Abstract
Background
The CANTOS trial showed that antagonism of interleukin-1β (IL-1β) reduces coronary heart disease in patients with a previous myocardial infarction and evidence of systemic inflammation, indicating that pathways required for IL-1β secretion increase cardiovascular risk. IL-1β and IL-18 are produced via the NLRP3 inflammasome in myeloid cells in response to cholesterol accumulation, but mechanisms linking NLRP3 inflammasome activation to atherogenesis are unclear. The cholesterol transporters ATP Binding Cassette A1 and G1 (ABCA1/G1) mediate cholesterol efflux to high-density-lipoprotein (HDL) and Abca1/g1 deficiency in myeloid cells leads to cholesterol accumulation.
Methods
To interrogate mechanisms connecting inflammasome activation with atherogenesis, we used mice with myeloid Abca1/g1 deficiency and concomitant deficiency of the inflammasome components Nlrp3 or Caspase-1/11. Bone marrow (BM) from these mice was transplanted into Ldlr−/− recipients, which were fed a Western-type diet (WTD).
Results
Myeloid Abca1/g1 deficiency increased plasma IL-18 levels in Ldlr−/− mice and induced IL-1β and IL-18 secretion in splenocytes, which was reversed by Nlrp3 or Caspase-1/11 deficiency, indicating activation of the NLRP3 inflammasome. Nlrp3 or Caspase-1/11 deficiency decreased atherosclerotic lesion size in myeloid Abca1/g1 deficient Ldlr−/− mice. Myeloid Abca1/g1 deficiency enhanced Caspase-1 cleavage not only in splenic monocytes and macrophages, but also in neutrophils, and dramatically enhanced neutrophil accumulation and neutrophil extracellular trap (NET) formation in atherosclerotic plaques, with reversal by Nlrp3 or Caspase-1/11 deficiency, suggesting that inflammasome activation promotes neutrophil recruitment and NETosis in atherosclerotic plaques. These effects appeared to be indirectly mediated by systemic inflammation leading to activation and accumulation of neutrophils in plaques. Myeloid Abca1/g1 deficiency also activated the non-canonical inflammasome causing increased susceptibility to LPS-induced mortality. Tangier Disease patients, who carry loss-of-function mutations in ABCA1 and have increased myeloid cholesterol content, showed a marked increase in plasma IL-1β and IL-18 levels.
Conclusion
Cholesterol accumulation in myeloid cells activates the NLRP3 inflammasome, which enhances neutrophil accumulation and NETosis in atherosclerotic plaques. Tangier Disease patients, who have increased myeloid cholesterol content, showed markers of inflammasome activation, suggesting human relevance.
Keywords: inflammasome, atherosclerosis, ABC transporter, neutrophil extracellular trap, high-density-lipoprotein
Introduction
Recent studies have implicated a role for the NLRP3 inflammasome in atherogenesis in patients at risk for developing cardiovascular disease (CVD). The CANTOS trial has shown that in patients with a previous myocardial infarction and evidence of systemic inflammation, antagonism of interleukin-1β (IL-1β), one of the major products of the inflammasome, reduces coronary heart disease.1 TET2 mutations that are associated with clonal hematopoiesis and increased CVD risk in humans2 activate the NLRP3 inflammasome in myeloid cells, enhancing IL-1β production,3 which appears to be key to their pro-atherogenic effects.3 Under specific conditions, the NLRP3 inflammasome may thus be activated and contribute to CVD. The products of the NLRP3 inflammasome IL-1β and IL-18 accelerate atherosclerosis in mice,4–8 but studies on the role of the NLRP3 inflammasome in atherogenesis have yielded mixed results, with deficiency of Nlrp3 or other inflammasome components either decreasing atherogenesis4, 9–12 or showing no effect.13, 14 The mechanisms and significance of NLRP3 inflammasome activation in atherosclerosis remain poorly understood.
The cholesterol transporters ATP Binding Cassette A1 and G1 (ABCA1 and ABCG1) mediate cholesterol efflux to apolipoprotein A1 (apoA1) and high-density-lipoprotein (HDL),15 and the capacity of HDL to mediate macrophage cholesterol efflux inversely correlates with CVD risk in humans.16 We recently found that mice with cholesterol accumulation in dendritic cells due to deficiency of ABCA1 and ABCG1 showed NLRP3 inflammasome activation contributing to their auto-immune phenotype,17 but the mechanisms of inflammasome activation were not determined. While myeloid Abca1/g1 deficiency did not induce auto-immunity,17 myeloid Abca1/g1 deficiency had been previously shown to increase atherosclerotic lesion area in mice.15 This led us to investigate the role of the inflammasome in atherogenesis in mice with myeloid Abca1/g1 deficiency.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request.
Animals
All mice were from Jackson Laboratories, unless indicated otherwise. Stock numbers are indicated. LysmCreAbca1fl/flAbcg1fl/fl were generated by crossbreeding Abca1fl/flAbcg1fl/fl (021067) with LysmCre mice (004781). Abca1fl/flAbcg1fl/flNlrp3−/−, LysmCreAbca1fl/flAbcg1fl/flNlrp3−/−, Abca1fl/flAbcg1fl/flCaspase1−/−11−/−, and LysmCreAbca1fl/flAbcg1fl/flCaspase1−/−11−/− mice were generated by crossbreeding of Abca1fl/flAbcg1fl/fl and LysmCreAbca1fl/flAbcg1fl/fl mice with Nlrp3−/− (021302) or Caspase1−/−11−/− mice (016621). Ldlr−/− mice (002207) were bought. Abca1fl/flAbcg1fl/fl mice are referred to as control mice, LysmCreAbca1fl/flAbcg1fl/fl mice as Myl-Abcdko mice, Abca1fl/flAbcg1fl/flNlrp3−/− mice as Nlrp3−/− mice, Abca1fl/flAbcg1fl/flCaspase1−/−11−/− mice as Caspase1−/−11−/− mice, LysmCreAbca1fl/flAbcg1fl/flNlrp3−/− mice as Myl-AbcdkoNlrp3−/− mice, and LysmCreAbca1fl/flAbcg1fl/flCaspase1−/−11−/− mice as Myl-AbcdkoCaspase1−/−11−/− mice. Abca1−/− mice were generated as described.18 All procedures followed were in accordance with the IACUC of Columbia University.
Patients
Tangier Disease patients and their family members (heterozygous ABCA1 mutation carriers) and age-and-gender-matched controls were recruited at the Academic Medical Center, Amsterdam, and at the University of Pennsylvania.19, 20 Subjects gave informed consent. Studies were approved by the respective IRBs. Data transfer was approved by the IRB of Columbia University.
Statistics
All data are presented as means ± SEM. The t-test was used to define differences between 2 datasets. For 3 or more datasets, One-way ANOVA was used with a Bonferroni multiple comparison post-test. For mortality studies, the Log-rank (Mantel-Cox) test was used.
To define differences between: (1) age-and-gender-matched controls, (2) ABCA1 heterozygous mutation carriers, and (3) Tangier Disease patients, mixed effects models with random intercepts were used taking into account data-clustering due to family members. Restricted maximum likelihood estimation and type 3 generalized score statistics were used for testing significance of the factor ‘group’ and data were (log-)transformed to meet model assumptions of normality. Significance levels for pairwise comparisons of estimated marginal means were Bonferroni-corrected to account for multiple testing.
An expanded version of the Methods section can be found in the online Data Supplement.
