterior uveitis. |
| Intermediate uveitis: inflammation predominantly in the vitreous humor. Neither the vitreous humor nor the anterior chamber technically are a part of the uveal tract, but leukocytes in either usually indicate a uveitis just as cells in the synovial fluid usually indicate a synovitis. |
| Keratic precipitates: the concretions of cells adherent to the endothelium of the cornea as seen with a slit lamp examination. Large concretions are called “granulomatous” and are seen in such diseases as sarcoidosis, tuberculosis, and herpes zoster infection. |
| Panuveitis: inflammation simultaneously in the anterior chamber, the vitreous humor and the retina and/or choroid. |
| Posterior synechiae: the adherence of the iris to the lens. This is a non-specific finding which is nonetheless much more common in some forms of uveitis (such as that associated with HLA B27 or in sarcoidosis) than in others. |
| Posterior uveitis: inflammation that involves the choroid and often adjacent structures such as the retina. |
| Retinal vasculitis: an abnormality of retinal vessels such as increased vascular permeability. Retinal vasculitis is a common feature of many forms of uveitis and does not correlate well with the occurrence of a systemic vasculitis. |
| SUN criteria: an acronym for the Standardization of Uveitis Nomenclature, an international consortium that helped to define terms related to uveitis. |
The co-existence of uveitis and arthritis is also not well understood despite how frequently this occurs as is shown in Table 1. Some specific forms of uveitis are discussed in Table 4. Both the eye and joint do share some biochemical similarities such as the presence of hyaluronic acid, type II collagen, and aggrecan. Uveitis and arthritis also occur together in several animal models such as the SKG mouse5, adjuvant arthritis in rats6, and aggrecan-induced arthritis in BALB/C mice7. The co-existence suggests a shared pathogenesis, but in the aggrecan model, mice which do not produce gamma interferon develop far more severe uveitis, while the arthritis is dramatically ameliorated7. Similarly characterizing the genetics of the uveitis associated with HLA B27 shows that ankylosing spondylitis and acute anterior uveitis share a variety of predisposing genes such as HLA B27 itself, the IL-23 receptor, and ERAP-1. At the same time, there are identifiable genes such as IL-6R, probably IL-10, and IL-18R1 that seem to influence solely the susceptibility to acute anterior uveitis8.
Table 4.
Forms of Uveitis of Potential Special Interest to Rheumatologists.
| Diagnosis | Comment | Lab Testing |
|---|---|---|
| Behcet’s disease | Typically a bilateral, recurrent, potentially blinding panuveitis associated with retinal vasculitis | No definitive test; strictly a clinical diagnosis |
| Birdshot retino-choroidopathy | A chronic, bilateral intermediate and posterior uveitis very strongly associated with HLA A29 and frequently treated by chronic immunosuppression | Almost all patients are HLA A29+ |
| Blau syndrome | A very rare, autosomal dominant form of uveitis associated with mutations in the nucleotide binding domain of NOD2, a gene which codes for an important component of the innate immune system which recognizes bacterial cell wall | Genotyping identifies the mutations known to be associated with this syndrome |
| HLA B27-related: | Typically, a sudden onset, self-limited (resolves within 3 months), anterior, recurrent, unilateral uveitis. Most uveitis from any cause is anterior and 50% of those with a sudden onset of anterior uveitis are HLA B27 positive, most of whom have some form of spondyloarthropathy. Usually can be managed by topical corticosteroids. | HLA B27 typing; sacroiliac imaging to help diagnose associated spondyloarthritis |
| Idiopathic | The most common diagnosis in most series from a uveitis clinic. The term is used to mean a form of uveitis that does not fit a diagnostic niche. Other suggested terms include non-classifiable, undifferentiated, or primary (as opposed to secondary to another condition such as ankylosing spondylitis). | A diagnosis made when all other diagnoses have been excluded |
| Juvenile idiopathic arthritis associated uveitis | Characteristically a bilateral, insidious onset, chronic (lasting longer than 3 months), anterior uveitis, especially likely in patients with JIA who have a pauci-articular, early onset, ANA positive form of arthritis | A +ANA is supportive but it is neither sensitive nor specific |
| Pars planitis | A relatively common form of uveitis that usually begins insidiously and causes inflammation in the vitreous humor resulting in prominent floaters. (However, the majority of patients with floaters do not have pars planitis.) The disease is named for the pars plana, an anatomic area immediately posterior to the ciliary body and a site where leukocytic concretions are found in this disease. | A clinical diagnosis based on the location of inflammation; occasionally associated with multiple sclerosis so a brain MRI is sometimes obtained. |
