Abstract
Background and Purpose
Concomitant acute ischemic lesions are detected in up to a quarter of patients with spontaneous intracerebral hemorrhage (ICH). Influence of bleeding pattern and intraventricular hemorrhage (IVH) on risk of ischemic lesions has not been investigated.
Methods
Retrospective cohort study of all 500 patients enrolled in the CLEAR III randomized controlled trial of thrombolytic removal of obstructive IVH using external ventricular drainage. The primary outcome measure was radiologically-confirmed ischemic lesions, as reported by the Safety Event Committee and confirmed by two neurologists. We assessed predictors of ischemic lesions including analysis of bleeding patterns (ICH, IVH and subarachnoid hemorrhage) on computed tomography scans (CT). Secondary outcomes were blinded assessment of mortality and modified Rankin scale (mRS) at 30 and 180 days.
Results
Ischemic lesions occurred in 23 (4.6%) during first 30 days after ICH. Independent risk factors associated with ischemic lesions in logistic regression models adjusted for confounders were higher IVH volume (p= 0.004) and persistent subarachnoid hemorrhage on CT scan (p=0.03). Patients with initial IVH volume ≥15mL had five times the odds of concomitant ischemic lesions compared to IVH volume <15mL. Patients with ischemic lesions had significantly higher odds of death at 1 and 6 months (but not poor outcome; mRS 4-6) compared to patients without concurrent ischemic lesions.
Conclusions
Occurrence of ischemic lesions in the acute phase of IVH is not uncommon, and is significantly associated with increased early and late mortality. Extra- parenchymal blood (larger IVH and visible SAH) is a strong predictor for development of concomitant ischemic lesions after ICH.
Keywords: intracerebral hemorrhage, intraventricular hemorrhage, ischemic lesions, concomitant ischemic strokes, CLEAR trial
Intracerebral hemorrhage (ICH) accounts for 10–15 % of all strokes and carries a disproportionately high risk of death or long-term disability.1 Concomitant acute ischemic lesions after ICH have been reported in up to 26.8% of patients with spontaneous ICH and may be under-reported.2–7 Although the exact etiology is unknown, theories proposed to explain ischemic lesions after ICH include aggressive blood pressure reduction, microangiopathy, and enlarged perivascular spaces.8 These hypotheses have yet to be convincingly proven. Regardless of the etiology, emerging data suggest that ICH patients with co-existing ischemic lesions have worse functional outcomes.8
Our current understanding of ischemic lesions in the setting of acute ICH, is based on single-center studies with small sample sizes. The role of vasospasm and cerebral infarction following isolated intraventricular hemorrhage (IVH) has been described;9 however, the influence of subarachnoid hemorrhage and bleeding pattern has not been evaluated in the IVH population. It is also unclear whether administration of intracerebral alteplase for enhanced removal of parenchymal clot and obstructive IVH impacts the incidence of such lesions. The aim of this study was to evaluate the influence of bleeding pattern and other risk factors on incidence of ischemic lesions in patients with spontaneous IVH, and to assess the relationship between ischemic lesions and IVH outcomes, using data from a large prospective randomized clinical trial.
METHODS
Study Design and Population
We performed a retrospective cohort study of patients enrolled in the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR) III 10 trial. The CLEAR III trial was a multicenter, double-blind, randomized study comparing 1mg of intraventricular alteplase (N=249) vs. saline (N=251) every 8 hours via a pragmatically placed external ventricular drain (EVD) for treatment of obstructive hypertensive IVH until third and fourth ventricles were radiographically open, IVH mass effect relieved, 80% of clot removed, or a maximum of 12 doses were received. The trial enrolled patients with supratentorial ICH volume <30 ml, Glasgow Coma Scale (GCS) score >3, age 18 to 80 years with historical modified Rankin score (mRS) of 0-1, and ICH verified by computed tomography (CT) scan performed within 24 hours of symptom onset. A conventional CT angiogram or magnetic resonance imaging/angiography (MRI/MRA) was obtained prior to enrollment to rule out aneurysm, arteriovenous malformation, or other vascular anomaly. Exclusion criteria were: (i) any infratentorial hemorrhage, (ii) coagulopathy, including platelet count <100,000, international normalized ratio outside the normal range, and prothrombin time (PT) or partial thromboplastin time (PTT) outside of normal range after reversal of anticoagulation. We analyzed incidence and etiology of ischemic lesions in all patients enrolled in CLEAR III. We only included ischemic lesions that occurred in the first 30 days after ICH/IVH onset and compared the clinical features of patients with ischemic lesions to those without. This study was approved by the Johns Hopkins Medical Institutions Review Board.
