Abstract
Half a million patients in the USA alone require treatment for burns annually. Following an extensive burn, it may not be possible to provide sufficient autografts in a single setting. Pig skin xenografts may provide temporary coverage. However, preformed xenoreactive antibodies in the human recipient activate complement, and thus result in rapid rejection of the graft. Because burn patients usually have some degree of immune dysfunction and are therefore at increased risk of infection, immunosuppressive therapy is undesirable. Genetic engineering of the pig has increased the survival of pig heart, kidney, islet, and corneal grafts in immunosuppressed non-human primates from minutes to months or occasionally years. We summarize the current status of research into skin xenotransplantation for burns, with special emphasis on developments in genetic engineering of pigs to protect the graft from immunological injury. A genetically-engineered pig skin graft now survives as long as an allograft and, importantly, rejection of a skin xenograft is not detrimental to a subsequent allograft. Nevertheless, currently, systemic immunosuppressive therapy would still be required to inhibit a cellular response, and so we discuss what further genetic manipulations could be carried out to inhibit the cellular response.
Keywords: burns, genetic-engineering, pig, skin, xenotransplantation
Introduction
Approximately half a million patients in the US require treatment for burns annually [1], with nearly 40,000 hospitalizations and 3,400 deaths [2]. Burns also remain an important source of casualty from military operations. Between March 2003 and October 2011, the United States Army Burn Center treated 1,037 patients as a result of wars in Iraq and Afghanistan, of whom 57% eventually underwent excision and grafting of burn wounds [3].
Skin transplantation
Standard methods of therapy for severe burns include early wound debridement, excision, and coverage by autologous split skin grafts. Following an extensive burn, it may not be possible to provide sufficient autografts in a single setting; the residual scars may remain unsatisfactory due to the lack of dermis [4]. If sufficient autologous skin is not available, (i) skin allografts from deceased human donors, (ii) cultured autologous keratinocytes and various artificial dermal substitutes, (iii) commercially-available acellular products, such as Alloderm, or (iv) skin xenografts may provide partial and/or temporary cover. Autograft skin has many advantages over other materials, such as normal physiological characteristics, full biocompatibility, plentiful supply, and convenience in usage.
Because of rejection by the patient`s immune system, which can take about 6–14 days for a cellular allograft, but may be much quicker for a pig xenograft [5] [6], skin allo- and xeno-grafts provide only a temporary covering as a biological dressing. However, they serve several functions. They (i) provide a clean, granulating area prior to autografting; (ii) protect an open wound from protein and water loss until autografting can be carried out; (iii) decrease the surface bacterial count; (iv) decrease pain at the site of an open wound; (v) can cover vital organs, and (vi) facilitate early movement of an affected part. At the very least, skin allo- and xeno-grafts promote re-epithelialization and prepare the wound bed for the autograft, increasing the healing rate when compared with traditional dressings [7, 8].
Skin allografts (Table 1)
Table 1.
Differences between allo (human) and xeno (wild-type pig) skin grafts, and bioengineered skin substitutes.
| Advantages | Disadvantages | |
|---|---|---|
| Allo-skin grafts from cadaver donors |
|
|
| Xeno-skin grafts from wild type pigs |
|
|
| Bioengineered skin substitutes |
|
|
Cadaver skin (cellular)
The gold standard for temporary coverage is a skin allograft [5] [8]. Like skin autografts, allografts undergo vascularization within 2–3 days, and are capable of providing a viable barrier in the early phase after a severe burn [9]. However, if left in place for a longer period of time, they will be rejected, which may cause excessive scarring. Therefore, they must eventually be replaced with an autograft. Despite their effectiveness, especially regarding wound bed vascularization, skin allografts are problematic with regard to (i) limited availability, (ii) the potential for transmission of pathogens, and (iii) cost [9–11].