| Primary intraocular lymphoma | A very rare form of uveitis, usually occurring in individuals over 45, and presenting as bilateral cells in the vitreous humor, sometimes with subretinal infiltrates, and often in association with central nervous system lymphoma | Definitive diagnosis is made by characterization of malignant cells in the vitreous humor or on retinal biopsy. |
| Sarcoidosis | A form of uveitis with “promiscuous” or highly varied presentations ranging from anterior uveitis to retinal vasculitis with or without chorioretinal lesions. Ocular inflammation and pulmonary disease are the two most common initial manifestations of sarcoidosis and it accounts for a relatively common systemic illness among patients in a uveitis clinic. | Chest computed tomography is the most sensitive test. Biopsy is rarely required if symmetric hilar or mediastinal adenopathy is present. ACE, lysozyme, and interleukin-2 receptor have questionable specificity and limited sensitivity. |
| Syphilis | Late secondary or tertiary syphilis are in the differential diagnosis for any patient labelled as having “idiopathic” uveitis. | FTA or comparable test is preferred. RPR can be negative in up to 40% of patients with uveitis secondary to syphilis. |
| Tubulo-interstitial nephritis with uveitis | A form of uveitis that is typically sudden onset, bilateral and mostly anterior with a variable amount of vitreous humor inflammation. The disease has an extremely strong association with HLA DRB1*0102. Patients with TINU are typically systemically ill with fever, myalgias and arthralgias as well as a markedly elevated sedimentation rate. | Elevation of urine beta2 microglobulin is a sensitive way to support the diagnosis. Renal biopsy is definitive but often not required. Serum blood urea nitrogen or serum creatinine have limited sensitivity. |
| Tuberculosis | A rare form of uveitis in the United States, but a very common form of uveitis in countries such as India or Saudi Arabia. Also a very difficult diagnosis to confirm as the organism is rarely cultured from the eye. The diagnosis should be considered if the patient has a risk factor for TB (such as being born outside the US or a history of incarceration) or if the illness does not respond to immunosuppression such as oral corticosteroids. | Culture is the ideal confirmatory test, but it is often negative. PCR, when available, is a good alternative.Interferon gamma release assays and skin test responses are useful in confirming exposure to tuberculosis, but neither establishes if active infection is present. |
| Vogt-Koyanagi-Harada syndrome | An autoimmune form of uveitis with the triggering antigen putatively being tyrosinase. Patients are almost always either Asian, native American, or Spanish speaking. The uveitis is a bilateral, severe panuveitis with serous retinal elevation. Additional symptoms may include headache, meningismus, eight nerve abnormalities, and vitiligo. | Fluorescein angiography and/or optical coherence tomography should show characteristic serous elevation of the retina. |
| Whipple’s disease | A rare but treatable cause of uveitis in association with arthritis. The presentation in the eye is generally cells in the vitreous humor. | Identification of the causative organism, Tropheryma whippeli, in the vitreous humor or elsewhere |
Abbreviations: ACE=angiotensin converting enzyme; FTA=fluorescent treponemal antibody; RPR=rapid plasma reagin; PCR=polymerase chain reaction.
DIFFERENTIAL DIAGNOSIS AND LABORATORY TESTING
The heterogeneity of uveitis has multiple implications. In terms of differential diagnosis, it is obviously critical to distinguish an infection from an immune-mediated cause of uveitis. Some infections, such as syphilis and tuberculosis, can be quite variable in terms of their presentation within the eye and frequently enter into the differential diagnosis. Some of the more common infectious causes of uveitis include herpes simplex, herpes zoster, toxoplasmosis, and cytomegalovirus (the latter usually in an immunocompromised host). Most infections of the uveal tract cause characteristic changes that can be recognized with a slit lamp examination or with indirect ophthalmoscopy. The “partnership” between a rheumatologist and ophthalmologist, in our opinion, is such that the rheumatologist must trust that the ophthalmologist has excluded an infection; both specialists must be aware that a patient who fails to respond to immunosuppression could have an overlooked infectious cause for her disease. Likewise, two other etiologies, masquerade syndromes and drug-induced disease, are relatively rare, but each has distinct therapeutic implications. The most common uveitis “masquerade” is probably a B cell lymphoma that is usually confined to the brain and the eye9, 10. It typically occurs bilaterally in patients who are over 45. This diagnosis can easily be missed. Medications do not usually cause uveitis, but possible culprits include iv bisphosphonates11, TNF inhibitors12, checkpoint inhibitors13 and several antibiotics14.