Measurements
Patient demographics and comorbidities were recorded at time of enrollment. The following baseline characteristics were collected: age, gender, stroke comorbidities, medication history particularly antithrombotic medications, and admission severity variables including blood pressure, and GCS and NIHSS scores. In-hospital events reported by the enrolling sites included any infection within the first 7 and 30 days, and intracranial and cerebral perfusion pressures (ICP, CPP) every 4 hours starting after EVD placement and for first 7 days after randomization.
Ischemic Event Monitoring
All ischemic lesions were reported by site investigators using standard Good Clinical Practice definitions (21 Code of Federal Regulations, Part 312, Investigational New Drug Application) and then independently reviewed by the coordinating center and the Medical and Intensive Care Unit Complications Monitors. Ischemic lesions were defined as any instance when cerebral infarction was diagnosed, using either clinical and/or radiologic criteria. Clinical criteria for cerebral infarction were based on the presence of focal and/or global neurologic deficits (sensory, motor, cranial nerve, or speech). Radiologic criteria (CT or MRI) for cerebral infarction were based on the presence of focal and/or global abnormalities in brain images that indicated regions which have lost viability. Intensity was graded as either asymptomatic or symptomatic.
Both imaging modalities, CT and MRI, have been used in prior studies to evaluate ischemia in the acute phase of ICH.11, 12 The Safety Event Committee (SEC) chairperson adjudicated all ischemic lesions.
Imaging analysis
Following diagnostic CT, the study protocol required a pre-randomization stabilization period up to 72 hours, during which patients underwent a CT scan at least 6 hours after diagnostic CT or EVD placement until stabilization of any intracranial bleeding was established. Post-randomization, all patients had daily head CT scans over the period of study agent administration (1-4 days), at 24 and 72 hours after last dose of study treatment and on day 30 (± 7 days). End of study treatment (EOT) was defined as 24 hours after last dose of study agent. MRI/MRA scans were not protocolized, and were performed in 9% (45/500) (MRI) and 7.4% (MRA) of CLEAR III patients, respectively. Two independent neurologists (W.C.Z and L.R.L) blinded to outcomes evaluated all ischemic lesions reported during the first 30 days in both trials and confirmed with neuroimaging. When there were discrepancies in the results, the two authors conferred and consensus was achieved. Ischemic lesion location/vascular territory and proximity to ICH (perihematomal, ipsilateral distant, contralateral), ischemic lesion appearance, and cerebral blood vessel evaluation (if available) were recorded. The association between ischemic lesions and hemorrhage patterns on CT scan was investigated by ICH and IVH volumes and %IVH removal at EOT, by presence of primary IVH (without radiographic evidence of ICH on any pre or post randomization CT), by ICH location (thalamic origin vs. non-thalamic) and by presence of and persistence of subarachnoid hemorrhage which was reported on all CT scans performed. Hematoma volumes were calculated using semi-automated planimetry.
Outcomes
Our primary outcome measure was occurrence of ischemic lesions defined on neuroimaging during the first 30 days after spontaneous IVH. Secondary outcomes were blinded assessments of mortality and poor outcome defined as mRS score of 4-6 at 30 days and 6 months.
Statistical analysis
We used the Mann-Whitney U test for continuous variables because data were not normally distributed. Categorical variables were analyzed using Pearson’s Chi square test (Fisher exact test when appropriate). We performed multivariable logistic regression to assess factors associated with ischemic brain lesions and the relationship between ischemic lesions and ICH outcomes. Covariates for the regression models were chosen based on bivariate logistic regression with a significance of p<0.1. Statistical analyses were performed using Stata (version 14.0, College Station, TX). All analyses were two- tailed, and significance level was determined by p<0.05.
RESULTS
Incidence and risk factors of concomitant ischemic lesions after ICH/IVH
Of 500 patients enrolled, concurrent ischemic lesions were noted in 23 (4.6%) in the first 30 days after ICH. All ischemic lesions were defined radiographically at a median of 10 (interquartile range [IQR] 5, 19) days post hemorrhage onset. Twelve patients with ischemic lesions (52%) were symptomatic.
