Abstract
Coronary allograft vasculopathy (CAV) is a major cause of mortality in late-stage orthotopic heart transplantation (OHT) patients. Recent evidence has shown that myocardial perfusion reserve (MPR) derived from vasodilator cardiovascular magnetic resonance imaging (vCMR) and global longitudinal strain (GLS) from transthoracic echocardiography (TTE) are useful to detect CAV. However, previous studies have not comprehensively addressed whether these parameters are confounded by allograft rejection, myocardial scar/fibrosis, or allograft dysfunction. Our aim was to determine whether changes in late post-OHT MPR and GLS are due to CAV or other confounding factors. Twenty OHT patients (time from transplant to vCMR was 8.1 ± 4.1 years) and 30 controls (10 healthy volunteers and 20 with prior myocardial infarction to provide perspective with regards to the severity of any abnormalities seen in post-OHT patients) underwent vasodilator vCMR from which MPR index (MPRi), left ventricular ejection fraction (LVEF), and burden of late gadolinium enhancement (LGE) were quantified. TTE was used to measure GLS. The presence of CAV was determined from invasive coronary angiograms using thrombolysis in myocardial infarction (TIMI) frame counts and grading severity per guidelines. Previous endomyocardial biopsies were reviewed to assess association with episodes of rejection. We examined the correlations between MPRi and GLS with markers of CAV, allograft function, scar/fibrosis, and rejection. MPRi was abnormal in post-OHT patients compared to both healthy volunteers and MI controls. While there was no relationship between MPRi or GLS and LVEF, episodes of rejection, or LGE burden, both MPRi and GLS were associated with TIMI frame counts and presence and severity of CAV. Additionally, MPRi correlated with GLS (R = 0.68, P = 0.0002). In conclusion, MPRi and GLS are abnormal in late-stage OHT and associated with CAV, but not related to allograft rejection, myocardial scar/fibrosis, or allograft dysfunction. Non-invasive monitoring of MPRi and GLS may be a useful strategy to detect CAV.
Keywords: Magnetic resonance imaging, Heart transplantation, Allograft vasculopathy, Myocardial perfusion, Longitudinal strain, Echocardiography
Introduction
Orthotopic heart transplantation (OHT) is an important treatment for patients with end-stage, refractory heart failure. Advances in immunosuppressive therapies have resulted over time in improved longevity of post-OHT recipients [1]. Presently, the leading causes of cardiovascular mortality in late post-OHT (> 10 years) patients include graft failure and coronary allograft vasculopathy (CAV) [2]. The diagnosis of CAV is typically confirmed with invasive coronary angiography, often with intravascular ultrasound (IVUS) to detect abnormal intimal thickening. Other invasive markers of CAV include abnormal coronary flow reserve or TIMI frame counts [3, 4]. Lately however, there is a growing interest to identify a robust, non-invasive technique to diagnose and monitor CAV.
Prior investigations with non-invasive modalities [vasodilator cardiovascular magnetic resonance (vCMR), myocardial contrast echocardiography, single-photon emission computed tomography (SPECT), and positron emission tomography (PET)] have reported conflicting results on how myocardial perfusion reserve (MPR) changes in the post-OHT patient with some suggesting no change and others suggesting a decrease over time [5–12]. More recent studies have shown that MPR derived from vCMR imaging is a promising tool to diagnose CAV when compared to invasive coronary angiography with IVUS [13]. In the transplanted heart with CAV, the capacity for coronary arteries and microcirculation to dilate is impaired, resulting in abnormal MPR [14]. However previous studies, especially in late post-OHT period, have not adequately addressed whether other variables such as allograft rejection, myocardial scar/ fibrosis, or allograft dysfunction are possible confounders responsible for reduced MPR.
Similar to reduced MPR by vCMR, abnormal global longitudinal strain (GLS) derived from transthoracic echocardiography (TTE) has been recently shown to be associated with CAV and coronary microvascular dysfunction [15–17] in studies from a single group of investigators. Moreover, the same group has reported that decreased GLS is associated with repeated episodes of allograft rejection [18]. However, it remains unknown whether GLS is similarly confounded by allograft rejection or myocardial scar/fibrosis. It is also not well known to what extent GLS and MPR are related.
Neither vCMR-derived MPR nor TTE-derived GLS have been adopted in routine clinical practice in the context of post-OHT CAV evaluation, thus highlighting the need to better understand both tools. Compared to invasive coronary angiography, a reliable non-invasive approach to CAV would be of great value. Specific advantages of vCMR include the ability to quantify myocardial perfusion and microvascular function [19–21], while TTE is nearly universal and could easily be used in this patient population. Accordingly, the aim of this study was to determine whether MPR and GLS are associated with markers of CAV and to what extent they are affected by other confounders (allograft rejection, myocardial scar/fibrosis, or allograft dysfunction), as well as whether they are associated with each other.
Methods
Population and study design
The study included 20 consecutive patients post-OHT (at least 1 year after transplant; mean 8.1 ± 4.1 years), who underwent vCMR, echocardiogram, and coronary angiogram over a period of 2 years and also had complete records of endomyocardial biopsies available. We used two different control groups who all underwent vCMR: 20 patients with a previous history of documented myocardial infarction (MI), confirmed by invasive coronary angiogram, who were successfully revascularizated, and 10 healthy volunteers with no history of cardiovascular disease. The control groups did not undergo echocardiogram. The MI control group was included to provide perspective with regards to the severity of any abnormalities seen in post-OHT patients in the context of a well-described cardiovascular disorder. All subjects were selected from a registry of studies performed at a single academic teaching hospital. The Institutional Review Board at the University of Chicago approved the study.
In order to determine the factors that govern changes in MPR and GLS, OHT patients were also required to have had:
vCMR examination, the results of which were used to determine MPR and presence of myocardial scar/fibrosis;
transthoracic echocardiography (TTE) examination, the results of which were used to determine GLS.
invasive coronary angiography, the results of which were used to detect CAV;
complete record of post-OHT endomyocardial biopsies to determine the relationship between rejection and MPR or GLS.