In rheumatology, distinguishing gout from rheumatoid arthritis as a cause of joint swelling will markedly change the therapeutic strategy. Most forms of non-infectious uveitis are approached by a treatment algorithm that is not impacted by the cause of the uveitis. Two prominent exceptions are Behcet’s disease and the anterior uveitis associated with juvenile idiopathic arthritis. For Behcet’s disease, a monoclonal antibody that inhibits TNF is used frequently because of the dramatic efficacy15. For the anterior uveitis associated with JIA, the more frequent use of methotrexate and/or a TNF inhibitor such as adalimumab has improved the prognosis for this disease markedly16.
The above considerations regarding differential diagnosis obviously impact the search for an etiology. The rheumatologist is often tasked with finding a systemic, immune-mediated cause, but should not be the practitioner who diagnoses an infection or masquerading malignancy as a cause. As is true of differential diagnosis in general, the history is key and pattern recognition aids greatly in the goal. Some forms of uveitis such as Behcet’s disease or Vogt-Koyanagi-Harada (VKH) syndrome are clinical diagnoses without a definitive laboratory test. Other diagnoses, like Crohn’s disease, ulcerative colitis, or sarcoidosis, can be established or supported by biopsy or imaging, but the procedure may be too costly, too toxic (radiation from a CT scan of a young person), too unlikely to yield positive results, or too uncomfortable (colonoscopy) to recommend on a routine basis. Our practice is to choose tests selectively based on clues gained from the examination or history. For example, multiple areas of serous elevation of the retina can be detected on examination and confirmed by testing such as optical coherence tomography or fluorescein angiography. The finding suggests a diagnosis of VKH.
The most common systemic disease associated with uveitis is spondyloarthritis. Roughly half of all patients with sudden onset, non-infectious, anterior uveitis in Europe or North America are HLA B27 positive17. Recent studies, one from an emergency room in Dublin, Ireland18 and another from Spain involving 798 subjects and a collaboration between rheumatologists and ophthalmologists19, concluded that roughly 80% of patients with B27-associated acute anterior uveitis have axial spondyloarthritis based on ASAS criteria. This observation held although both studies excluded any patient with uveitis who had a known spondyloarthropathy. Spondyloarthritis is less common among those who have anterior uveitis and are HLA B27 negative, but the diagnosis remains surprisingly frequent19. Older studies using more stringent criteria to diagnose spondyloarthritis had also concluded that spondyloarthropathy was endemic among patients with anterior uveitis4, 20. Rheumatologists who are unaware of this association will frequently fail to recognize the clinical significance of the chronic inflammatory back pain that afflicts many with anterior uveitis.
Another critical cause of uveitis to appreciate is sarcoidosis. In a study from the Cleveland Clinic, 57% of women over 61 years of age with idiopathic uveitis had normal chest x-rays but chest CT evidence for sarcoidosis21. We have recently found a similar, slightly lower yield by performing chest CTs on patients with idiopathic uveitis over 40 years of age22. In addition, we noted that 21% of those discovered to have sarcoidosis on chest CT also had cardiac sarcoidosis with associated ventricular tachycardia22. Thus, recognition of the systemic illness had potentially life-saving implications. A study from Japan reached a similar conclusion about uveitis and cardiac sarcoidosis23.
While most systemic rheumatic diseases associated with uveitis such as Behçet’s disease are diagnosed on the basis of the clinical presentation, tubulointerstitial nephritis with uveitis (TINU) is another of those easily overlooked entities. Rheumatologists might be asked to see a patient with TINU because the patient has bilateral red eyes and photophobia from the anterior uveitis and the patient is typically systemically ill with fever, myalgias, a markedly elevated erythrocyte sedimentation rate, mild anemia, and mildly abnormal liver enzymes24, 25. Unless one is cognizant of the diagnosis and requests a measurement of beta2 microglobulin in the urine, the diagnosis is frequently overlooked.
In virtually every series of patients with uveitis, a diagnosis of “idiopathic” disease is the most common etiology noted26. Other terms to describe idiopathic uveitis include non-classifiable, primary uveitis or undifferentiated disease27.