We found no differences in demographics and comorbidities between patients with and without ischemic lesions (Table 1). Patients with ischemic lesions had significantly higher IVH volumes (38 ml [21, 48] vs. 27 ml [12, 35], p=0.003) but similar ICH volumes (7 ml [1, 18] vs. 8 ml [3, 15] p=0.86) at randomization compared to those without ischemic lesions (Figure 1). Furthermore, median IVH volume at EOT was also significantly higher in the ischemic lesion group compared to the non-ischemic lesion group (p=0.02) (Table 2). ICH location (thalamic vs. non-thalamic) was not associated with ischemic lesions (p=0.52). Visible SAH which had a cortical distribution (Figure 2) was present in 3.8% (19/500) of CLEAR III patients on diagnostic CT and in 27% (135/500) at randomization (day 2-4 after presentation). SAH was present on at least one head CT in 41.8% (209/500) of patients and on all head CT’s in 17% (85/500) of patients within the first 6 days after randomization. The presence of visible SAH at randomization and persistent visible SAH on all head CT scans post randomization performed during the first 7 days, were significantly associated with ischemic lesions vs. their absence (47.8% vs. 26%; p=0.02 and 39.1% vs. 15.9%; p=0.004, respectively). Cerebral vasospasm defined by cerebral vessel imaging was found in 17% of patients with ischemic lesions (4/23). The brain vessel imaging was done as part of the work-up for ischemic lesions. No significant differences were found in stroke risk factors or comorbidities between groups including pre-admission use of anticoagulation or antiplatetelet agents, admission and randomization systolic and diastolic blood pressure, and change in blood pressure during this interval. Study group assignment and in-hospital complications were not significantly different between patients with and without ischemic lesions with exception of site reported infection in the first 30 days which showed a trend to higher frequency in subjects with ischemic lesions compared to those without (69.6% vs. 48.8%; p=0.06).
Table 1.
Demographics, comorbidities and clinical characteristics at baseline in patients with intracerebral and intraventricular hemorrhage.
| Demographic variables | No ischemic lesions (N= 477) | Ischemic lesions (N= 23) | p value |
|---|---|---|---|
| Age in Years, median [IQR] | 59 [51, 67] | 59 [52, 66] | 0.73 |
| Gender: Female, n (%) | 212 (44.4) | 10 (43.5) | 0.93 |
| Race, n (%) | |||
| Caucasian | 12 (52.2) | 240 (50.3) | 0.25 |
| African American | 163 (34.2) | 7 (30.4) | |
| Hispanic/Latino | 52 (10.9) | 1 (4.4) | |
| Other | 22 (4.6) | 3 (13.0) | |
| Baseline variables | |||
| Tobacco Use, n (%) | 127 (26.6) | 5 (21.7) | 0.60 |
| Alcohol use, n (%) | 19 (3.9) | 1 (4.4) | 0.93 |
| Hypertension, n (%) | 445 (93.3) | 20 (86.9) | 0.21 |
| Diabetes Mellitus, n (%) | 51 (10.7) | 3 (13.1) | 0.72 |
| On Antiplatelet at Registration, n (%) | 122 (25.6) | 6 (26.1) | 0.96 |
| Anticoagulated at Registration, n (%) | 47 (9.8) | 2 (8.7) | 0.85 |
| History of Myocardial Infarction, n (%) | 29 (6.1) | 0 (0) | 0.38 |
| Atrial Fibrillation, n (%) | 10 (2.1) | 1 (4.4) | 0.40 |
| Hyperlipidemia, n (%) | 463 (97.1) | 22 (95.6) | 0.51 |
| GCS at screening Total, median [IQR] | 10 [7,14] | 9 [5,14] | 0.28 |
| Index Clot Location, n (%) | |||
| Thalamus | 281 (58.9) | 12 (52.2) | 0.15 |
| Putamen | 15 (3.1) | 2 (8.7) | |
| Caudate | 85 (17.8) | 2 (8.7) | |
| Primary IVH | 42 (8.8) | 3 (13.0) | |
| Stability CT: IVH Volume* (mL), median [IQR] | 27 [12, 35] | 38 [21, 48] | 0.003 |
| Stability CT: presence of SAH, n (%) | 124 (26) | 11(47.8) | 0.02 |
| Stability CT: ICH Volume* (mL), median [IQR] | 8 [3, 15] | 7 [1, 18] | 0.86 |
| Admission SBP, median [IQR] | 194 [167, 220] | 199 [163, 218] | 0.93 |
| Admission DBP, median [IQR] | 108 [90, 124] | 100 [88, 123] | 0.39 |
Abbreviations: IQR: interquartile range; n: number; SBP: systolic blood pressure; DBP: diastolic blood pressure; GCS: Glasgow Coma score; ICH: intracerebral hemorrhage; IVH: intraventricular hemorrhage; SAH: subarachnoid hemorrhage.
Stability volume calculated by head computed tomography (last one prior to enrollment).
Figure 1.