In order to understand the factors responsible for the changes in MPR and GLS, we employed a multiparametric strategy. To this effect, vCMR was used to determine whether scar/fibrosis is related to MPR or GLS, endomyocardial biopsies were examined to evaluate whether rejection is related to MPR or GLS, and coronary angiograms were analyzed to examine the relationship between markers of CAV and MPR or GLS.
vCMR protocol
Each subject underwent a vCMR study, after clinical evaluation, in which baseline demographics, current symptoms, past medical history, exercise capacity, and current medications were assessed. Patients were excluded if they had an implantable cardioverter-defibrillator, pacemaker or other standard contraindications to gadolinium-enhanced vCMR, such as claustrophobia, severe reactive airway disease, high-grade atrioventricular (AV) nodal block, or GFR < 30 ml/min/1.73 m2. A 12-lead electrocardiogram was performed in all patients prior to vCMR imaging to rule out high-degree AV nodal block. Patients were asked to stop all nitrates and caffeine-containing products 12 h before the vCMR exam.
Each vCMR study was performed using a 1.5-T MRI scanner (Achieva, Philips, Best, Netherlands) and a 5-element phased array cardiac coil. Retrospectively gated cine-CMR images were obtained using a steady-state free precession (SSFP) pulse sequence (temporal resolution ~ 40 ms). Standard long-axis views, including four-chamber, two-chamber, and three-chamber, were obtained. In addition, one series of short-axis slices (6 mm thickness, 4 mm gap) spanning from left ventricular (LV) base to apex was acquired. Myocardial perfusion imaging was performed during vasodilator stress using a hybrid gradient echo/echo planar imaging sequence (voxel size ~ 2.5 × 2.5 mm, slice thickness 10 mm, flip angle 20°, delay time 80 ms, and SENSE factor 1.3). Images of three LV short-axis slices were acquired during first pass of gadolinium-contrast agent (Omniscan™ or MultiHance™, 0.05–0.1 mmol/ kg, depending on GFR, injected at 4 ml/s, IV) 1 min after regadenoson administration (Lexiscan 0.4 mg IV bolus, Astellas Pharma, Deerfield, IL) and then again during recovery, 15 min after the administration of aminophylline (75 mg IV). Late gadolinium enhancement (LGE) images of the same cine-CMR short- and long-axis views were obtained 5 min after the second infusion of contrast, using a T1-weighted gradient echo pulse sequence with a phase sensitive inversion recovery reconstruction (TR 4.5 ms, TE 2.2 ms, TI 250–300 ms, flip angle 30°, flip angle 5°, voxel size 2 × 2 × 10 mm, SENSE factor 2, no gap). A Look-Locker sequence was used to select an inversion time between 200 and 250 ms for optimal nulling of normal myocardium. Heart rate (HR) was monitored continuously, and blood pressure (BP) was recorded at rest, hyperemia, and during recovery.
vCMR image analysis
Cine perfusion and LGE vCMR images were analyzed using commercial software (Medis, Leiden, Netherlands). Short-axis slices were used to measure left and right ventricular end-diastolic (first phase of the R-wave triggered acquisition) and end-systolic (the phase with the smallest ventricular cavity area in the majority of slices) volumes, LV mass, and left and right ventricular ejection fraction using the Simpson method of disks [22]. All volumes and masses were indexed for body surface area. Detection of regional wall motion abnormalities was primarily based on the short-axis view and confirmed in orthogonal long-axis views. The burden of LGE was quantified using commercially available software (Medis, Leiden, Netherlands) using the full-width, half-maximum (FWHM) technique and was expressed as a fraction (%) of LV mass [23].
Time-intensity curves generated from stress and recovery perfusion images were used to determine myocardial perfusion reserve (MPR), which was calculated as the upslope ratio of stress to recovery (Fig. 1). Myocardial perfusion reserve index (MPRi) was determined by normalizing MPR to the LV cavity upslope to account for differences in contrast volume and cardiac output during different imaging conditions [24]. Due to denervation of the transplanted heart and the potential for differences in HR and BP between the post-OHT patients and controls after administration of regadenoson, MPRi was also normalized to the rate-pressure-product measured during stress [25]. The equation used to determine MPRi was:
Fig. 1.
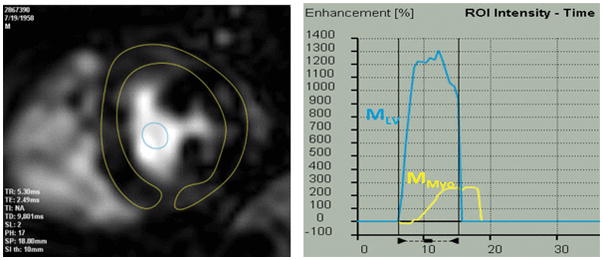
Vasodilator stress CMR image obtained in a post-OHT patient with regions of interest drawn in the myocardium and the left ventricular cavity (left) used to generate regional time-intensity curves (right), from which upslopes were derived for MPR calculation (see text for details)
MPRi was determined for the post-OHT patients and the healthy controls by tracing an entire myocardium in the mid-left ventricular myocardial slice (global perfusion). For the MI controls, MPRi was assessed in a segment remote to any ischemic or infarcted territory, based on LGE and invasive coronary angiography.
Echocardiography
Comprehensive two-dimensional TTE was performed using an iE33 imaging system (Phillips Healthcare, Andover, MA) with either an S5 or X5 transducer. Frame rate was maximized by increasing the depth and decreasing the sector width. The focal point was optimized for the midventricular level of the apical views. Left ventricular ejection fraction (LVEF) was quantified using the biplane method of discs (modified Simpson’s rule) in the apical two- and four-chamber views [26]. The TTE dataset closest to the vCMR was analyzed. Strain analysis was performed using QLAB 10 (Phillips Healthcare, Andover, MA). Endocardial contours were manually corrected to optimize tracking throughout the cardiac cycle, and segments with inadequate tissue tracking were excluded from analysis. Longitudinal strain was measured in apical two-, three-, and four-chamber views and then averaged to obtain GLS.
Invasive coronary angiography
As part of routine surveillance for CAV, all patients underwent regularly scheduled invasive coronary angiograms (time from coronary angiogram to vCMR ≤ 6 months in 30% of patients). Using either a radial or femoral arterial approach, standard catheters were used to access the left and right coronary systems. Injection of contrast during cine fluoroscopy was performed in multiple orthogonal views and recorded at a frame rate of either 15 or 30 frames per second. The coronary angiogram most proximal in time to the vCMR study was reviewed by an experienced interventional cardiologist (JEB) blinded to all other clinical variables. CAV was graded according to standardized International Society of Heart and Lung Transplantation (ISHLT) criteria [27] as none (CAV 0), mild (CAV 1), moderate (CAV 2), or severe (CAV 3).