Despite the breadth of the differential diagnosis for uveitis, the rheumatologist should be able to take a relatively targeted approach to laboratory testing28. The examination by the ophthalmologist should have identified any suspicion for a masquerade syndrome or an infectious cause. The detailed history should point to most of the possible associated systemic, immune-mediated diseases. If history and exam have failed to point to a probable cause, we screen for syphilis since this infection can be latent for many years and its uveal manifestations are protean. In addition, we obtain a chest x-ray as sarcoidosis might be asymptomatic in the lungs and this is also useful as a screen for tuberculosis. The sensitivity for either ocular tuberculosis or sarcoidosis using chest x-ray is probably 50% or worse. Because of the extensive radiation, we usually do not obtain a chest CT scan to search for sarcoid unless the patient is over 40 years of age22. In the US, we also do not screen for tuberculosis exposure unless the patient has a specific risk factor for tuberculosis such as birth outside the United States or a history of incarceration29. Additional tests might be useful to monitor therapy such as a complete blood count and metabolic panel. Targeted testing is also useful if the presentation suggests a specific entity. For example, we often test for HLA B27 if the patient presents with unilateral, acute, anterior uveitis. We check urine for beta 2 microglobulin for patients who present with a bilateral, sudden onset, anterior uveitis. We consider multiple sclerosis if the patient relates neurological symptoms that might be explained by this diagnosis.
THERAPY
The heterogeneity of uveitis has impacted the ability to design clinical trials. The relative rarity of vision threatening uveitis is such that different etiologies are usually combined into one clinical trial. However, the manifestations of a disease such as birdshot retinochoroidopathy are such that the endpoint for treating it differ greatly from the endpoint in treating an inflammation such as Behcet’s disease. This challenge in trial design may have contributed to the failure of promising therapies such as voclosporine (a congener of cyclosporine), secukinumab (anti IL-17A)30, 31, gevokizumab (anti IL-1 beta)32 or intravitreal rapamycin33 to show consistent benefit in clinical uveitis trials.
Most practitioners treat uveitis initially, especially anterior uveitis, with topical corticosteroids. Although these are frequently effective, their penetration posterior to the lens is limited. In addition, use of topical corticosteroids can be complicated by cataractogenesis or elevated intraocular pressure. One formulation, difluprednate, has greater ability to treat inflammation posterior to the lens, but it also has a greater tendency to cause a cataract or glaucoma. If topical corticosteroids fail, a locally injected corticosteroid such as triamcinolone can be useful. However, in addition to being an uncomfortable injection, risks include cataract, glaucoma, lid ptosis, and rarely retinal detachment. Triamcinolone can be injected directly into the vitreous humor where it has increased benefit and increased risk of intraocular infection or hemorrhage. Oral corticosteroids represent an additional option, but long-term use has toxicity well known to rheumatologists34. Antimetabolites including mycophenolate mofetil, methotrexate, and azathioprine are popular corticosteroid-steroid sparing drugs used by uveitis experts35. Additional options include calcineurin antagonists such as cyclosporine or tacrolimus36, alkylators like cyclophosphamide, or long-lasting corticosteroid implants delivered either by surgery or injection.
The rheumatologist has a major role to play in managing the therapy of a subset of patients with ocular inflammatory disease. Many ophthalmologists are comfortable prescribing oral corticosteroids but rarely resort to steroid sparing medications37. The dosage of prednisone or its equivalent is often such that the treatment has considerable morbidity. An NIH sponsored trial known as MUST (Multicenter Uveitis Steroid Trial) for patients with non-infectious, intermediate, posterior or panuveitis recently showed superior efficacy for systemic immunosuppression as with anti-metabolites compared to a sustained release of fluocinolone into the vitreous humor of the eye. This conclusion was based on visual acuity with 7 years of follow-up38.
Progress in approval of new therapies for uveitis is hampered by the relative rarity of specific forms of uveitis, by the variety of presumed causes, and by the heterogeneity of outcomes that might define successful therapy. In two time to treatment failure trials reported in 2016, adalimumab showed clear-cut benefit for non-infectious intermediate, posterior or panuveitis39, 40. In most instances, adalimumab is indicated for patients who have failed therapy with oral corticosteroids as well as another oral immunosuppressant such as methotrexate or mycophenolate mofetil. The responsiveness of Behcet’s disease to adalimumab or infliximab is such that the authors’ sometimes consider such therapy without a trial of an anti-metabolite41. Multiple sclerosis (MS) can be associated with intermediate uveitis42. As MS is a relative contraindication to inhibiting TNF, this diagnosis may need to be excluded prior to starting adalimumab therapy for uveitis.