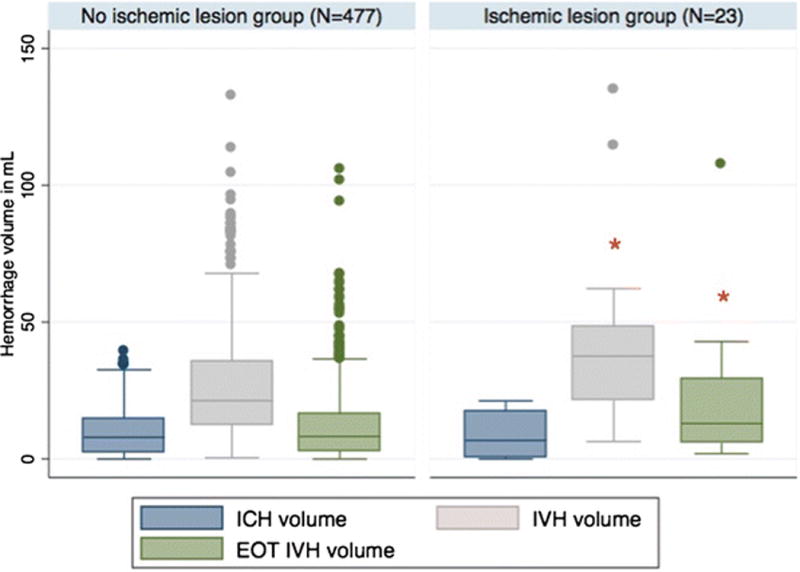
Box plot of intraventricular and intracerebral hemorrhage volume (IVH and ICH, respectively) at randomization and end of treatment (EOT) IVH volume in patients with and without concomitant ischemic lesions.
Table 2.
In-hospital complications and clinical characteristics during the first 30 days of hospital stay.
| Clinical characteristics | No ischemic lesions (N= 23) | Ischemic lesions (N= 477) | p value |
|---|---|---|---|
| Randomization SBP, median [IQR] | 145 [130, 158] | 149 [138, 161] | 0.35 |
| Randomization DBP, median [IQR] | 108 [90, 124] | 100 [88, 123] | 0.40 |
| SBP change*, median [IQR] | 49 [22, 78] | 45 [26,64] | 0.68 |
| DBP change§, median [IQR] | 36 [19, 54] | 28 [21, 52] | 0.51 |
| Randomization NIHSS, median [IQR] | 19 [11, 36] | 19 [13, 36] | 0.57 |
| NIHSS at 7 days, median [IQR] | 17 [8, 27] | 20 [13, 39] | 0.08 |
| NIHSS at 30 days, median [IQR] | 11 [3, 19] | 14 [4, 24] | 0.62 |
| Any infection in the first 7 days, n (%) | 92 (19.3) | 8 (34.8) | 0.10 |
| Any infection in the first 30 days, n (%) | 233 (48.8) | 16 (69.6) | 0.06 |
| Any CPP readings below 70 mm Hg, n (%) | 293 (61.7) | 15 (65.2) | 0.73 |
| Any ICP readings above 20 mm Hg, n (%) | 347 (72.9) | 16 (69.6) | 0.72 |
| Presence of SAH on all head CTs#, n (%) | 76 (15.9) | 9 (39.1) | 0.004 |
| Alteplase administration in CLEAR III, n (%) | 236 (49.4) | 13 (56.5) | 0.50 |
| Number doses of study agent if Alteplase, median [IQR] | 0 [0,5] | 3 [0,5] | 0.64 |
| EOT IVH Volume+ (mL), median [IQR] | 8.3 [3, 16] | 13 [6, 29] | 0.02 |
| Greater than 80% IVH removal at EOT, n (%) | 49 (10.3) | 4 (17.4) | 0.28 |
Abbreviations: CI: confidence interval; CPP: cerebral perfusion pressure; EOT: end of treatment; ICP: intracerebral pressure; IVH: intraventricular hemorrhage’ IQR: interquartile range; N: number; SAH: subarachnoid hemorrhage.
SBP difference from admission to randomization.
DBP difference from admission to randomization.
Presence of SAH in all head CT’s post-randomization during first 7 days
Figure 2.
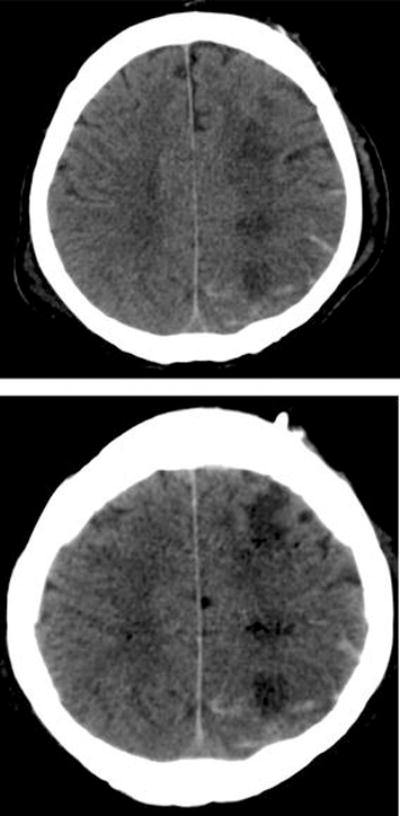
Head computed tomography (CT) of a patient who presented with intracerebral and intraventricular hemorrhage. The ischemic lesion and subarachnoid hemorrhage were not seen on admission CT, but were present on subsequent scans after redistribution of intraventricular hemorrhage into the subarachnoid space.