TIMI frame count analysis was also performed by an experienced interventional cardiologist (JEB) who was blinded to all other study variables, using methodology outlined previously [28]. The number of cine frames required for contrast to reach the distal bifurcation of the left anterior descending artery (LAD) was determined using Centricity Cardio Enterprise (GE Healthcare, Milwaukee, WI). For cineangiograms recorded at 15 frames/s, the calculated TIMI frame count was multiplied by 2.
Endomyocardial biopsy and rejection analysis
Surveillance endomyocardial biopsies were routinely performed in post-OHT patients. Using fluoroscopic guidance, a bioptome was advanced into the right ventricle where 3–4 biopsy specimens of the septum were obtained for pathologic analysis to assess cellular or humoral rejection. Cellular rejection was reported per the 1990 standardized grading method set forth by the ISHLT [29]. The severity of rejection was classified as none (Grade 0), mild (Grades 1A or 1B), moderate (Grades 2 or 3A), or severe (Grades 3B or 4). The absolute number of cellular rejection episodes was determined by counting all rejection episodes noted on endomyocardial biopsy. Additionally, humoral rejection was assessed on each endomyocardial biopsy by the presence or absence of C4d staining.
Statistical analysis
Categorical variables were expressed as percentages and continuous variables as mean ± SD or medians with first and third quartiles. One-way ANOVA analysis was used to test the differences in vCMR characteristics while Student’s unpaired t test was used to test the significance of the difference between the individual groups when necessary. The differences in MPRi between post-OHT, normal controls, and MI controls were tested using a Mann Whitney test. The median, first, and third quartile were used to create Whisker plots of MPRi. Analysis of rejection, CAV, and allograft function was carried out using linear regression analysis (Microsoft Excel 2011) and/or a Student’s unpaired t test (STATA 12, College Station, TX, USA). A P-value of < 0.05 was considered statistically significant. Multivariable regression analysis was used to evaluate the relationship between MPRi or GLS and TIMI frame counts adjusted for time from transplant to vCMR.
Results
Patient demographics and CMR characteristics
Demographic data is shown in Table 1. As expected, both the post-OHT group and the MI controls had multiple known risk factors associated with coronary disease, compared to the normal control group. Baseline vCMR characteristics are shown in Table 2. As expected, the OHT group had similar LVEF compared to normal controls, but elevated LV mass index. Similarly, MI controls had elevated LV mass index but also reduced LVEF.
Table 1.
Patient demographics of study population and control groups
| Patient characteristics | OHT | Normal controls | MI controls |
|---|---|---|---|
| Total patients | 20 | 10 | 20 |
| Male | 14 (70%) | 6 (60%) | 9 (45%) |
| Mean age ± SD (years) | 57 ± 12 | 42 ± 13 | 65 ± 11 |
| Race | |||
| Caucasian | 11 (55%) | 10 (100%) | 10 (50%) |
| African American | 9 (45%) | 0 (0%) | 10 (50%) |
| LV systolic dysfunction | 0 (0%) | 0 (0%) | 10 (50%) |
| Hypertension | 18 (90%) | 0 (0%) | 17 (85%) |
| LDL (mg/dl) | 90 ± 36 | 68 ± 10 | 80 ± 38 |
| Diabetes | 10 (50%) | 0 (0%) | 6 (30%) |
| Chronic kidney disease (GFR < 60 ml/min/1.73 m2) | 8 (40%) | 0 (0%) | 7 (35%) |
| Mean GFR | 66 ± 22 | 101 ± 17 | 94 ± 75 |
| Overweight (BMI > 25) | 17 (85%) | 1 (10%) | 14 (70%) |
| Current/former smoker | 9 (45%) | 1 (10%) | 10 (50%) |
OHT orthotopic heart transplant, MI myocardial infarction
Table 2.
Vasodilator cardiovascular magnetic resonance imaging (vCMR) characteristics of study population and control groups
| vCMR characteristics | OHT | Normal controls | MI controls | P-value (3-way comparison) |
|---|---|---|---|---|
| LV ejection fraction (%) | 59.9 ± 6.5# | 63.0 ± 5.3^ | 50.8 ± 15.0#,^ | < 0.01 |
| LV end systolic index (ml/m2) | 26.5 ± 10.4 | 33.4 ± 11.1 | 48.5 ± 34.5 | 0.02 |
| LV end diastolic index (ml/m2) | 62.5 ± 15.8*,# | 90.0 ± 30.0* | 90.6 ± 34.9# | < 0.01 |
| LV mass index (mg/m2) | 58.4 ± 13.3* | 35.4 ± 18.4*,^ | 64.6 ± 21.8^ | < 0.01 |
OHT orthotopic heart transplant, MI myocardial infarction
P < 0.05 for OHT versus normal controls,
P < 0.05 for OHT versus MI controls,
P < 0.05 for normal controls versus MI controls
Hemodynamics response and myocardial perfusion reserve
During hyperemia and recovery, there were no major differences among the study groups in the hemodynamic response (Fig. 2). The BP at baseline of the post-OHT, normal, and MI groups was 133/83 (± 13/9), 122/64 (± 19/12), and 129/75 (± 14/9) mmHg and the baseline HR was 82 (± 9), 64 (± 11), and 71 (± 16) bpm, respectively. Plots of MPRi normalized to the rate-pressure-product for the three groups are shown in Fig. 3. Significant differences were noted between OHT and normal controls and between OHT and MI controls, while the difference between normal and MI controls was not significant.
Fig. 2.
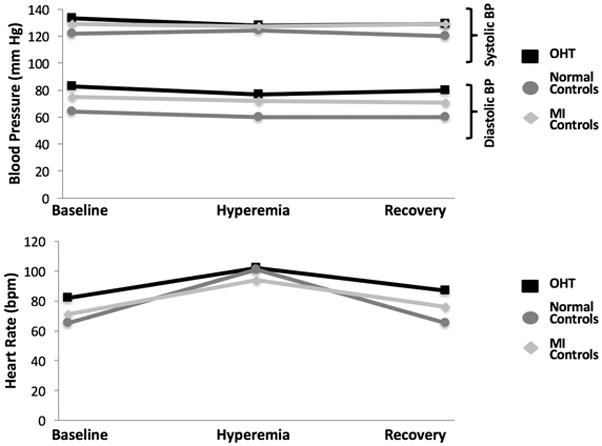
Hemodynamic parameters (blood pressure and heart rate) in response to regadenoson during vCMR at baseline, hyperemia, and recovery
Fig. 3.