It is rare to prescribe a biologic for anterior uveitis, since most patients with anterior uveitis can be controlled with topical medication alone. An exception is the uveitis characteristically associated with one subset of patients with juvenile idiopathic arthritis (JIA). The typical JIA patient with chronic uveitis has disease that begins between the ages of two and eight and affects a few joints. The majority have a positive ANA and are female. The SYCAMORE study tested the efficacy of adalimumab for patients with JIA whose uveitis was active despite methotrexate and topical corticosteroids43. The trial was halted early since the evidence for benefit was apparent. Another randomized controlled trial used a different outcome measure, quantification of protein in the anterior chamber of the eye, and also concluded that adalimumab was useful for the chronic anterior uveitis associated with JIA44.
These successes have encouraged additional uveitis trials such as one assessing the benefit of the Jak inhibitor, filgotinib, for indications similar to those supporting the use of adalimumab. Many emerging biologics, however, have not been studied rigorously for possible benefit in the treatment of uveitis. And while uveitis is increasingly targeted in therapeutic trials, a search of the website, clinicaltrials.gov in February, 2018, identified only 7 current or prior randomized controlled trials for uveitis. A similar search for rheumatoid arthritis identified 54 trials. Finally, although some ophthalmologists are comfortable prescribing immunosuppression without close physician collaboration, virtually all ophthalmologists lack an infrastructure that is prepared to deal with the systemic infectious complications which are rare but unavoidable when one suppresses the immune system.
To aid physicians who care for patients with uveitis, several international groups have recently offered guidelines to assist in the care of patients with ocular inflammatory diseases45, 46. The FOCUS (Fundamentals of Care for Uveitis) group consisted of 146 international experts who graded the strength of the evidence and who used consensus methodology.45
Patients with spondyloarthritis tend to have recurrent episodes of uveitis. As most of these episodes are anterior, last for no more than 3 months, and can be managed with topical corticosteroids alone, the issue of prophylaxis often does not arise. Several medications do reduce the frequency of uveitis. The use of sulfasalazine to prevent attacks of uveitis in patients with spondyloarthritis is supported by randomized controlled trials47, 48. Monoclonal antibodies that neutralize TNF, especially adalimumab or infliximab, also prevent attacks of uveitis49, but these are not generally prescribed if the sole reason is to prevent attacks of anterior uveitis. At this time, extensive data on how treatments such as secukinumab or tofacitinib affect recurrent uveitis in patients with spondylitis have not been published.
In the future, therapies delivered locally to the eye might become the treatment of choice for ocular inflammation. Locally injected corticosteroid into the eye itself can be very effective, but the therapy is limited, in part because the medication frequently causes cataract and glaucoma. Gene therapy for inherited retinal degeneration has now been FDA approved and is successful in part because the injected gene enters a confined space with minimal worries about expression in other tissues50. In contrast to polyarticular rheumatoid arthritis, successful gene therapy for ocular inflammation needs to target no more than two locations. In the decades ahead, one or more locally delivered inhibitors of inflammation might become standard of care for uveitis.
CONCLUSION
We recognize the challenges to familiarize oneself with a group of diseases which one cannot fully assess by using the tools in a conventional rheumatology clinic. And we empathize with the time requirements that impair optimal management of a patient whose illness requires two or more subspecialists to confer. Mechanisms that facilitate communication with an ophthalmologist include interdisciplinary clinics and case conferences to discuss patients whose illness lies in the interstices between these two disciplines. The gratification of practicing medicine derives primarily from the opportunity to improve the welfare of our patients. That welfare is best served if we as rheumatologists share our knowledge and experience, while welcoming the collaboration of ophthalmologists who quite literally have a different view of our patients and their disease. Just as a conductor of a symphony orchestra coordinates multiple musicians, physicians can and should achieve a similar collaboration.