In the logistic regression model factors associated with higher odds of ischemic lesions after ICH were: IVH volume (Odds Ratio [OR]: 1.02; 95% confidence interval [CI]: 1.01-1.03, p=0.004) and presence of SAH on all post-randomization head CT’s (OR 2.75, 1.1-7.1, p=0.03). The presence of SAH only on the randomization CT did not reach statistical significance (OR 2.25, 0.94-5.4, p=0.07). Patients with an IVH volume ≥15 ml had five times the odds of having a concomitant ischemic lesion (OR 5.05[1.17-21.82]) compared to patients with IVH volumes <15 ml. Patients with IVH volume < 15 ml had no significant increase in the odds of concomitant ischemic lesions (OR 4.31 [0.99-18.66]).
Characteristics of patients with new ischemic lesions after ICH
Patients with ischemic lesions had non-significantly higher NIHSS at day 7 and 30. In nineteen (19/23) subjects, ischemic lesions were identified on head CT and in four (4/23), on brain MRI. All ischemic lesions were distant from the hematoma except two that were within the perihematoma edema region. In nine subjects, ischemic lesions occurred contralateral to side of ICH, in seven, the ischemic lesions were bilateral, in four, ischemic lesions were ipsilateral but distant from the ICH. The three remaining patients had primary IVH.
Fifteen of 29 patients with ischemic lesions (52%) were symptomatic. The most common presumed etiologies of ischemic stroke according to the TOAST classification were cardioembolism (11/29), followed by large vessel atherosclerosis (5/29). Stroke of other determined etiology, such as vasospasm defined by cerebral vessel imaging occurred in 14% (4/29) of patients, and transtentorial herniation due to intracranial hypertension in 5% (2/29) of patients (Figure 3).
Figure 3.
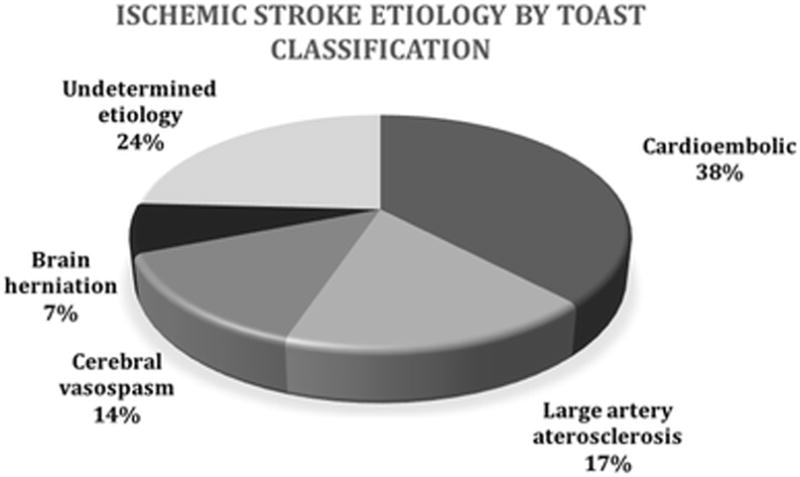
Classification of ischemic strokes by presumed etiology according to TOAST criteria.
Ischemic lesions and ICH outcomes
Patients with ischemic lesions had higher odds for death at 1 month (OR 5.52, 2.04-14.95; p = 0.001) and at 6 months (OR 3.97; 1.65-9.56; p = 0.002) in the logistic regression model adjusted for confounders (Table 3). There was no significant association of ischemic lesion with poor outcome at 1 and 6 months.
Table 3.