Results
Myeloid Abca1/g1 deficiency activates the NLRP3 inflammasome
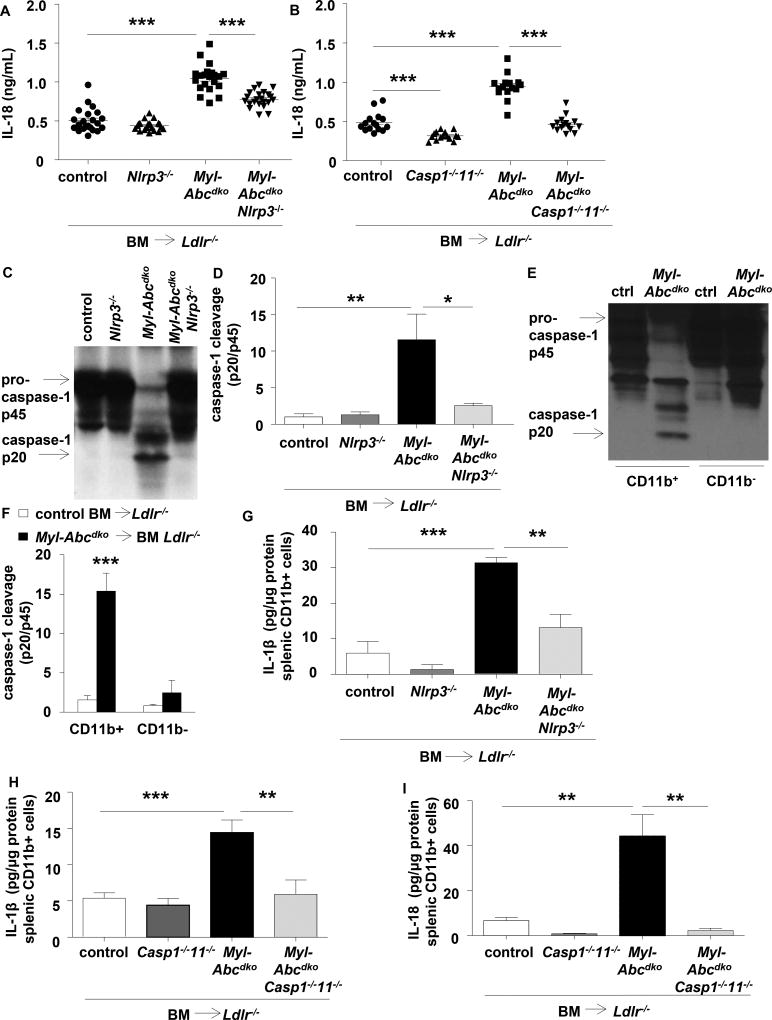
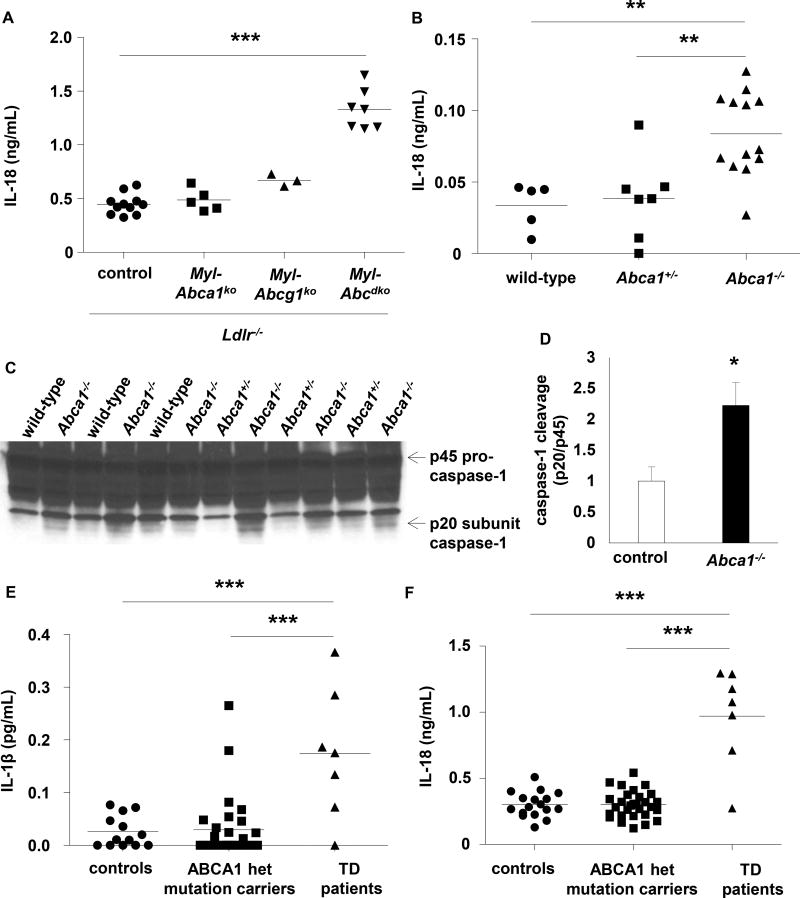
Ldlr−/− mice were transplanted with LysmCreAbca1fl/flAbcg1fl/fl (Myl-Abcdko) or Abca1fl/flAbcg1fl/fl (control) bone marrow (BM). Myeloid Abca1/g1 deficiency modestly increased IL-18 levels on a chow diet, and markedly on the Western type diet (WTD) (Supplementary Figure 1A–B). To assess whether this was inflammasome dependent, we generated Myl-AbcdkoNlrp3−/− and Myl-AbcdkoCaspase1−/−11−/− mice, and transplanted their BM into Ldlr−/− mice. Nlrp3 and Caspase-1/11 deficiency markedly decreased plasma IL-18 levels in WTD-fed Myl-Abcdko BM transplanted (BMT) Ldlr−/− mice (Figure 1A–B). These data were obtained in female mice; male mice showed similar phenotypes (not shown). Myeloid Abca1/g1 deficiency led to Caspase-1 cleavage in splenic homogenates, a hallmark of inflammasome activation, with reversal by Nlrp3 deficiency (Figure 1C–D and Supplementary Figure 1C); specificity was shown by the absence of signal in Caspase1−/−11−/− mice (Supplementary Figure 1D). We observed marked Caspase-1 cleavage in myeloid Abca1/g1 deficient CD11b+ cells (Figure 1E–F), comprising inflammatory macrophages, monocytes and neutrophils (Supplementary Figure 1E). Myeloid Abca1/g1 deficiency also enhanced IL-1β and IL-18 secretion in CD11b+ cells, with reversal by Nlrp3 or Caspase-1/11 deficiency (Figure 1G–I). Thus, several lines of evidence indicate that myeloid Abca1/g1 deficiency activates the NLRP3 inflammasome in vivo.
Figure 1. Myeloid Abca1/g1 deficiency enhances NLRP3 inflammasome activation in Ldlr−/− mice.
Ldlr−/− mice were transplanted with bone marrow (BM) from control, Myl-Abcdko, Nlrp3−/−, Myl-AbcdkoNlrp3−/−, Caspase1−/−11−/−, or Myl-AbcdkoCaspase1−/−11−/− mice and fed Western type diet (WTD) for 4 weeks. Genotypes of BM donors are indicated on the graphs. (A–B) Plasma IL-18 levels (n=12–20; each datapoint represents one mouse). (C–D) Caspase-1 cleavage and quantification in total splenocytes, and (E–F) splenic CD11b+ and CD11b− cells (n=6). (G–I) IL-1β and IL-18 secretion in splenic CD11b+ cells. (n=6). (F). *P<0.05, **P<0.01, ***P<0.001 by t-test, or (A–B, D, G–I) one-way ANOVA with Bonferroni post-test.