Acknowledgments
JTR receives support from the William and Mary Bauman Foundation, the Stan and Madelle Rosenfeld Family Trust, the Spondylitis Association of America, Research to Prevent Blindness, and NIH Grant RO1 EY 026572. JTR has received consulting fees from Abbvie, Gilead, Regeneron, Topivert, Cavtherx, Mallinckrodt, UCB, Novartis, and Eyevensys. He receives laboratory support from the Alcon Research Institute, the Rheumatology Research Foundation, the Spondylitis Association of America, and Pfizer. He holds stock in Novartis. He receives royalties from UpToDate. ADD is partly supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital, National Health Service Foundation Trust, and University College London Institute of Ophthalmology. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or the Department of Health. ADD consults for Abbvie, Astellas, Sanofi, and Quark Pharmaceuticals. No funding agency or pharmaceutical company contributed to the content of this report and none has been privy to the content.
Contributor Information
James T. Rosenbaum, Departments of Ophthalmology, Medicine, and Cell Biology, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, L467Ad, Portland, Oregon 97239; Legacy Devers Eye Institute, 1040 NW 22nd Ave, Portland, Oregon 97210
Andrew D. Dick, Director of UCL-Institute of Ophthalmology and Duke Elder Chair of Ophthalmology, University College London, Institute of Ophthalmology, London, UK; Professor of Ophthalmology, University of Bristol, Bristol Eye Hospital, Bristol, UK; National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital and University College London, Institute of Ophthalmology, London, UK.
References
- 1.Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, et al. Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmol. 2016;134:1237–45. doi: 10.1001/jamaophthalmol.2016.3229. [DOI] [PubMed] [Google Scholar]
- 2.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–8. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 3.Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N Engl J Med. 2015;372:2423–7. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbaum JT. Characterization of uveitis associated with spondyloarthritis. J Rheumatol. 1989;16:792–6. [PubMed] [Google Scholar]
- 5.Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, et al. Beta-glucan triggers spondylarthritis and Crohn’s disease-like ileitis in SKG mice. Arthritis Rheum. 2012;64:2211–22. doi: 10.1002/art.34423. [DOI] [PubMed] [Google Scholar]
- 6.Petty RE, Johnston W, McCormick AQ, Hunt DWC, Rootman J, Rollins DF. Uveitis and arthritis induced by adjuvant: Clinical, immunologic and histologic characteristics. J Rheumatol. 1989;16:499–505. [PubMed] [Google Scholar]
- 7.Kezic JM, Davey MP, Glant TT, Rosenbaum JT, Rosenzweig HL. IFN-gamma regulates discordant mechanisms of uveitis versus joint and axial disease in a murine model resembling spondyloarthritis. Arthritis Rheum. 2011;64:762–71. doi: 10.1002/art.33404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson PC, Claushuis TA, Cortes A, Martin TM, Evans DM, Leo P, et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol. 2015;67:140–51. doi: 10.1002/art.38873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JR, Braziel RM, Paoletti S, Lipp M, Uguccioni M, Rosenbaum JT. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101:815–21. doi: 10.1182/blood-2002-05-1576. [DOI] [PubMed] [Google Scholar]
- 10.Smith JR, Rosenbaum JT, Wilson DJ, Doolittle ND, Siegal T, Neuwelt EA, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709–16. doi: 10.1016/s0161-6420(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 11.Patel DV, Horne A, House M, Reid IR, McGhee CN. The incidence of acute anterior uveitis after intravenous zoledronate. Ophthalmology. 2013;120:773–6. doi: 10.1016/j.ophtha.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Lim LL, Fraunfelder FW, Rosenbaum JT. Do tumor necrosis factor inhibitors cause uveitis? A registry-based study. Arthritis Rheum. 2007;56:3248–52. doi: 10.1002/art.22918. [DOI] [PubMed] [Google Scholar]
- 13.Conrady CD, Larochelle M, Pecen P, Palestine A, Shakoor A, Singh A. Checkpoint inhibitor-induced uveitis: a case series. Graefes Arch Clin Exp Ophthalmol. 2017 doi: 10.1007/s00417-017-3835-2. [DOI] [PubMed] [Google Scholar]