Logistic regression analysis showing the relationship between concomitant ischemic lesions and outcomes.*
| Outcome | No ischemic lesion n (%) | Ischemic lesion n (%) | Unadjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value |
|---|---|---|---|---|---|---|
| Mortality at 1 month | 48 (10.06) | 10 (43.48) | 6.87 (2.86-16.52) | <0.001 | 5.52 (2.04- 14.95) | 0.001 |
| Mortality at 6 months | 106 (22.22) | 13 (56.52) | 4.45 (1.94-10.66) | <0.000 | 3.97 (1.65-9.56) | 0.002 |
| Modified Rankin | 382 (80.93) | 21 (95.45) | 4.94 (0.66-37.26) | 0.121 | 3.68 (0.33-41.26) | 0.29 |
| Scale Score 4-6 at 1 month | ||||||
| Modified Rankin Scale Score 4-6 at 6 months | 248 (52.99) | 16 (69.57) | 2.02 (0.82-5.02) | 0.126 | 1.52 (0.53-4.38) | 0.43 |
Each model was adjusted for age, Glasgow Coma scale, stability intracerebral and intraventricular hemorrhage volume, and thalamic ICH location.
Abbreviations: N: number; CI: confidence interval; OR: odds ratio
DISCUSSION
In this retrospective cohort study of patients with large obstructive IVH with or without spontaneous ICH, we report an incidence rate of 4.6% for concomitant ischemic lesions. The presence of ischemic lesions was independently associated with higher odds for early and late mortality. Factors associated with incident ischemic lesions were larger IVH volume (≥15 ml) and persistence of SAH for one week. Furthermore, hemodynamic factors, including blood pressure, prior use of antiplatelet agents or anticoagulants, and intraventricular administration of alteplase were not associated with ischemic lesions.
Prior studies have reported an increased incidence of ischemic lesions following acute ICH. Arsava et al.5 reported an incidence of 17.4% in a retrospective cohort of 86 patients who had MRI scans of the brain performed within 14 days of symptom onset. Similarly, Kang et al.7 reported an incidence of 26.8% when MRI was performed 5 days after ICH onset. The low incidence of ischemic lesions in our study is probably an underestimation since not all patients underwent MRI scans, which were only required by the study protocol when clinically indicated, and nearly half of incident ischemic lesions (48%) were asymptomatic.
SAH was present on 27% of CT scans in CLEAR III at randomization and was significantly more frequent in subjects who developed ischemic lesions especially if persistent during the acute treatment period. Furthermore, 17% of patients with ischemic lesions had vasospasm identified on brain vessel imaging done as part of the lesion work up, suggesting a possible association between occurrence of ischemic lesions and cerebral vasospasm associated with IVH and SAH. Redistribution of blood to the subarachnoid space from the ventricular compartment may cause cerebral vasospasm and delayed cerebral ischemia and has been reported in several case reports with isolated IVH.9, 13–17 Ischemic lesions occurred at a median of 10 days after IVH/ICH in CLEAR III. Despite reliance on CT scans to diagnose these lesions and inherent delays in visualization, the apparent delay in lesion onset also supports a possible hypothesis of blood redistribution to the subarachnoid space over the first few days after hemorrhage onset causing delayed cerebral vasospasm which may have resulted in ischemic lesions in the subacute phase. A single center prospective observational study of 62 patients with spontaneous ICH and IVH reported vasospasm, defined by transcranial Doppler (TCD) ultrasound, in 37% of patients which was significantly associated with delayed cerebral ischemia in a similar time interval to our study.13 It is not known whether radiographically/TCD defined vasospasm behaves similar to that associated with aneurysmal SAH, or whether it requires similar intervention. Ischemic lesion incidence was not different between alteplase and saline treatment groups, and subjects with ischemic lesions had significantly more IVH clot burden on EOT CT. It is not known whether rapid lysis of IVH and removal through an EVD increases or decreases persistence of cortical SAH and potential for ischemic injury.
It should be emphasized that the most frequent etiology of the ischemic lesions in this cohort was cardioembolic, followed by large vessel atherosclerosis. These results differ somewhat from prior studies which have identified small vessel ischemic disease as the most prevalent etiology based on MRI diagnosis and frequent association with white matter changes and cerebral microbleeds (6,7). Vasospasm, defined by cerebral vessel imaging was the third most common etiology; therefore vasospasm surveillance in IVH patients may be warranted, but only after more common etiologies have been considered. Aggressive systolic BP reduction in the acute phase of ICH has previously been reported as a risk factor for ischemic lesions after ICH.5 We did not find a relationship between diagnostic or enrollment BP and ischemic lesions in this study that treated subjects according to American Heart Association blood pressure guidelines or equivalent.18 Our findings corroborate the results of a pooled study of INTERACT 1 and 2, where a policy of initial BP control was not associated with white matter lesions on CT scans.12 The INTERACT cohort did not find an association between ischemic lesions and ICH/IVH volume. Due to trial design, patients in the CLEAR III cohort had significantly greater median IVH volume on admission compared to other ICH trials (21.7 ml [12.6, 36.9] vs. 3 mL on Interact I and II) and smaller ICH volume (7.9 ml [2.4, 15] vs. 11 mL [6, 20] on Interact II).