NLRP3 inflammasome activation accelerates atherogenesis in myeloid Abca1/g1 deficiency
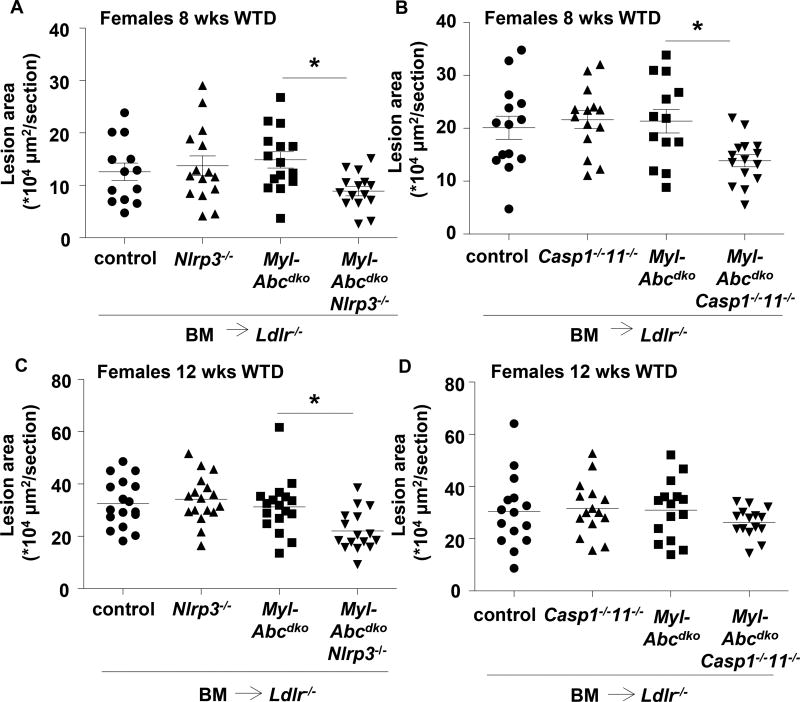
To compare the effect of inflammasome activation on lesion development in Ldlr−/− mice transplanted with control versus Myl-Abcdko BM at comparable stages of lesion development, we fed mice the WTD which resulted in 50% lower levels of plasma cholesterol in Myl-Abcdko BM transplanted Ldlr−/− mice (Supplementary Table 1) and thus similar lesion area in control and Myl-Abcdko BMT Ldlr−/− mice (Figure 2A–B). Surprisingly, there was no effect of Nlrp3 or Caspase-1/11 deficiency on atherosclerosis in control mice. In female Myl-Abcdko BMT Ldlr−/− mice after 8 weeks of WTD, Nlrp3 deficiency and Caspase-1/11 deficiency both decreased atherosclerotic lesion area by ~50% (Figure 2A–B). After 12 weeks of WTD, Nlrp3 deficiency decreased plasma IL-18 and atherosclerotic lesion area in Myl-Abcdko BMT Ldlr−/− mice, whereas Caspase-1/11 deficiency reduced IL-18 but not lesion area (Figure 2C–D and Supplementary Figure 2A–B). Inflammasome activation thus clearly increased atherosclerosis in Myl-Abcdko BMT Ldlr−/− at 8 weeks, and to a lesser extent at 12 weeks.
Figure 2. Inflammasome activation accelerates atherosclerosis in myeloid Abca1/g1 deficiency.
Ldlr−/− mice were transplanted with BM from control, Myl-Abcdko, Nlrp3−/−, Myl-AbcdkoNlrp3−/−, Caspase1−/−11−/−, or Myl-AbcdkoCaspase1−/−11−/− mice, and fed WTD for 8 or 12 weeks, as indicated. (A–D) Sections were stained with haematoxylin-eosin (H&E) and atherosclerotic lesion area was measured at the level of the aortic root (n=13–17 mice per group). In (A–D) each datapoint represents a single mouse. (A–D) *P<0.05 by one-way ANOVA with Bonferroni post-test.
NLRP3 inflammasome activation in myeloid Abca1/g1 deficiency promotes neutrophil accumulation and NETosis in atherosclerotic plaques
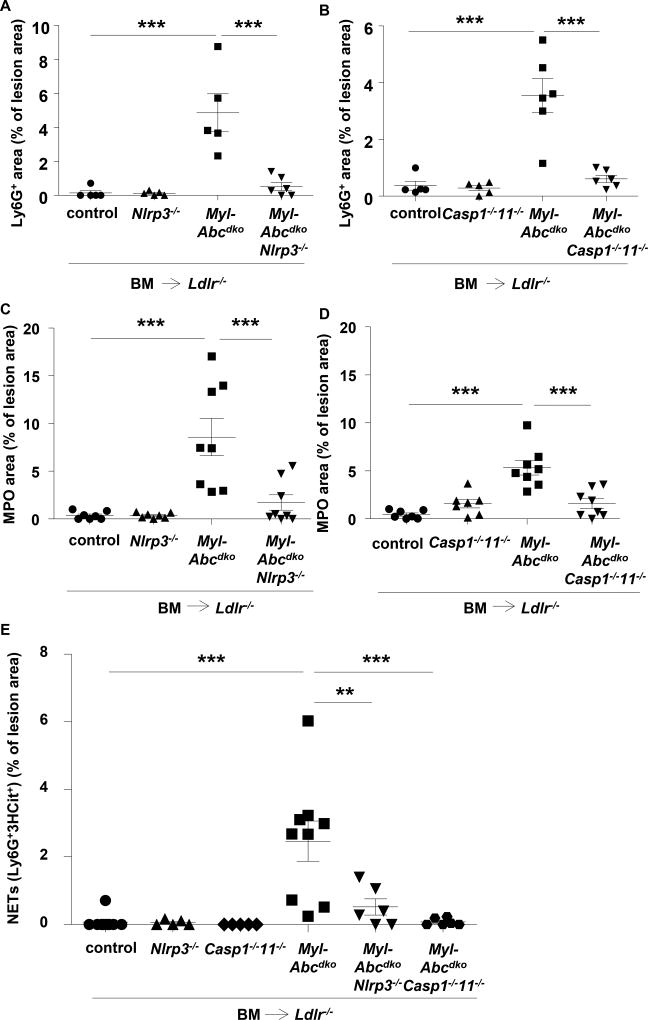
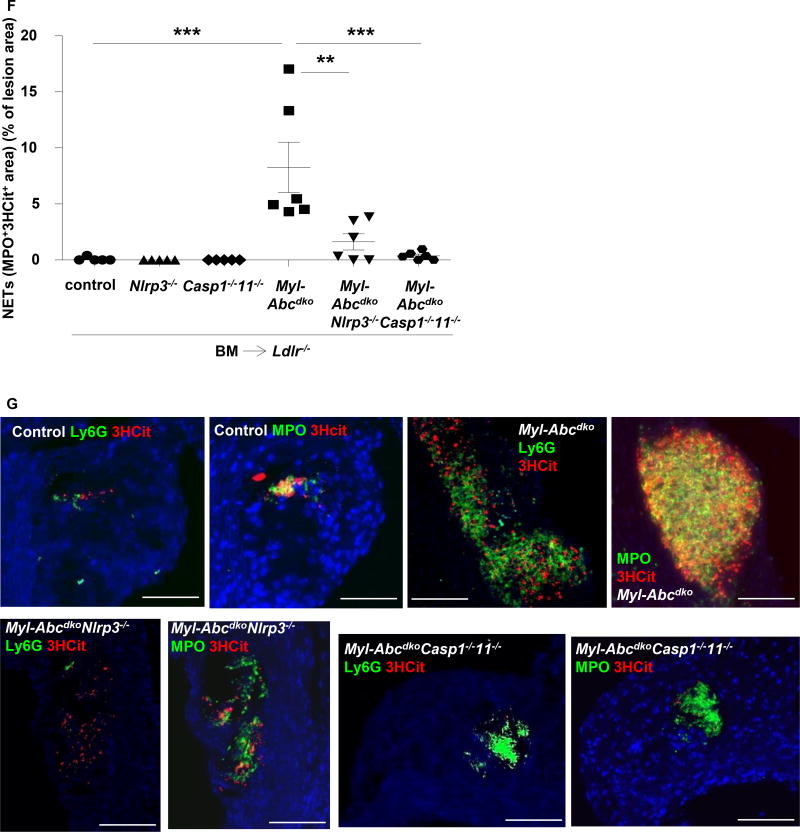
We examined the mechanism underlying the effect of the inflammasome on atherogenesis. Myeloid Abca1/g1 deficiency increased blood monocyte and neutrophil levels by 2-fold (Supplementary Figure 3).15 Nlrp3 deficiency did not reduce blood monocytes but decreased blood neutrophil levels in Myl-Abcdko BMT Ldlr−/− mice (Supplementary Figure 3), potentially due to decreased IL-1β secretion as seen in Myl-AbcdkoNlrp3−/− CD11b+ splenocytes (Figure 1G). To assess neutrophils, we stained atherosclerotic plaques for Ly6G and myeloperoxidase (MPO), which is released upon neutrophil activation.21 Myeloid Abca1/g1 deficiency dramatically increased Ly6G and MPO staining in plaques, indicating neutrophil accumulation and activation in cellular areas as well as necrotic cores. Nlrp3 or Caspase-1/11 deficiency reversed these increases (Figure 3A–D and Supplementary Figure 4). MPO may also be released from macrophages upon activation,21 but in adjacent sections, MPO positive areas corresponded to Ly6G, and not Mac-2 positive areas (Supplementary Figure 4), suggesting neutrophil activation. There was also prominent formation of neutrophil extracellular traps (NETs) as shown by staining for citrullinated histones overlapping with Ly6G or MPO staining and this was also reversed by deficiency of inflammasome components (Figure 3E–G and Supplementary Figure 4). After 12 weeks of WTD, MPO staining in lesions as well as NETosis had decreased (Supplementary Figure 5), consistent with previous findings showing that neutrophils mainly contribute to early lesion development,22, 23 and likely explaining the more pronounced effect of inflammasome activation on lesion area at the earlier timepoint. To assess whether NETosis was due to a cell-intrinsic effect, we isolated neutrophils and studied spontaneous NETosis in cell culture. At 4 hours after isolation, we observed overlap in staining for citrullinated histones with DAPI in Ly6G+ neutrophils (Supplementary Figure 6), suggesting NETosis. However, this was not affected by myeloid Abca1/g1 deficiency or Caspase-1/11 deficiency (Supplementary Figure 6). Stimulation with PMA led to very high levels of NETosis, which were not affected by myeloid Abca1/g1 deficiency (not shown). We also investigated whether plaque CD11b+ cells or Ly6G+ neutrophils showed inflammasome activation. Myeloid Abca1/g1 deficiency did not enhance Caspase-1 or Caspase-11 cleavage in the CD11b+, CD11b−, Ly6G+ or Ly6G− fractions of the aorta (n=3 samples per group with 2 aortas per group pooled) (Supplementary Figure 7A–C), suggesting that myeloid Abca1/g1 deficiency was not activating the inflammasome directly in plaques.