- 14.Eadie B, Etminan M, Mikelberg FS. Risk for Uveitis With Oral Moxifloxacin: A Comparative Safety Study. JAMA Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2014.3598. [DOI] [PubMed] [Google Scholar]
- 15.Ohno S, Nakamura S, Hori S, Shimakawa M, Kawashima H, Mochizuki M, et al. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behcet’s disease with refractory uveoretinitis. J Rheumatol. 2004;31:1362–8. [PubMed] [Google Scholar]
- 16.Ramanan AV, Dick AD, Benton D, Compeyrot-Lacassagne S, Dawoud D, Hardwick B, et al. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE Trial) Trials. 2014;15:14. doi: 10.1186/1745-6215-15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewerton DA, Caffrey M, Nicholls A, Walters D, James DC. Acute anterior uveitis and HL-A 27. Lancet. 1973;302:994–6. doi: 10.1016/s0140-6736(73)91090-8. [DOI] [PubMed] [Google Scholar]
- 18.Haroon M, O’Rourke M, Ramasamy P, Murphy CC, FitzGerald O. A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: the DUET (Dublin Uveitis Evaluation Tool) Ann Rheum Dis. 2015;74:1990–5. doi: 10.1136/annrheumdis-2014-205358. [DOI] [PubMed] [Google Scholar]
- 19.Juanola X, Loza Santamaria E, Cordero-Coma M, Group SW Description and Prevalence of Spondyloarthritis in Patients with Anterior Uveitis: The SENTINEL Interdisciplinary Collaborative Project. Ophthalmology. 2016;123:1632–6. doi: 10.1016/j.ophtha.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Monnet D, Breban M, Hudry C, Dougados M, Brezin AP. Ophthalmic findings and frequency of extraocular manifestations in patients with HLA-B27 uveitis: a study of 175 cases. Ophthalmology. 2004;111:802–9. doi: 10.1016/j.ophtha.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser PK, Lowder CY, Sullivan P, Sanislo SR, Kosmorsky GS, Meziano MA, et al. Chest computerized tomography in the evaluation of uveitis in elderly women. Am J Ophthalmol. 2002;133:499–505. doi: 10.1016/s0002-9394(02)01333-8. [DOI] [PubMed] [Google Scholar]
- 22.Han YS, Rivera-Grana E, Salek S, Rosenbaum JT. Distinguishing Uveitis Secondary to Sarcoidosis From Idiopathic Disease: Cardiac Implications. JAMA Ophthalmol. 2018 doi: 10.1001/jamaophthalmol.2017.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umazume A, Kezuka T, Okunuki Y, Ooshita M, Usui Y, Hirano M, et al. Prediction of severe cardiac involvement by fundus lesion in sarcoidosis. Jpn J Ophthalmol. 2014;58:81–5. doi: 10.1007/s10384-013-0288-y. [DOI] [PubMed] [Google Scholar]
- 24.Mandeville JT, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol. 2001;46:195–208. doi: 10.1016/s0039-6257(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 25.Mackensen F, Smith J, Rosenbaum JT. Enhanced recognition, treatment, and prognosis of tubulointerstitial nephritis and uveitis syndrome. Ophthalmology. 2007;114:995–9. doi: 10.1016/j.ophtha.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum JT. Nibbling away at the diagnosis of idiopathic uveitis. JAMA Ophthalmol. 2015;133:146–7. doi: 10.1001/jamaophthalmol.2014.4272. [DOI] [PubMed] [Google Scholar]
- 27.Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol. 2013;156:228–36. doi: 10.1016/j.ajo.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbaum JT, Wernick R. Selection and interpretation of laboratory tests for patients with uveitis. Int Ophthalmol Clin. 1990;30:238–43. doi: 10.1097/00004397-199030040-00002. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum JT, Wernick R. The utility of routine screening of patients with uveitis for systemic lupus erythematosus or tuberculosis. A Bayesian analysis. Arch Ophthalmol. 1990;108:1291–3. doi: 10.1001/archopht.1990.01070110107034. [DOI] [PubMed] [Google Scholar]
- 30.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120:777–87. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122:939–48. doi: 10.1016/j.ophtha.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Tugal-Tutkun IM, Kadayifcilar SM, Khairallah MM, Lee SCMP, Ozdal P, Ozyazgan Y, et al. Safety and Efficacy of Gevokizumab in Patients with Behcet’s Disease Uveitis: Results of an Exploratory Phase 2 Study. Ocul Immunol Inflamm. 2017;25:62–70. doi: 10.3109/09273948.2015.1092558. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen QD, Sadiq MA, Soliman MK, Agarwal A, Do DV, Sepah YJ. The Effect of Different Dosing Schedules of Intravitreal Sirolimus, a Mammalian Target of Rapamycin (mTOR) Inhibitor, in the Treatment of Non-Infectious Uveitis (An American Ophthalmological Society Thesis) Trans Am Ophthalmol Soc. 2016;114:T3. [PMC free article] [PubMed] [Google Scholar]