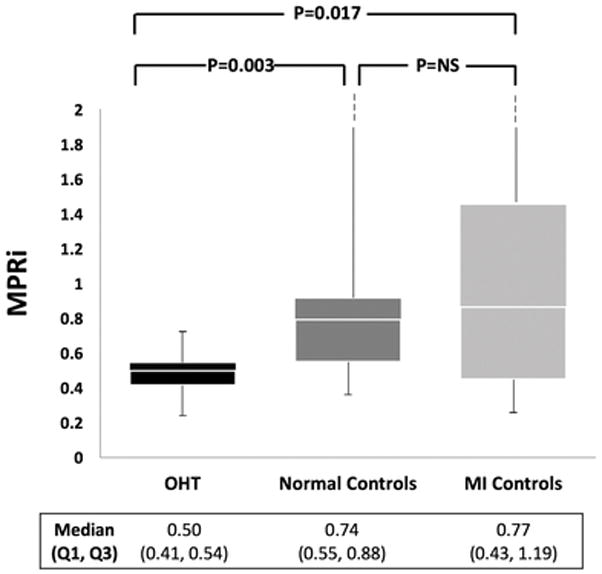
Myocardial perfusion reserve index (MPRi) derived from vCMR of study population and control groups
Late gadolinium enhancement
Five post-OHT patients had qualitative evidence of LGE. In none of them was the pattern of LGE consistent with prior myocardial infarction, i.e. subendocardial or transmural LGE in a coronary territory. The relationships between MPRi and GLS with quantitative burden of LGE were both weak (Fig. 4), without reaching statistical significance, but demonstrated opposite trends: a positive trend with MPRi and a negative trend with GLS.
Fig. 4.
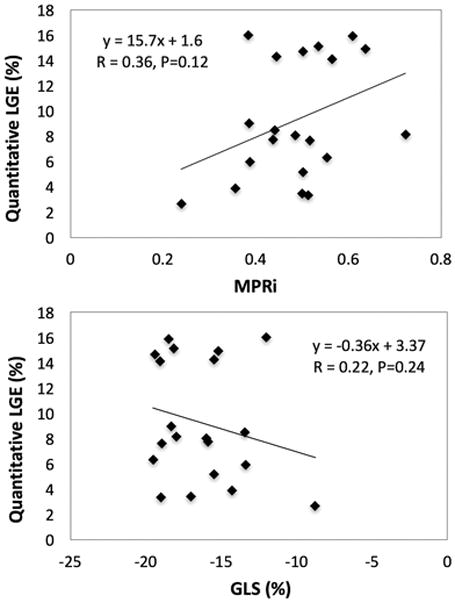
Relationship between quantitative late gadolinium enhancement (LGE) and MPRi or GLS
Endomyocardial biopsy
19 of 20 post-OHT patients experienced at least one episode of cellular rejection and two patients experienced humoral rejection. There were no clear relationships between MPRi or GLS with rejection parameters. Specifically, only weak correlations without statistical significance were noted between MPRi or GLS and the total number of cellular rejection episodes (R = 0.16, P = 0.50; R = 0.32, P = 0.41) as well as between MPRi or GLS and the total combined number of cellular and humoral rejection episodes (R = 0.14, P = 0.55; R = 0.25, P = 0.45). There was no significant difference between the MPRi or GLS in patients with no or any history of mild rejection (Grade 0, 1A, 1B) versus patients with moderate or severe rejection (Grade 2, 3A, 3B, 4) (P = 0.65; P = 0.37). Additionally, there was no difference in the mean MPRi or GLS of patients with any history of Grade 0 or 1A rejection and patients with Grade 1B rejection or higher (P = 0.80; P = 0.78).
Coronary allograft vasculopathy
The mean TIMI frame count for the post-OHT sample was 43 ± 16 frames. There was a moderate correlation between MPRi and TIMI frame count of the LAD (R = −0.68, P = 0.0009) (Fig. 5, top) and between MPRi and time from OHT to vCMR (R = 0.50, P = 0.03). In multivariate analysis controlling for the time from OHT to vCMR, the correlation between MPRi and TIMI frame count persisted (R = 0.70, P = 0.003). MPRi was higher in patients with TIMI frame count of 0–30 and also 31–45, compared to those with ≥ 46 frames (Fig. 5, middle). Additionally, MPRi in patients with CAV 0 or 1 (not significant or mild, N = 17) was higher than in patients with CAV 2 or 3 (moderate or severe, N = 3) (Fig. 5, bottom). There was a good correlation between GLS and TIMI frame count of the LAD (Fig. 6, top) and a significant difference was noted in GLS between CAV grades (Fig. 6, bottom).
Fig. 5.
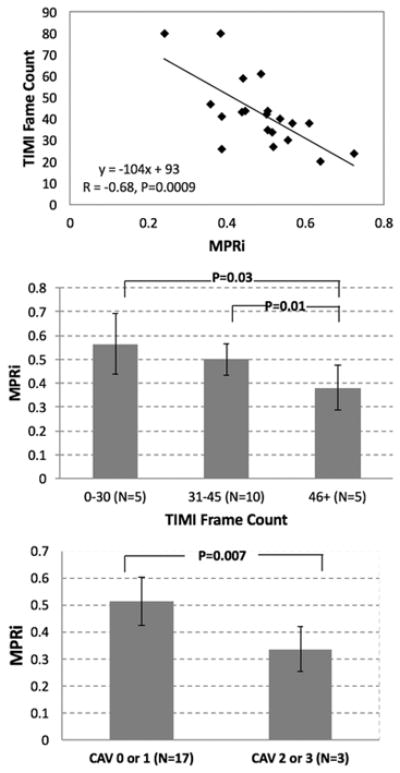
Relationship between MPRi and thrombolysis in myocardial infarction (TIMI) frame count represented continuously (top) and grouped according to TIMI frame count 0–30, 31–45, and 46+ frames (middle). Relationship between MRPi and International Society for Heart & Lung Transplantation (ISHLT) grading of coronary allograft vasculopathy (CAV) (bottom)
Fig. 6.