Figure 3. Inflammasome activation promotes neutrophil accumulation and neutrophil extracellular trap formation in atherosclerotic lesions of myeloid Abca1/g1 deficient mice.
Ldlr−/− mice were transplanted with BM from control, Myl-Abcdko, Nlrp3−/−, Myl-AbcdkoNlrp3−/−, Caspase1−/−11−/−, or Myl-AbcdkoCaspase1−/−11−/− mice, and fed WTD for 8 weeks. (A–D) In atherosclerotic lesions of mice, neutrophils were stained using Ly6G (A–B) and activated neutrophils using myeloperoxidase (MPO) (C–D) Ly6G+ and MPO+ percentage of lesion size was quantified (n=5–8 mice per group). (E–F) Lesions were also stained for citrullinated histones 2, 8, and 17 (3HCit). To assess neutrophil extracellular traps (NETs), the overlap of Ly6G and 3HCit (E) or MPO and 3HCit (F) was quantified (n=5–7 mice per group). (G) Representative examples are shown. In (A–F) each datapoint represents one mouse. **P<0.01, ***P<0.001 by one-way ANOVA with Bonferroni post-test. Scale bars represent 100 µm.
The genetic evidence (Figure 3) shows for the first time that lesional neutrophil recruitment and NETosis may be promoted downstream of the inflammasome, likely driving early atherogenesis.24 However, myeloid Abca1/g1 deficiency rather caused NETosis in atherosclerotic plaques (Figure 3E–G and Supplementary Figure 4–5) by enhancing neutrophil recruitment or activation than via cell-intrinsic effects in neutrophils.
Tangier Disease patients with ABCA1 loss-of-function show markers of inflammasome activation
Combined deficiency of Abca1 and Abcg1 represents an extreme model of defective cholesterol efflux. We thus questioned whether deficiency of both cholesterol transporters was required for inflammasome activation. We found that myeloid Abca1 or Abcg1 deficiency alone did not affect plasma IL-18 levels in WTD fed Ldlr−/− mice (Figure 4A). However, whole body Abca1 deficiency did activate the inflammasome (Figure 4B–D), likely because this causes both low plasma HDL, eliminating the acceptor for macrophage ABCG1 mediated cholesterol efflux, and myeloid Abca1 deficiency. Tangier Disease (TD) patients are homozygous for an ABCA1 loss-of-function mutation.25 To assess potential inflammasome activation in TD subjects, we measured plasma IL-1β and IL-18 levels. This showed marked increases compared to gender and age matched controls19, 20 and ABCA1 heterozygotes (Figure 4E–F and Supplementary Table 2), suggesting that cholesterol efflux pathways also suppress inflammasome activation in humans.
Figure 4. Mice with whole body Abca1 deficiency and humans with homozygous loss-of-function mutations for ABCA1 show signs of inflammasome activation.
(A) Ldlr−/− mice were transplanted with BM from control, Myl-Abca1ko, Myl-Abcg1ko, or Myl-Abcdko mice, and fed WTD for 4 weeks. Plasma IL-18 levels were assessed. (B–D) Wild-type, Abca1+/−, and Abca1−/− mice were fed WTD for 4 weeks and plasma IL-18 levels (B) and caspase-1 cleavage in total splenocytes (C–D) were assessed. In (A–B), each datapoint represents one mouse. Controls in (D) represent wild-type and Abca1+/− mice. (E–F) IL-1β (E) and IL-18 (F) levels were measured in plasma of Tangier Disease (TD) patients carrying a homozygous loss-of-function mutation for ABCA1, and gender and age matched heterozygous ABCA1 mutation carriers and controls. Each datapoint represents one patient or control. *P<0.05, **P<0.01, ***P<0.001 by t-test (D) or one-way ANOVA with Bonferroni post-test (A–B) or a mixed effects model with random intercepts taking into account data-clustering due to family members (E–F).