- 34.Miloslavsky EM, Naden RP, Bijlsma JW, Brogan PA, Brown ES, Brunetta P, et al. Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-210002. [DOI] [PubMed] [Google Scholar]
- 35.Esterberg E, Acharya NR. Corticosteroid-sparing therapy: practice patterns among uveitis specialists. J Ophthalmic Inflamm Infect. 2012;2:21–8. doi: 10.1007/s12348-011-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee RW, Greenwood R, Taylor H, Amer R, Biester S, Heissigerova J, et al. A randomized trial of tacrolimus versus tacrolimus and prednisone for the maintenance of disease remission in noninfectious uveitis. Ophthalmology. 2012;119:1223–30. doi: 10.1016/j.ophtha.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen QD, Hatef E, Kayen B, Macahilig CP, Ibrahim M, Wang J, et al. A cross-sectional study of the current treatment patterns in noninfectious uveitis among specialists in the United States. Ophthalmology. 2011;118:184–90. doi: 10.1016/j.ophtha.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 38.Writing Committee for the Multicenter Uveitis Steroid Treatment T, Follow-up Study Research G. Kempen JH, Altaweel MM, Holbrook JT, Sugar EA, et al. Association Between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs Systemic Anti-inflammatory Therapy and Visual Acuity at 7 Years Among Patients With Intermediate, Posterior, or Panuveitis. JAMA. 2017;317:1993–2005. doi: 10.1001/jama.2017.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in Patients with Active Noninfectious Uveitis. N Engl J Med. 2016;375:932–43. doi: 10.1056/NEJMoa1509852. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–92. doi: 10.1016/S0140-6736(16)31339-3. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum JT. Blind insight: eyeing anti-tumor necrosis factor treatment in uveitis associated with Behcet’s disese. J Rheumatol. 2004;31:1241–3. [PubMed] [Google Scholar]
- 42.Messenger W, Hildebrandt L, Mackensen F, Suhler E, Becker M, Rosenbaum JT. Characterisation of uveitis in association with multiple sclerosis. Br J Ophthalmol. 2015;99:205–9. doi: 10.1136/bjophthalmol-2014-305518. [DOI] [PubMed] [Google Scholar]
- 43.Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot-Lacassagne S, et al. Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N Engl J Med. 2017;376:1637–46. doi: 10.1056/NEJMoa1614160. [DOI] [PubMed] [Google Scholar]
- 44.Quartier P, Baptiste A, Despert V, Allain-Launay E, Kone-Paut I, Belot A, et al. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2017-212089. [DOI] [PubMed] [Google Scholar]
- 45.Dick AD, Rosenbaum JT, Al-Dhibi HA, Belfort R, Jr, Brezin AP, Chee SP, et al. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis: Fundamentals of Care for Uveitis (FOCUS) Initiative. Ophthalmology. 2018 doi: 10.1016/j.ophtha.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Wakefield D, McCluskey P, Wildner G, Thurau S, Carr G, Chee SP, et al. Inflammatory eye disease: Pre-treatment assessment of patients prior to commencing immunosuppressive and biologic therapy: Recommendations from an expert committee. Autoimmun Rev. 2017;16:213–22. doi: 10.1016/j.autrev.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Munoz-Fernandez S, Hidalgo V, Fernandez-Melon J, Schlincker A, Bonilla G, Ruiz-Sancho D, et al. Sulfasalazine reduces the number of flares of acute anterior uveitis over a one-year period. J Rheumatol. 2003;30:1277–9. [PubMed] [Google Scholar]
- 48.Dougados M, Berenbaum F, Maetzel A, Amor B. Prevention of acute anterior uveitis associated with spondyloarthropathy induced by salazosulfapyridine. Rev Rhum. 1993;60:81–3. [PubMed] [Google Scholar]
- 49.Lie E, Lindstrom U, Zverkova-Sandstrom T, Olsen IC, Forsblad-d’Elia H, Askling J, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis. 2017;76:1515–21. doi: 10.1136/annrheumdis-2016-210931. [DOI] [PubMed] [Google Scholar]
- 50.Trapani I, Banfi S, Simonelli F, Surace EM, Auricchio A. Gene therapy of inherited retinal degenerations: prospects and challenges. Hum Gene Ther. 2015;26:193–200. doi: 10.1089/hum.2015.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