This study found that occurrence of ischemic lesions impacted mortality, but not functional outcome. These results differ from the literature. Two longitudinal studies investigated influence of DWI lesions on functional outcomes after ICH. Garg et al. reported a 5-fold increase in risk of poor functional outcomes (defined as mRS 4–6) at 3 months adjusting for known predictors (age, initial stroke severity, and ICH score).19, 20 A single-center study evaluating outcomes at 1 year observed >6- fold increased likelihood of poor outcomes in ICH patients with DWI lesions compared to those without, independent of stroke severity.20 In our cohort, just over half (52%) of ischemic lesions were symptomatic and therefore may have impacted the likelihood for a meaningful recovery and possibly affected decision for withdrawal of life support. Of 10 subjects with ischemic lesions who died, 7 had withdrawal of life support. The remaining 13 subjects with ischemic lesions who survived likely constituted too small a number to significantly impact functional outcome at later time points. Moreover, smaller subcortical ischemic lesions appear to be more common than large vessel cortical strokes and therefore the impact on functional outcome may be relatively small and masked by the overwhelming injury from the hemorrhage. Finally, we suspect underreporting of ischemic lesions in this study. Studies with protocolized MRI may show more ischemic lesions and potentially greater impact on functional outcomes. The clinical importance of identifying such lesions is both to identify possible need for additional preventive treatment based on etiology in a high risk population, and to be aware of potential impact on functional outcome which may not be assessed with scales such as the mRS. Moreover, silent infarcts in older patients are known biomarkers for severity of cerebrovascular disease, and predict subsequent risk of stroke and vascular dementia.21, 22
The strengths of our study include a large number of patients enrolled in a large multicenter randomized study with well-defined inclusion criteria, pre-established time points for neuroimaging, safety event monitoring, and blinded assessment of outcome. Our study does have some important limitations predominantly related to the retrospective study design. Although we had pre-specified criteria for definition of ischemic stroke, this study lacked protocolized MR imaging and evaluation for vasospasm. This may have led to an underestimation of ischemic lesions, and the significant association between baseline IVH volume and ischemic lesions may have been stronger, had protocolized MR imaging been performed. CT remains the preferred imaging modality for assessment of acute SAH which may not have been accurately assessed on MRI. Finally, workup for suspected cerebral ischemia was at the discretion of the treating physicians at participating institutions, which limited our ability to determine the lesion etiology in this cohort. As a result, many patients with ischemic lesions did not have brain MRI and/or vessel imaging or TCD at the time of lesion occurrence, which may have influenced the low incidence of cerebral vasospasm reported. However, screening for vasospasm in the setting of ICH/IVH is not standard of care, which may need to be revisited if prospective studies confirm that vasospasm and associated DCI are an important cause of ischemic lesions in this population.
In summary, incident ischemic lesions after ICH with large IVH are not uncommon, and are significantly associated with increased early and late mortality. Mass effect from ICH volume, strict blood pressure control, stroke risk factors, and use of intraventricular alteplase do not appear to be major contributors to acute ischemic lesions in this population. Instead baseline IVH volume (≥15 ml) and presence of SAH were important predisposing factors for such lesions. Further investigation of the role of SAH and recirculation of IVH with potential for vasospasm seems warranted as a potential treatable mechanism of ischemic injury when IVH is significant.
Acknowledgments
Funding/Support: Dr. Murthy is supported by the American Academy of Neurology, American Brain Foundation, and the Leon Levy Neuroscience Foundation. Dr. Hanley was awarded significant research support through grant numbers 5U01NS062851 for Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR) III. Dr. Ziai is supported by grants 5U01NS062851 and 1U01NS08082. The other authors report no disclosures.
Footnotes
Author Contributions:
Drs. Rivera-Lara and Ziai had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rivera-Lara, Hanley, Ziai.
Acquisition, analysis, or interpretation of data: Rivera-Lara, Murthy, Ziai.
Drafting of the manuscript: Rivera-Lara, Ziai.
Critical revision of the manuscript for important intellectual content: Rivera-Lara,
Murthy, Dlugash, McBee, Aldrich, Caron, Awad, Hanley, Ziai.
Statistical analysis: Rivera-Lara, Ziai.
Administrative, technical, or material support: Ziai.
Study supervision: Ziai.
Conflict of Interest Disclosures: The authors report no conflicts of interest.