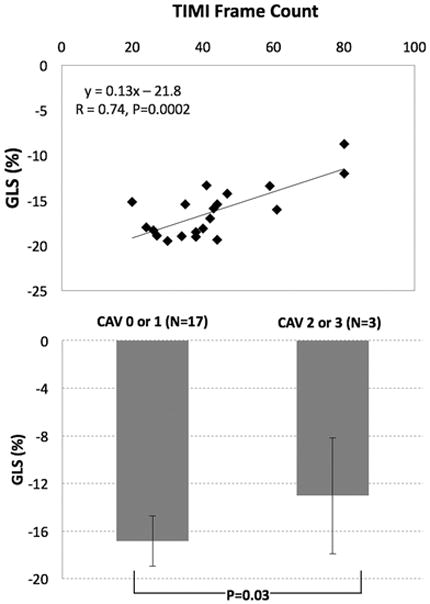
Relationship between global longitudinal strain (GLS) and TIMI frame counts (top) and CAV grades (bottom)
Echocardiography
In the OHT group, mean LVEF measured by TTE and vCMR was 63 ± 6% and 60 ± 6%, and there were no regional LV wall motion abnormalities detected on either imaging modality. Mean GLS was - 16.3 ± 2.9%, which is outside the expected normal range [26]. Of note, a good correlation was observed between MPRi and GLS (Fig. 7).
Fig. 7.
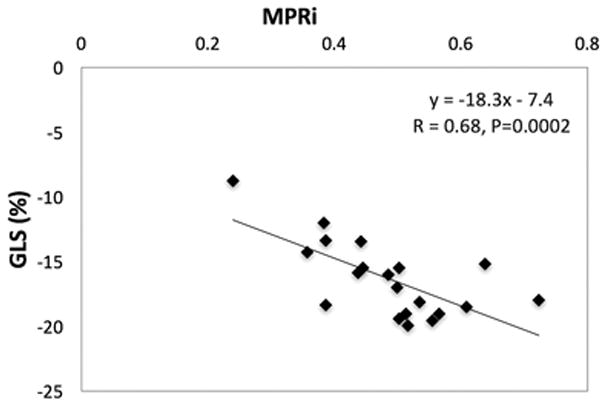
Relationship between MPRi and GLS
Discussion
While vCMR has been shown to accurately diagnose patients with coronary artery disease of the native heart [30–33], less is known about the transplanted heart. Previous small vCMR studies have shown that abnormal MPRi or other vCMR parameters are associated with CAV diagnosed by invasive coronary angiography and/or endomyocardial biopsy [13, 34–38]. However, potential confounding factors have not been systematically investigated. Moreover, GLS is a well-documented and sensitive marker of myocardial deformation [39]. In post-OHT patients, GLS has been previously noted to be a prognostic marker of adverse outcomes and recently has been shown to play a role in patients with CAV compared to other echocardiographic parameters such as LVEF or tissue Doppler parameters of LV function [40, 41]. Similar to vCMR-derived MPRi, little is known about the potential confounders of abnormal GLS in this patient population. There are several potential explanations for the reduced MPRi and GLS magnitude noted in the post-OHT population, including CAV, rejection, myocardial scar/fibrosis, and allograft dysfunction. To our knowledge, this is the first study that comprehensively evaluated these factors that could potentially reduce MPRi or GLS magnitude and thus confound the association between these parameters and CAV. We found that CAV is the likely explanation for reduced MPRi and GLS magnitude, rather than the other aforementioned factors.
While previous studies have examined myocardial perfusion reserve with other non-invasive techniques such as PET, few studies have used vCMR in post-OHT patients. In a PET investigation of post-OHT patients free of epicardial CAD, MPR was impaired early after OHT (< 3 months), but ultimately recovered > 9 months post-transplantation [5]. In another PET study of post-OHT patients without epicardial CAD, resting myocardial perfusion was highest 1 year after transplantation before subsequently declining [6]. Our study shows that MPR is abnormally reduced in the late post-transplant period, compared to normal and post-MI controls.
It is known that nearly 50% of patients 10 years post-OHT have evidence of CAV [42]. CAV is postulated to impact the coronary microcirculation potentially before the development of epicardial disease. In this study, a correlation between surrogate markers of CAV and MPRi and GLS was noted. Using the ISHLT grading criteria, patients with no or mild CAV had a higher MPRi compared to those with more significant CAV. Additionally, we confirmed the results of other smaller studies that showed that elevated TIMI frame counts correlated with microvascular disease in both native and transplanted hearts [3, 43–45]. We also found that worsening in MPRi and GLS was associated with worsening in TIMI frame counts. However, unlike our study, none of the previous studies robustly excluded other potential explanations for abnormal MPRi.
Specifically, in our study, quantitative LGE did not correlate with either MPRi or GLS. Previous studies have yielded mixed results when assessing the correlation between LGE and CAV, with some suggesting no relationship [13, 34], while others describing a potential association [46]. In contrast, unique to our study is the fact that we also explored the relationship between rejection data and MPRi and GLS. While several previous studies have suggested a relationship between CAV and historical rejection episodes, these studies have not measured MPRi, and conflicting data exist regarding this relationship [35, 47–49]. With respect to GLS, several previous studies have noted an association between GLS and rejection [15, 18, 50, 51]. These studies, however, did not systematically examine CAV as a potential explanation for reduced GLS. Since CAV is likely due to a combination of immunological and non-immunological mediated factors, there is likely some downstream effect with multiple episodes of rejection and the development of CAV. Nevertheless, we did not observe any relationship between the total number or severity of rejection episodes and MPRi or GLS.
While abnormal LVEF has prognostic value in post-OHT patients, those with normal LVEF are more difficult to risk stratify. In our patients without clinically evident allograft dysfunction, GLS and MPRi were found to be better correlated with surrogate markers of CAV, and not other potential confounders. Interestingly, we also observed a correlation between GLS and MPRi. This relationship may be explained by the fact that perfusion abnormalities of the subendocardium are more likely to be detected with GLS, which is highly dependent on the longitudinal fibers in the subendocardial layer of the heart. Further studies specifically examining subendocardial MPRi and GLS may show a stronger correlation. Also, additional studies evaluating the role of CMR derived strain either using strain-encoded MRI or feature tracking may add incremental value to myocardial perfusion analysis.