NLRP3 inflammasome activation is cholesterol dependent
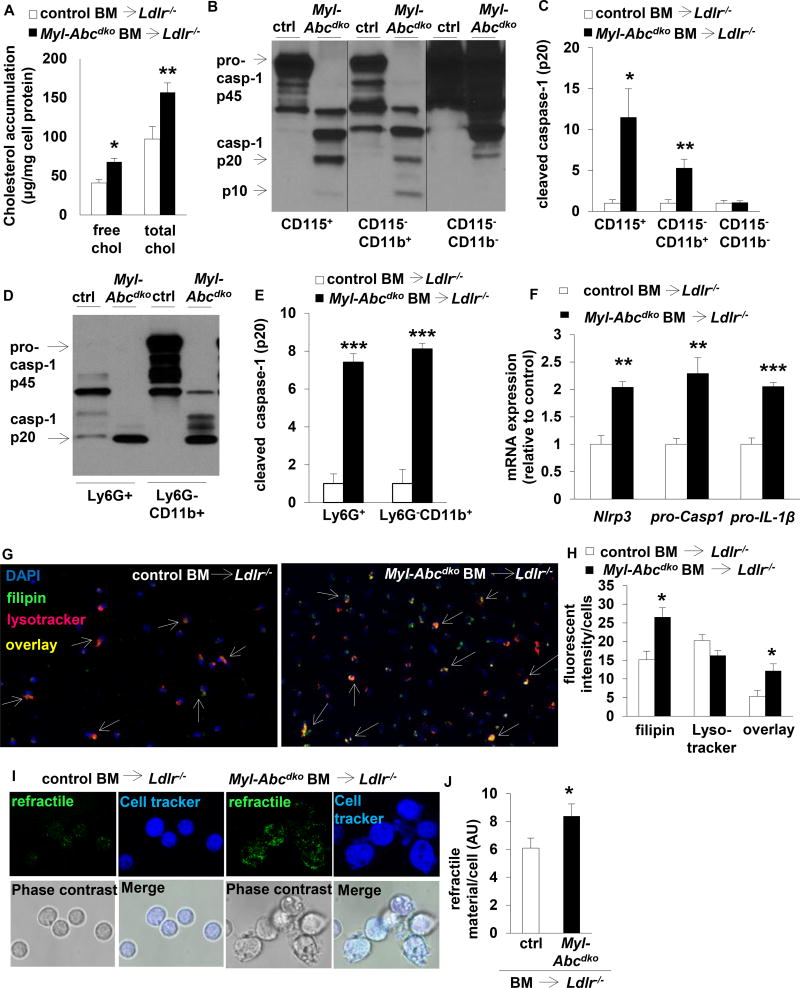
Myeloid Abca1/g1 deficiency increases cholesterol accumulation in macrophages and monocytes on the chow diet, and moreso in Ldlr−/− mice fed WTD.15 Myeloid Abca1/g1 deficiency also enhanced free and esterified cholesterol accumulation in splenic neutrophils (Figure 5A).
Figure 5. Inflammasome activation in myeloid Abca1/g1 deficiency is cholesterol-dependent.
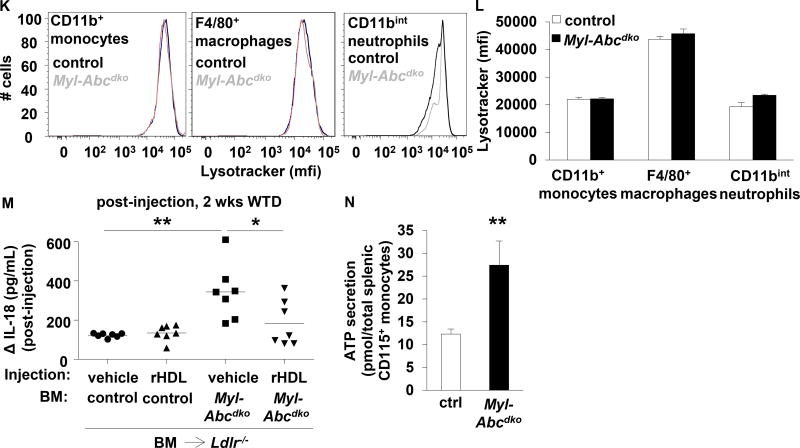
Ldlr−/− mice were transplanted with BM from control or Myl-Abcdko mice, as indicated, and fed WTD for 8 weeks unless indicated otherwise. Genotypes of BM donors are indicated on the graphs. (A) Splenic Ly6G+ cells were isolated, lipids extracted, and free cholesterol and total cholesterol levels were assessed. (B–E) Caspase-1 cleavage and quantification in (B–C) splenic CD115+, CD115−CD11b+, and CD115−CD11b− cells, and (D–E) splenic Ly6G+, and Ly6G−CD11b+ cells. (F) Inflammasome priming in splenic CD11b+ cells. (n=6). (G–H) Splenic CD115+CD11b+ cells were isolated and stained with lysotracker and filipin. Arrowheads indicate overlay between lysotracker and filipin (G). Representative examples (G) and quantification (H) are shown. (I–J) Refractile material was assessed in total splenocytes using confocal microscopy. Representative examples (I) and quantification (J) are shown. (K–L) Lysotracker staining was assessed using flow cytometry in CD11b+ monocytes, F4/80+ macrophages, and CD11bint neutrophils, gated as in Suppl Fig 8A. (M) Mice were fed WTD for 1 week, injected with reconstituted HDL (rHDL; CSL-111; 120 mg/kg) and the increase in plasma IL-18 levels was assessed at 1 week after injection (n=7 mice per group; each datapoint represents one mouse). (N) ATP secretion from total splenic CD115+ monocytes was assessed using luciferase assay. n=6 (A, C, E–F, H, J, L, N), or n=7 (M) mice per group. (A, C, E–F, H, J, L, N) *P<0.05, **P<0.01, ***P<0.001 by t-test, or (M) one-way ANOVA with Bonferroni post-test.