References
- 1.Andrews CM, Jauch EC, Hemphill JC, 3rd, Smith WS, Weingart SD. Emergency neurological life support: Intracerebral hemorrhage. Neurocrit Care. 2012;17(Suppl 1):S37–46. doi: 10.1007/s12028-012-9757-2. [DOI] [PubMed] [Google Scholar]
- 2.Prabhakaran S, Gupta R, Ouyang B, John S, Temes RE, Mohammad Y, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage: A diffusion-weighted imaging study. Stroke. 2010;41:89–94. doi: 10.1161/STROKEAHA.109.566257. [DOI] [PubMed] [Google Scholar]
- 3.Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon RS, Kidwell CS. Neuroimaging demonstration of evolving small vessel ischemic injury in cerebral amyloid angiopathy. Stroke. 2009;40:e675–677. doi: 10.1161/STROKEAHA.109.552935. [DOI] [PubMed] [Google Scholar]
- 5.Arsava EM, Kayim-Yildiz O, Oguz KK, Akpinar E, Topcuoglu MA. Elevated admission blood pressure and acute ischemic lesions in spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2013;22:250–254. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: Multicentre cross-sectional magnetic resonance imaging study. Brain. 2011;134:2376–2386. doi: 10.1093/brain/awr172. [DOI] [PubMed] [Google Scholar]
- 7.Kang DW, Han MK, Kim HJ, Yun SC, Jeon SB, Bae HJ, et al. New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology. 2012;79:848–855. doi: 10.1212/WNL.0b013e3182648a79. [DOI] [PubMed] [Google Scholar]
- 8.Prabhakaran S, Naidech AM. Ischemic brain injury after intracerebral hemorrhage: A critical review. Stroke. 2012;43:2258–2263. doi: 10.1161/STROKEAHA.112.655910. [DOI] [PubMed] [Google Scholar]
- 9.Gerard E, Frontera JA, Wright CB. Vasospasm and cerebral infarction following isolated intraventricular hemorrhage. Neurocrit Care. 2007;7:257–259. doi: 10.1007/s12028-007-0057-1. [DOI] [PubMed] [Google Scholar]
- 10.Ziai WC, Tuhrim S, Lane K, McBee N, Lees K, Dawson J, et al. A multicenter, randomized, double-blinded, placebo-controlled phase iii study of clot lysis evaluation of accelerated resolution of intraventricular hemorrhage (clear iii) Int J Stroke. 2014;9:536–542. doi: 10.1111/ijs.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidwell CS, Rosand J, Norato G, Dixon S, Worrall BB, James ML, et al. Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology. 2017;88:782–788. doi: 10.1212/WNL.0000000000003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato S, Delcourt C, Heeley E, Arima H, Zhang S, Al-Shahi Salman R, et al. Significance of cerebral small-vessel disease in acute intracerebral hemorrhage. Stroke. 2016;47:701–707. doi: 10.1161/STROKEAHA.115.012147. [DOI] [PubMed] [Google Scholar]
- 13.Regula JU, Schill J, Ringleb PA, Sykora M. Cerebral vasospasm and delayed cerebral ischemia in intraventricular hemorrhage. Neurocrit Care. 2014;20:460–465. doi: 10.1007/s12028-013-9897-z. [DOI] [PubMed] [Google Scholar]
- 14.Dull C, Torbey MT. Cerebral vasospasm associated with intraventricular hemorrhage. Neurocrit Care. 2005;3:150–152. doi: 10.1385/NCC:3:2:150. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Takayama H, Mihara B, Kawase T. Severe vasospasm caused by repeated intraventricular haemorrhage from small arteriovenous malformation. Acta Neurochir (Wien) 2002;144:405–406. doi: 10.1007/s007010200059. [DOI] [PubMed] [Google Scholar]
- 16.Pendharkar AV, Guzman R, Dodd R, Cornfield D, Edwards MS. Successful treatment of severe cerebral vasospasm following hemorrhage of an arteriovenous malformation. Case report. J Neurosurg Pediatr. 2009;4:266–269. doi: 10.3171/2009.4.PEDS09126. [DOI] [PubMed] [Google Scholar]
- 17.Yokobori S, Watanabe A, Nakae R, Onda H, Fuse A, Kushimoto S, et al. Cerebral vasospasms after intraventricular hemorrhage from an arteriovenous malformation: Case report. Neurol Med Chir (Tokyo) 2010;50:320–323. doi: 10.2176/nmc.50.320. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 19.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on mri, and outcomes after intracerebral hemorrhage. Stroke. 2012;43:67–71. doi: 10.1161/STROKEAHA.111.629493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess REMR, Gibbons MC, Fokar A, Shara NM, Forrester L, et al. Presence of dwi lesions is the strongest predictor of poor year 1 outcome in patients with primary intracerebral hemorrhage. Stroke. 2011;42 [Google Scholar]
- 21.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, et al. Silent mri infarcts and the risk of future stroke: The cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 22.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. The New England journal of medicine. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]