The use of non-invasive imaging has been advocated for the detection of CAV. The diagnosis of CAV can allow for treatment with more aggressive immunosuppression or novel medical therapies to halt, or at least slow down, its progression [52]. Our findings suggest that both vCMR and TTE may be potentially useful as non-invasive tools for the diagnosis of late CAV. Further work to examine patients in the early post-OHT period are needed.
Limitations
There are several limitations to the study. The study is retrospective with a relatively small sample size. Additionally, our sample population is relatively heterogeneous in terms of their time course post-transplant. While we sought to examine patients’ late post-OHT, there was nonetheless a wide range of time. Ideally all imaging modalities would have been performed within a short time window, but due to the retrospective analysis in our study, this was not the case. Also, at the time of the study, T1 mapping was not routinely performed, which has been shown to be useful to detect scar/fibrosis. Furthermore, The LGE contrast agents, while not approved by the Food and Drug Administration for CMR, were widely used in the United States and Europe at the time of this study. Also, while invasive coronary angiography with IVUS is the “gold standard” to diagnose CAV, our lab was not routinely performing IVUS at the time of the study. Therefore, we utilized another marker of microcirculatory dysfunction, namely TIMI frame count, as a surrogate for CAV [3]. Lastly, in our vCMR protocol, imaging was first performed under hyperemic conditions, while resting perfusion evaluation was performed after the reversal of hyperemia with aminophylline. Although this sequence has been shown to underestimate MPR [53], this is unlikely to have impact on our results since this protocol was used in all patients and controls. In our MPR calculations, we used the upslope technique rather than a fully quantitative technique involving deconvolution, which could have been more accurate. However, the fact that we were able to find a relationship despite using the upslope technique only supports the notion that the relationship between MPR and CAV is real and possibly underestimated in our study.
Conclusion
To the best of our knowledge, this is the first multimodality imaging study examining a variety of hypotheses governing reduced MPRi and GLS in a population of patients late after transplantation. We found that MPRi and GLS are both abnormal in > 1-year post-OHT patients, which is likely due to underlying CAV, but is unrelated to rejection episodes, presence of LGE, or reduced LVEF. Assessment of MPRi or GLS may be a useful strategy to detect CAV.
Acknowledgments
The study was funded by a T32 Cardiovascular Sciences Training Grant (5T32HL7381) (AN). The authors received research grants from Philips Healthcare (RML, ARP) and Astellas Pharma (VM and ARP) for other unrelated studies.
Abbreviations
- vCMR
Vasodilator cardiac magnetic resonance
- MPRi
Myocardial perfusion reserve index
- OHT
Orthotopic heart transplant
- CAV
Coronary allograft vasculopathy
- LGE
Late gadolinium enhancement
- GLS
Global longitudinal strain
Footnotes
Compliance with ethical standards
Conflict of interest: There are no conflicts of interest among the authors.
References
- 1.Furiasse N, Kobashigawa JA. Immunosuppression and adult heart transplantation: emerging therapies and opportunities. Expert Rev Cardiovasc Ther. 2017;15:59–69. doi: 10.1080/14779072.2017.1267565. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The registry of the International Society for Heart and Lung transplantation: Twenty-eighth adult heart transplant report-2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Baris N, Sipahi I, Kapadia SR, Nicholls SJ, Erinc K, Gulel O, Crowe TD, Hobbs R, Yamani MH, Taylor DO, Smedira N, Starling RC, Nissen SE, Tuzcu EM. Coronary angiography for follow-up of heart transplant recipients: insights from TIMI frame count and TIMI myocardial perfusion grade. J Heart Lung Transplant. 2007;26:593–597. doi: 10.1016/j.healun.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Sun ZH, Rashmizal H, Xu L. Molecular imaging of plaques in coronary arteries with PET and SPECT. J Geriatr Cardiol. 2014;11:259–273. doi: 10.11909/j.issn.1671-5411.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preumont N, Berkenboom G, Vachiery J, Jansens J, Antoine M, Wikler D, Damhaut P, Degré S, Lenaers A, Goldman S. Early alterations of myocardial blood flow reserve in heart transplant recipients with angiographically normal coronary arteries. J Heart Lung Transplant. 2000;19:538–545. doi: 10.1016/s1053-2498(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 6.Kushwaha SS, Narula J, Narula N, Zervos G, Semigran MJ, Fischman AJ, Alpert NA, Dec GW, Gewirtz H. Pattern of changes over time in myocardial blood flow and microvascular dilator capacity in patients with normally functioning cardiac allografts. Am J Cardiol. 1998;82:1377–1381. doi: 10.1016/s0002-9149(98)00645-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhao XM, Delbeke D, Sandler MP, Yeoh TK, Votaw JR, Frist WH. Nitrogen-13-ammonia and PET to detect allograft coronary artery disease after heart transplantation: comparison with coronary angiography. J Nucl Med. 1995;36:982–987. [PubMed] [Google Scholar]
- 8.Senneff MJ, Hartman J, Sobel BE, Geltman EM, Bergmann SR. Persistence of coronary vasodilator responsivity after cardiac transplantation. Am J Cardiol. 1993;71:333–338. doi: 10.1016/0002-9149(93)90801-i. [DOI] [PubMed] [Google Scholar]
- 9.Rechavia E, Araujo LI, De Silva R, Kushwaha SS, Lammertsma AA, Jones T, Mitchell A, Maseri A, Yacoub MH. Dipyridamole vasodilator response after human orthotopic heart transplantation: quantification by oxygen-15-labeled water and positron emission tomography. J Am Coll Cardiol. 1992;19:100–106. doi: 10.1016/0735-1097(92)90058-u. [DOI] [PubMed] [Google Scholar]