Further analysis of splenocytes showed that myeloid Abca1/g1 deficiency induced Caspase-1 cleavage in monocytes (CD115+ and Ly6G−CD11b+), macrophages (CD115−CD11b+ and Ly6G−CD11b+), and neutrophils (Ly6G+ and CD115−CD11b+) (Figure 5B–E and Supplemental Figure 8A); CD11b+ cells and Ly6G+ neutrophils from myeloid Abca1/g1 deficient BMT Ldlr−/− mice showed increased Nlrp3, pro-Caspase-1, and pro-IL-1β mRNA expression compared to controls, indicative of inflammasome priming (Figure 5F and Supplementary Figure 8B). Myeloid Abca1/g1 deficiency also enhanced Nlrp3 and pro-IL-1β mRNA expression and Caspase-1 and IL-18 cleavage in thioglycollate-elicited macrophages (Supplementary Figure 8C–D). Thus, myeloid Abca1/g1 deficiency enhances inflammasome priming, likely reflecting enhanced Toll like receptor (TLR) activation.26
In addition to the priming signal, inflammasome activation in macrophages requires a second signal, potentially including lysosomal free cholesterol accumulation, cholesterol crystals, mitochondrial ROS, or ATP.4, 27, 28 Myeloid Abca1/g1 deficiency enhanced free cholesterol accumulation in lysosomes of splenic monocytes/macrophages (Figure 5G–H) and free cholesterol accumulation in neutrophils (Figure 5A). To assess the presence of cholesterol crystals, we used confocal reflectance microscopy in splenic cell suspensions while keeping the samples at 37°C to avoid cooling, which may result in artefactual formation of crystalline cholesteryl esters.29 We observed a 37% increase in refractile material in splenic cells of WTD fed Myl-Abcdko BMT Ldlr−/− mice, perhaps suggesting minor cholesterol micro-crystal formation (Figure 5I–J). Free cholesterol accumulation in lysosomes may activate the inflammasome, by increasing lysosomal pH, compromising lysosomal function.30 However, lysotracker and lysosensor stainings were unchanged (Figure 5G–H, K–L, and Supplementary Figure 9A–B) suggesting that the lysosomal pH remained unaffected. We further assessed the contribution of cholesterol accumulation to inflammasome activation by injecting mice with reconstituted HDL (rHDL; CSL-111), which promotes cholesterol efflux from Abca1/g1 deficient macrophages by passive efflux mechanisms.15 rHDL injections reduced the increase in IL-18 plasma levels in WTD fed Myl-Abcdko BMT Ldlr−/− mice by ~50% (Figure 5M), while Myl-Abcdko groups had similar plasma IL-18 levels before injection (Supplementary Figure 9C). Myeloid Abca1/g1 deficiency did not affect levels of mitochondrial ROS in splenic monocytes, macrophages, and neutrophils (Supplementary Figure 9D–E), or the numbers of splenic apoptotic or necrotic cells that release ATP (Supplementary Figure 9F–H). Monocytes secrete ATP and thus require only a priming signal for inflammasome activation.27 Myeloid Abca1/g1 deficiency increased ATP secretion from total splenic monocytes by 2-fold (Figure 5N); however this was simply proportional to a 2-fold increase in the splenic monocyte population of Myl-Abcdko BMT Ldlr−/− mice compared to controls (Supplementary Figure 9A and I). Moreover, myeloid Abca1/g1 deficiency enhanced the secretion of IL-1β and IL-18 in LPS-treated bone marrow-derived macrophages at the same concentration of ATP as controls (Supplementary Figure 9J–K). This result is consistent with increased inflammasome priming in Abca1/g1 deficient macrophages, due to an enhanced LPS-response.31 Thus, our findings suggest a major contribution of cellular cholesterol accumulation to inflammasome activation in Myl-Abcdko BMT Ldlr−/− mice, in the absence of overt cholesterol crystal formation, lysosomal dysfunction, or increased ATP production, implicating a novel cholesterol-sensing mechanism that provides a second signal of inflammasome activation.
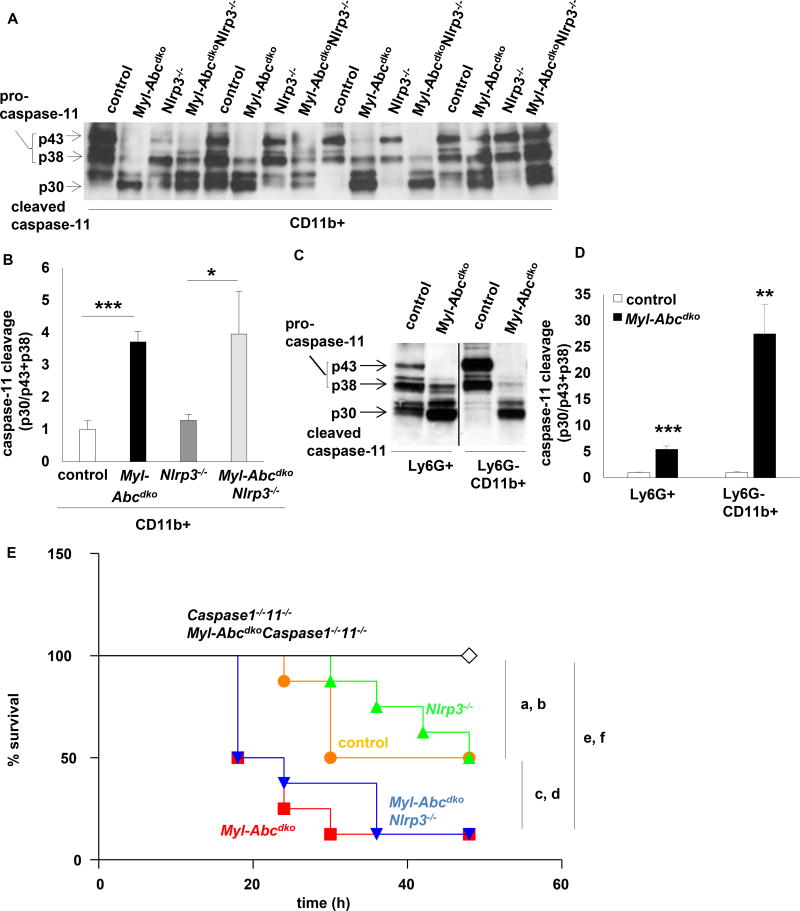
Myeloid Abca1/g1 deficiency activates the NLRP3 inflammasome by inducing the non-canonical inflammasome
Non-canonical inflammasome activation induces Caspase-11 cleavage, leading to activation of the NLRP3 inflammasome.32 We found that myeloid Abca1/g1 deficiency enhanced Caspase-11 cleavage in splenic CD11b+ cells moderately on the chow diet (Supplementary Figure 10A)15 and markedly in Ldlr−/− mice fed Western type diet. Consistent with previous studies of the non-canonical inflammasome,32 this was independent of Nlrp3 (Figure 6A–B). Abca1/g1 deficiency enhanced Caspase-11 cleavage in Ly6G+ neutrophils and Ly6G22CD11b+ monocytes/macrophages (Figure 6C–D and Supplementary Figure 10B). The only activator described for non-canonical inflammasome activation in macrophages is cytosolic LPS.32 Our data suggest a distinct mechanism of Caspase-11 activation in splenic Myl-Abcdko Ly6G+ neutrophils and Ly6G−CD11b+ monocytes and macrophages, likely related to sensing of cellular cholesterol accumulation. Caspase11−/− mice are protected from LPS-induced mortality (septic shock).32 To substantiate our findings that myeloid Abca1/g1 deficiency activates the non-canonical inflammasome pathway, we carried out an LPS mortality study. We used a chow diet to reduce variability in response, which nonetheless is associated with a moderate level of lipid accumulation in monocytes/macrophages,15 inflammasome activation (Supplementary Figure 1A), and enhanced Caspase-11 cleavage in splenic CD11b+ cells (Supplementary Figure 8A). Myeloid Abca1/g1 deficiency enhanced LPS-induced mortality, which was dependent on Caspase-1/11, but independent of Nlrp3 expression (Figure 6E). This pattern is considered a hallmark of non-canonical inflammasome activation,32 and indicates that NLRP3 inflammasome activation is downstream of the non-canonical inflammasome in mice with myeloid Abca1/g1 deficiency.
Figure 6. Myeloid Abca1/g1 deficiency activates the non-canonical inflammasome.
(A–D) Ldlr−/− mice were transplanted with BM from control, Myl-Abcdko, Nlrp3−/−, or Myl-AbcdkoNlrp3−/− mice (n=6 per group), and fed WTD for 8 weeks. Genotypes of BM donors are indicated on the graph. Caspase-11 cleavage was assessed in splenic CD11b+ cells (A–B) or Ly6G+ and Ly6G−CD11b+ cells (C–D) using Western blot, and quantified (B, D). (B) *P<0.05, **P<0.01, ***P<0.001 by one-way ANOVA with Bonferroni post-test or (D) t-test. (E) Mice of the indicated genotypes (n=8 per group) were fed a chow diet and injected with LPS at 8 weeks of age (20 mg/kg; i.p.). Mortality was assessed every 6 hours over a 48 h time period. aP<0.05 control compared to Caspase1−/−11−/−; bP<0.05 Nlrp3−/− compared to Caspase1−/−11−/−; cP<0.01 control compared to Myl-Abcdko; dP<0.01 Nlrp3−/− compared to Myl-AbcdkoNlrp3−/−; eP<0.001 Myl-Abcdko compared to Myl-AbcdkoCaspase1−/−11−/−; fP<0.001 Myl-AbcdkoNlrp3−/− compared to Myl-AbcdkoCaspase1−/−11−/−, Log-rank (Mantel-Cox) test. Reported significance values are nominal and uncorrected.
Discussion
Our findings indicate that cholesterol accumulation in Abca1/g1 deficient myeloid cells activates the NLRP3 inflammasome promoting neutrophil infiltration and NETosis in atherosclerotic lesions. In contrast to the current view of a cholesterol crystal stimulated activation of the macrophage inflammasome within atherosclerotic lesions,4 our findings suggest a systemic effect of myeloid inflammasome activation indirectly stimulating neutrophil activation and entry in plaques, leading to prominent NETosis. Moreover, inflammasome activation appears to involve both increased levels of NLRP3 inflammasome components, likely a TLR4-mediated priming effect, and a membrane cholesterol sensing mechanism that leads to non-canonical inflammasome activation upstream of the NLRP3 inflammasome and increases susceptibility to LPS-induced death.
NETosis has been shown to promote atherosclerotic plaque development in mice,33, 34 and has been associated with features of plaque instability or erosion in humans.24, 35–37 Mouse studies have indicated a role of NETosis in the development of advanced atherosclerotic lesions33, 34 and have shown that NETosis can promote macrophage inflammasome activation.34 In contrast, our studies show prominent neutrophil accumulation and NETosis in early but not late atherosclerotic lesions, consistent with a significant body of literature indicating a role for neutrophils in early atherogenesis.22, 23, 38 Moreover, in our model, abolition of lesional neutrophil accumulation and NETosis by Caspase-1/11 or Nlrp3 deficiency shows for the first time that neutrophil recruitment and NETosis can be induced downstream of inflammasome activation. A potential explanation for these findings would be that neutrophil cholesterol accumulation in mice with myeloid Abca1/g1 deficiency led to inflammasome activation which in turn promoted NETosis as a mode of neutrophil death. However, several observations suggest that NETosis may not be due to a cell-intrinsic effect in neutrophils. Myeloid Abca1/g1 deficiency did not enhance Caspase-1 or Caspase-11 cleavage in neutrophils of atherosclerotic plaques or spontaneous NETosis in isolated blood neutrophils. NETosis may thus be due to enhanced neutrophil recruitment or activation as a systemic consequence of inflammasome activation in myeloid cells rather than a cell-intrinsic effect in neutrophils. NLRP3 inflammasome slightly enhanced neutrophilia in myeloid Abca1/g1 deficient Ldlr−/− mice, consistent with NLRP3 inflammasome dependent IL-1β secretion. IL-1β may also promote binding and entry of myeloid cells into plaques.6, 39 Our findings are thus more consistent with systemic effects of NLRP3 inflammasome activation than local effects in atherosclerotic lesions.
Earlier studies have emphasized the role of cholesterol crystals in inflammasome activation and NETosis.34 However, our studies in Abca1/g1 deficient macrophages point to a role of endosomal and membrane cholesterol accumulation without obvious cholesterol crystal formation or lysosomal damage. Moreover, in vivo the major effects of the inflammasome were seen in early lesions when cholesterol crystals are not readily observed. In contrast to the predictions of phase diagrams based on the lipid composition of macrophage foam cell predominant atherosclerotic lesions,40 confocal reflectance microscopy studies have suggested abundant cholesterol crystals in early mouse atherosclerotic plaques.4 However, some of this crystalline material may have been formed during sample processing due to the ready crystallization of cholesteryl esters in refrigerated samples.
In contrast to some previous studies,4, 9–12 we found no effect of hematopoietic Nlrp3 or Caspase-1/11 deficiency on atherogenesis in Ldlr−/− mice. Based on our data in Ldlr−/− mice showing that hematopoietic Nlrp3 deficiency did not reduce plasma IL-18 levels, while Caspase-1/11 deficiency had only a small effect, we attribute these findings to a lack of NLRP3 inflammasome activation. Studies showing that the NLRP3 inflammasome only contributed to atherogenesis in the setting of enhanced activation due to mitochondrial ROS accumulation or the TET2 mutation, but not in the control Ldlr−/− condition,3, 14 support this interpretation. Several NLRP3 inflammasome components also did not affect atherogenesis in Apoe−/− mice fed a diet containing 1.25% cholesterol.13 While the lack of an effect has been attributed to the high cholesterol content of the diet,41 the absence of inflammasome activation in Apoe−/− mice or differences in the microbiome could also explain these results. The microbiome from Nlrp3−/− and Nlrp6−/− mice has been reported to enhance inflammation via MyD88 and TRIF pathways,42 which could produce variable effects and complicate the interpretation of atherosclerosis results in compound knockout mice.
We found increased inflammasome priming in Abca1/g1 deficient splenic monocytes, macrophages, and neutrophils, which likely contributed to NLRP3 inflammasome activation. Excessive membrane cholesterol content in Abca1/g1 deficient macrophages enhanced the interaction between TLR4 and MD-2, stimulating TLR4 induced gene expression.31 Interestingly, it has also been shown that cholesterol may interact with phospholipids in membranes to propagate inflammatory signals induced by LPS,43 consistent with excessive membrane cholesterol accumulation enhancing inflammasome priming as we observed. Enhanced cholesterol synthesis in cells deficient in cholesterol-25-hydroxylase has been associated with activation of the AIM-2 inflammasome due to mitochondrial damage enhancing mitochondrial DNA release into the cytosol.44 Mitochondrial damage was also associated with accumulation of mitoROS,44 which activates the NLRP3 inflammasome.28 We did not observe enhanced mitoROS accumulation in our myeloid Abca1/g1 deficient model, suggesting a distinct activation mechanism. In sum, these findings suggest that cholesterol accumulation in myeloid cells can activate the inflammasome via several pathways, including inflammasome priming, mitochondrial damage, and Caspase-11 cleavage.
Our study points to a major role of the non-canonical inflammasome initiated by Caspase-11 cleavage acting upstream of the NLRP3 inflammasome in monocytes, macrophages, and neutrophils. Although cytosolic LPS accumulation has been implicated as an activation mechanism of the non-canonical inflammasome,32 upstream signals and sensors relevant to metabolic disease are uncertain45 and could be related to excessive membrane cholesterol content. Our findings suggest that non-canonical inflammasome activation contributes to NLRP3 inflammasome activation and atherogenesis in Myl-Abcdko BMT Ldlr−/− mice fed WTD and to sepsis in Myl-Abcdko mice on chow diet.
The elevated levels of plasma IL-1β and IL-18 in TD patients suggest that myeloid cholesterol accumulation may also contribute to inflammasome activation in humans. TD patients sometimes present with premature atherosclerotic cardiovascular disease;46 however, the more consistent phenotype among adult TD patients is peripheral neuropathy.25 A recent study has shown that defective myelin clearance due to macrophage Abca1/g1 deficiency promotes inflammasome activation and limits remyelination in the central nervous system in aged mice.47 Together with our findings this suggests that macrophage inflammasome activation may be involved in the pathogenesis of peripheral neuropathy in TD. TD patients could also be susceptible to a sepsis-induced hyperinflammatory state due to inflammasome activation. Although TD is rare, low levels of ABCA1/G1 in monocyte/macrophages along with low HDL levels may commonly occur in humans with poorly controlled diabetes,48 chronic kidney disease,49 or simply with ageing.47 Targeting the NLRP3 or non-canonical inflammasome, or their products, in susceptible patients, represents an attractive area for future atherosclerosis research.