- 10.Preumont N, Lenaers A, Goldman S, Vachiery JL, Wikler D, Damhaut P, Degré S, Berkenboom G. Coronary vasomotility and myocardial blood flow early after heart transplantation. Am J Cardiol. 1996;78:550–554. doi: 10.1016/s0002-9149(96)00363-3. [DOI] [PubMed] [Google Scholar]
- 11.McGinn AL, Wilson RF, Olivari MT, Homans DC, White CW. Coronary vasodilator reserve after human orthotopic cardiac transplantation. Circulation. 1988;78:1200–1209. doi: 10.1161/01.cir.78.5.1200. [DOI] [PubMed] [Google Scholar]
- 12.Krivokapich J, Stevenson LW, Kobashigawa J, Huang SC, Schelbert HR. Quantification of absolute myocardial perfusion at rest and during exercise with positron emission tomography after human cardiac transplantation. J Am Coll Cardiol. 1991;18:512–517. doi: 10.1016/0735-1097(91)90608-c. [DOI] [PubMed] [Google Scholar]
- 13.Miller CA, Sarma J, Naish JH, Yonan N, Williams SG, Shaw SM, Clark D, Pearce K, Stout M, Potluri R, Borg A, Coutts G, Chowdhary S, McCann GP, Parker GJ, Ray SG, Schmitt M. Multiparametric cardiovascular magnetic resonance assessment of cardiac allograft vasculopathy. J Am Coll Cardiol. 2014;63:799–808. doi: 10.1016/j.jacc.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 14.Grupper A, Gewirtz H, Kushwaha S. Reinnervation post-Heart transplantation. Eur Heart J. 2017 doi: 10.1093/eurheartj/ehw604. [DOI] [PubMed] [Google Scholar]
- 15.Clemmensen TS, Løgstrup BB, Eiskjær H, Poulsen SH. Evaluation of longitudinal myocardial deformation by 2-dimensional speckle-tracking echocardiography in heart transplant recipients: relation to coronary allograft vasculopathy. J Heart Lung Transplant. 2015;34:195–203. doi: 10.1016/j.healun.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Clemmensen TS, Eiskjær H, Løgstrup BB, Tolbod LP, Harms HJ, Bouchelouche K, Hoff C, Frøkiær J, Poulsen SH. Noninvasive detection of cardiac allograft vasculopathy by stress exercise echocardiographic assessment of myocardial deformation. J Am Soc Echocardiogr. 2016;29:480–490. doi: 10.1016/j.echo.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Clemmensen TS, Løgstrup BB, Eiskjaer H, Poulsen SH. Coronary flow reserve predicts longitudinal myocardial deformation capacity in heart-transplanted patients. Echocardiography. 2016;33:562–571. doi: 10.1111/echo.13123. [DOI] [PubMed] [Google Scholar]
- 18.Clemmensen TS, Løgstrup BB, Eiskjaer H, Høyer S, Poulsen SH. The long-term influence of repetitive cellular cardiac rejections on left ventricular longitudinal myocardial deformation in heart transplant recipients. Transpl Int. 2015;28:475–484. doi: 10.1111/tri.12520. [DOI] [PubMed] [Google Scholar]
- 19.Wöhrle J, Nusser T, Merkle N, Kestler HA, Grebe OC, Marx N, Höher M, Kochs M, Hombach V. Myocardial perfusion reserve in cardiovascular magnetic resonance: correlation to coronary microvascular dysfunction. J Cardiovasc Magn Reson. 2006;8:781–787. doi: 10.1080/10976640600737649. [DOI] [PubMed] [Google Scholar]
- 20.Patel AR, Epstein FH, Kramer CM. Evaluation of the microcirculation: advances in cardiac magnetic resonance perfusion imaging. J Nucl Cardiol. 2008;15:698–708. doi: 10.1016/j.nuclcard.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narang A, Mor-Avi V, Bhave NM, Tarroni G, Corsi C, Davidson MH, Lang RM, Patel AR. Large high-density lipoprotein particle number is independently associated with microvascular function in patients with well-controlled low-density lipoprotein concentration: a vasodilator stress magnetic resonance perfusion study. J Clin Lipidol. 2016;10:314–322. doi: 10.1016/j.jacl.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 23.Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Yu Z, Addetia K, Mor-Avi V, Moss JD, Hogarth DK, Sweiss NJ, Lang RM, Patel AR. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016;9:e003738. doi: 10.1161/CIRCIMAGING.115.003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerosch-Herold M, Seethamraju RT, Swingen CM, Wilke NM, Stillman AE. Analysis of myocardial perfusion MRI. J Magn Reson Imaging. 2004;19:758–770. doi: 10.1002/jmri.20065. [DOI] [PubMed] [Google Scholar]
- 25.Czernin J, Müller P, Chan S, Brunken RC, Porenta G, Krivokapich J, Chen K, Chan A, Phelps ME, Schelbert HR. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62–69. doi: 10.1161/01.cir.88.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Gibson CM, Cannon CP, Daley WL, Dodge JT, Alexander B, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 29.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 30.Lockie T, Ishida M, Perera D, Chiribiri A, De Silva K, Kozerke S, Marber M, Nagel E, Rezavi R, Redwood S, Plein S. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. J Am Coll Cardiol. 2011;57:70–75. doi: 10.1016/j.jacc.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Zhou T, Yang LF, Peng ZH, Ding J, Sun G. Diagnostic accuracy of myocardial magnetic resonance perfusion to diagnose ischemic stenosis with fractional flow reserve as reference: systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:1098–1105. doi: 10.1016/j.jcmg.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E, Hoffmann U, Leiner T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8(1):e002666. doi: 10.1161/CIRCIMAGING.114.002666. [DOI] [PubMed] [Google Scholar]
- 33.Pontone G, Andreini D, Bertella E, Loguercio M, Guglielmo M, Baggiano A, Aquaro GD, Mushtaq S, Salerni S, Gripari P, Rossi C, Segurini C, Conte E, Beltrama V, Giovannardi M, Veglia F, Guaricci AI, Bartorelli AL, Agostoni P, Pepi M, Masci PG. Prognostic value of dipyridamole stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a mid-term follow-up study. Eur Radiol. 2015;26(7):2155–2165. doi: 10.1007/s00330-015-4064-x. [DOI] [PubMed] [Google Scholar]
- 34.Chih S, Ross HJ, Alba AC, Fan CS, Manlhiot C, Crean AM. Perfusion cardiac magnetic resonance imaging as a rule-out test for cardiac allograft vasculopathy. Am J Transplant. 2016;16(10):3007–3015. doi: 10.1111/ajt.13839. [DOI] [PubMed] [Google Scholar]