Supplementary Material
Clinical Perspective.
What is new?
Deficiency of ATP Binding Cassette Transporters A1 and G1 (ABCA1/G1) mediated cholesterol efflux pathways in myeloid cells activates the NLRP3 inflammasome as a consequence of excessive cholesterol accumulation.
NLRP3 inflammasome activation enhances neutrophil accumulation and neutrophil extracellular trap formation in atherosclerotic plaques, accelerating atherogenesis.
Tangier Disease patients with ABCA1 loss-of-function, who have increased myeloid cholesterol content, show markers of inflammasome activation. Inflammasome activation may underlie CVD and peripheral neuropathy in these patients.
What are the clinical implications?
Targeting the NLRP3 inflammasome in patients who show low expression of ABC transporters, such as patients with diabetes, chronic kidney disease or the elderly, may represent an attractive therapeutic option.
Acknowledgments
Sources of Funding
This work has been supported by NIH grants R01HL107653 (to A.R.T.), R01HL117334 and R35HL135799 (to K.J.M), R01HL113147 and K24HL107643 (to M.P.R. and H.Z.), K99HL130574 (to H.Z.), T32HL007343 (to M.M.M.), Netherlands Organization of Scientific Research VIDI-grant 917.15.350 (to M.W.), and a Rosalind Franklin Fellowship from the University Medical Center Groningen (to M.W.). Flow cytometry experiments were performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, NIH under awards S10RR027050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Disclosures
A.R.T. reports being a consultant to Amgen, CSL, Staten Biotech, and Dalcor. The other authors report no conflicts of interest.
References
- 1.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with tet2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein e-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 6.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 7.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 8.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein e(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 9.Gage J, Hasu M, Thabet M, Whitman SC. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein e-null mice. Can J Cardiol. 2012;28:222–229. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Hendrikx T, Jeurissen ML, van Gorp PJ, Gijbels MJ, Walenbergh SM, Houben T, van Gorp R, Pottgens CC, Stienstra R, Netea MG, Hofker MH, Donners MM, Shiri-Sverdlov R. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in ldlr(−/−) mice. FEBS J. 2015;282:2327–2338. doi: 10.1111/febs.13279. [DOI] [PubMed] [Google Scholar]
- 11.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S, Takahashi M. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in western diet-fed apolipoprotein e-deficient mice. Biochem Biophys Res Commun. 2012;425:162–168. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 12.Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, Mai J, Virtue A, Lopez-Pastrana J, Meng S, Tilley D, Monroy MA, Choi ET, Thomas CJ, Jiang X, Wang H, Yang XF. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J. Atherosclerosis in apoe-deficient mice progresses independently of the nlrp3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumurkhuu G, Shimada K, Dagvadorj J, Crother TR, Zhang W, Luthringer D, Gottlieb RA, Chen S, Arditi M. Ogg1-dependent DNA repair regulates nlrp3 inflammasome and prevents atherosclerosis. Circ Res. 2016;119:e76–90. doi: 10.1161/CIRCRESAHA.116.308362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of atp-binding cassette transporters a1 and g1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, Wang N, Randolph GJ, D'Agati VD, Yvan-Charvet L, Tall AR. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 2017;25:1294–1304. e1296. doi: 10.1016/j.cmet.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bochem AE, van der Valk FM, Tolani S, Stroes ES, Westerterp M, Tall AR. Increased systemic and plaque inflammation in abca1 mutation carriers with attenuation by statins. Arterioscler Thromb Vasc Biol. 2015;35:1663–1669. doi: 10.1161/ATVBAHA.114.304959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Xue C, Shah R, Bermingham K, Hinkle CC, Li W, Rodrigues A, Tabita-Martinez J, Millar JS, Cuchel M, Pashos EE, Liu Y, Yan R, Yang W, Gosai SJ, VanDorn D, Chou ST, Gregory BD, Morrisey EE, Li M, Rader DJ, Reilly MP. Functional analysis and transcriptomic profiling of ipsc-derived macrophages and their application in modeling mendelian disease. Circ Res. 2015;117:17–28. doi: 10.1161/CIRCRESAHA.117.305860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, Moskowitz MA, Weissleder R. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci USA. 2008;105:18584–18589. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 23.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 24.Doring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–743. doi: 10.1161/CIRCRESAHA.116.309692. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer EJ, Santos RD, Asztalos BF. Marked hdl deficiency and premature coronary heart disease. Curr Opin Lipidol. 2010;21:289–297. doi: 10.1097/MOL.0b013e32833c1ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of abca1 and abcg1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of il-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in nlrp3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 29.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Revs. Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. Cd36 coordinates nlrp3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 33.Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, Guo C, Grenn RC, Thompson PR, Eitzman DT, Kaplan MJ. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer B. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33:2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P. Tlr2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kithcart AP, Libby P. Unfriendly fire from neutrophils promiscuously potentiates cardiovascular inflammation. Circ Res. 2017;121:1029–1031. doi: 10.1161/CIRCRESAHA.117.311867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived cramp reduces atherosclerosis in mice. Circ Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 39.Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: Biological basis of cantos and beyond. J Am Coll Cardiol. 2017;70:2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Small DM, Shipley GG. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974;185:222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]
- 41.Sheedy FJ, Moore KJ. Il-1 signaling in atherosclerosis: Sibling rivalry. Nat Immunol. 2013;14:1030–1032. doi: 10.1038/ni.2711. [DOI] [PubMed] [Google Scholar]
- 42.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of nafld and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–298. doi: 10.1038/nature20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dang EV, McDonald JG, Russell DW, Cyster JG. Oxysterol restraint of cholesterol synthesis prevents aim2 inflammasome activation. Cell. 2017;171:1057–1071. e1011. doi: 10.1016/j.cell.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Razek O, Sadananda SN, Li X, Cermakova L, Frohlich J, Brunham LR. Increased prevalence of clinical and subclinical atherosclerosis in patients with damaging mutations in abca1 or apoa1. J Clin Lipidol. 2018;12:116–121. doi: 10.1016/j.jacl.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lutjohann D, Mobius W, Simons M. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359:684–688. doi: 10.1126/science.aan4183. [DOI] [PubMed] [Google Scholar]
- 48.Patel DC, Albrecht C, Pavitt D, Paul V, Pourreyron C, Newman SP, Godsland IF, Valabhji J, Johnston DG. Type 2 diabetes is associated with reduced atp-binding cassette transporter a1 gene expression, protein and function. PloS one. 2011;6:e22142. doi: 10.1371/journal.pone.0022142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganda A, Yvan-Charvet L, Zhang Y, Lai EJ, Regunathan-Shenk R, Hussain FN, Avasare R, Chakraborty B, Febus AJ, Vernocchi L, Lantigua R, Wang Y, Shi X, Hsieh J, Murphy AJ, Wang N, Bijl N, Gordon KM, de Miguel MH, Singer JR, Hogan J, Cremers S, Magnusson M, Melander O, Gerszten RE, Tall AR. Plasma metabolite profiles, cellular cholesterol efflux, and non-traditional cardiovascular risk in patients with ckd. J Mol Cell Cardiol. 2017;112:114–122. doi: 10.1016/j.yjmcc.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.