- 35.Erbel C, Mukhammadaminova N, Gleissner CA, Osman NF, Hofmann NP, Steuer C, Akhavanpoor M, Wangler S, Celik S, Doesch AO, Voss A, Buss SJ, Schnabel PA, Katus HA, Korosoglou G. Myocardial perfusion reserve and strain-encoded cmr for evaluation of cardiac allograft microvasculopathy. JACC Cardiovasc Imaging. 2016;9:255–266. doi: 10.1016/j.jcmg.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Muehling OM, Wilke NM, Panse P, Jerosch-Herold M, Wilson BV, Wilson RF, Miller LW. Reduced myocardial perfusion reserve and transmural perfusion gradient in heart transplant arteriopathy assessed by magnetic resonance imaging. J Am Coll Cardiol. 2003;42:1054–1060. doi: 10.1016/s0735-1097(03)00924-0. [DOI] [PubMed] [Google Scholar]
- 37.Korosoglou G, Osman NF, Dengler TJ, Riedle N, Steen H, Lehrke S, Giannitsis E, Katus HA. Strain-encoded cardiac magnetic resonance for the evaluation of chronic allograft vasculopathy in transplant recipients. Am J Transplant. 2009;9:2587–2596. doi: 10.1111/j.1600-6143.2009.02769.x. [DOI] [PubMed] [Google Scholar]
- 38.Mirelis JG, García-Pavía P, Cavero MA, González-López E, Echavarria-Pinto M, Pastrana M, Segovia J, Oteo JF, Alonso-Pulpón L, Escaned J. Magnetic resonance for noninvasive detection of microcirculatory disease associated with allograft vasculopathy: intracoronary measurement validation. Rev Esp Cardiol. 2015;68:571–578. doi: 10.1016/j.rec.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 39.Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol. 2017;69:1043–1056. doi: 10.1016/j.jacc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Sarvari SI, Gjesdal O, Gude E, Arora S, Andreassen AK, Gullestad L, Geiran O, Edvardsen T. Early postoperative left ventricular function by echocardiographic strain is a predictor of 1-year mortality in heart transplant recipients. J Am Soc Echocardiogr. 2012;25:1007–1014. doi: 10.1016/j.echo.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Clemmensen TS, Eiskjær H, Løgstrup BB, Ilkjær LB, Poulsen SH. Left ventricular global longitudinal strain predicts major adverse cardiac events and all-cause mortality in heart transplant patients. J Heart Lung Transplant. 2017;36:567–576. doi: 10.1016/j.healun.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, Levvey BJ, Meiser B, Rossano JW, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant. 2015;34:1244–1254. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Kunadian V, Harrigan C, Zorkun C, Palmer AM, Ogando KJ, Biller LH, Lord EE, Williams SP, Lew ME, Ciaglo LN, Buros JL, Marble SJ, Gibson WJ, Gibson CM. Use of the TIMI frame count in the assessment of coronary artery blood flow and microvascular function over the past 15 years. J Thromb Thrombolysis. 2009;27:316–328. doi: 10.1007/s11239-008-0220-3. [DOI] [PubMed] [Google Scholar]
- 44.Sun H, Fukumoto Y, Ito A, Shimokawa H, Sunagawa K. Coronary microvascular dysfunction in patients with microvascular angina: analysis by TIMI frame count. J Cardiovasc Pharmacol. 2005;46:622–626. doi: 10.1097/01.fjc.0000181291.96086.ae. [DOI] [PubMed] [Google Scholar]
- 45.Petersen JW, Johnson BD, Kip KE, Anderson RD, Handberg EM, Sharaf B, Mehta PK, Kelsey SF, Merz CN, Pepine CJ. TIMI frame count and adverse events in women with no obstructive coronary disease: a pilot study from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) PLoS ONE. 2014;9:e96630. doi: 10.1371/journal.pone.0096630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braggion-Santos MF, Lossnitzer D, Buss S, Lehrke S, Doesch A, Giannitsis E, Korosoglou G, Katus HA, Steen H. Late gadolinium enhancement assessed by cardiac magnetic resonance imaging in heart transplant recipients with different stages of cardiac allograft vasculopathy. Eur Heart J Cardiovasc Imaging. 2014;15:1125–1132. doi: 10.1093/ehjci/jeu090. [DOI] [PubMed] [Google Scholar]
- 47.Dong L, Maehara A, Nazif TM, Pollack AT, Saito S, Rabbani LE, Apfelbaum MA, Dalton K, Moses JW, Jorde UP, Xu K, Mintz GS, Mancini DM, Weisz G. Optical coherence tomographic evaluation of transplant coronary artery vasculopathy with correlation to cellular rejection. Circ Cardiovasc Interv. 2014;7:199–206. doi: 10.1161/CIRCINTERVENTIONS.113.000949. [DOI] [PubMed] [Google Scholar]
- 48.Zakliczynski M, Nozynski J, Konecka-Mrowka D, Pyka L, Trybunia D, Swierad M, Maruszewski M, Zembala M. Quilty effect correlates with biopsy-proven acute cellular rejection but does not predict transplanted heart coronary artery vasculopathy. J Heart Lung Transplant. 2009;28:255–259. doi: 10.1016/j.healun.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Hammond EH, Yowell RL, Price GD, Menlove RL, Olsen SL, O’Connell JB, Bristow MR, Doty DB, Millar RC, Karwande SV. Vascular rejection and its relationship to allograft coronary artery disease. J Heart Lung Transplant. 1992;11:S111–S119. [PubMed] [Google Scholar]
- 50.Sera F, Kato TS, Farr M, Russo C, Jin Z, Marboe CC, Di Tullio MR, Mancini D, Homma S. Left ventricular longitudinal strain by speckle-tracking echocardiography is associated with treatment-requiring cardiac allograft rejection. J Card Fail. 2014;20:359–364. doi: 10.1016/j.cardfail.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Romano G, Raffa GM, Licata P, Tuzzolino F, Baravoglia CH, Sciacca S, Scardulla C, Pilato M, Lancellotti P, Clemenza F, Bellavia D. Can multiple previous treatment-requiring rejections affect biventricular myocardial function in heart transplant recipients? A two-dimensional speckle-tracking study. Int J Cardiol. 2016;209:54–56. doi: 10.1016/j.ijcard.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 52.Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RS. Allograft vasculopathy: the Achilles’ heel of heart transplantation. J Am Coll Cardiol. 2016;68:80–91. doi: 10.1016/j.jacc.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 53.Bhave NM, Freed BH, Yodwut C, Kolanczyk D, Dill K, Lang RM, Mor-Avi V, Patel AR. Considerations when measuring myocardial perfusion reserve by cardiovascular magnetic resonance using regadenoson. J Cardiovasc Magn Reson. 2012;14:89. doi: 10.1186/1532-429X-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]