Commercialized acellular dermal matrices from cadaver skin
Acellular dermal matrices are derived from human dermis, treated to remove all immunogenic elements (keratinocytes, fibroblasts, vascular endothelium, and smooth muscle) [4]. At least five different manufacturing processes and products (Alloderm, DermaMatrix, GlyaDerm, GraftJacket, and SureDerm) are currently registered for wound care [4]. However, most of these matrices remain antigenic [12], which may lead to poor graft survival. Moreover, these products are expensive [4].
Immunobiology of skin allotransplantation
The skin is the largest organ of the body and is highly immunogenic. When a viable skin allograft is transplanted onto a healthy recipient, rejection occurs within 6 to 14 days, predominantly by CD8+T cells, leading to destruction of the graft [5, 6]. Initially, the epidermal Langerhans cells play an important role in the rejection process. As revascularization occurs, these cells migrate from the donor skin graft to the draining lymph nodes of the recipient where they activate T cells [6]. The dermis also contains a population of dendritic cells possessing similar properties. Pretreatment of the allogeneic skin with agents that suppress the function of Langerhans cells and dendritic cells may delay the rejection process [6] [13], as may the pathologically-suppressed immune system of the patient with burns [14] [15].
Use of cultured epidermal allografts, which lack Langerhans cells and dendritic cells, has been attempted, but did not result in prolonged or permanent coverage [16]. Allogeneic sheets of cultured keratinocytes containing no Langerhans cells became infiltrated by the recipient`s Langerhans cells and T cells [17], which may ultimately lead to rejection.
Bioengineered skin substitutes (Table 1)
Several kinds of bioengineered skin substitutes are being explored [18, 19], including biosynthetic skin substitutes and autologous cultured/non-cultured skin engineering products [18]. They are designed to close the wound, temporarily or permanently, providing a mechanical barrier to infection and fluid loss. They also possess some of the biological and pharmacological properties of human skin which allow and/or promote new tissue growth under ideal conditions for healing [18].
Cell-based approaches for more permanent coverage have made progress. Culture-based options, such as Epicel® (Genzyme, Cambridge, MA, USA), use a small biopsy of the patient`s skin to provide keratinocytes, which are expanded in number over 2–3 weeks into a confluent epidermal autograft. However, because cultured autologous keratinocytes require weeks to grow before being ready for application in a patient, they are not ideal for the treatment of burns. Furthermore, they may yield a thin, delicate graft that is easily injured. Moreover, bioengineered skin substitutes are expensive, and thus currently not widely used, especially in developing countries [20].
Skin xenografts (Table 1)
Fresh [21], cryopreserved [22], or glutaraldehyde-preserved [22] pig skin has been used widely as a temporary dressing for burns. Historically, skin from several species (e.g., chicken, rat, pigeon, cat, dog, frog, cow, pig) has been used as a biologic dressing in humans [5, 23, 24]. Pig skin is similar to human skin in its histologic structure, and is an inexpensive and readily-available source [5, 9, 10, 25]. Silvetti et al. demonstrated the beneficial effects on wound healing of bovine embryonic skin xenografts, applied as a temporary wound dressing [5, 26].
Some researchers have discussed the theoretical risk of zoonotic infectious microorganisms being transferred from the graft to the patient, especially porcine endogenous retroviruses and Clostridium difficile [5, 27]. However, no evidence of the transfer of porcine endogenous retroviruses has been reported [28–30], and C. difficile infection, while common in burn patients, has not been demonstrated to be of zoonotic origin [31]. Furthermore, genetically-engineered pigs bred specifically for xenotransplantation (see below) will be housed under clean, biosecure conditions so the risk of zoonotic disease transmission is further decreased.
Pig skin is most commonly preserved with glutaraldehyde, its protein cross-linking properties having both a germicidal and preservative effect on the tissues [22]. However, this ‘fixed’ skin fails to vascularize as the cells in the graft are no longer viable, and thus the graft functions only as a biological dressing. When transplanted to humans or Old World nonhuman primates (NHPs), unpreserved pig skin is susceptible to early graft failure from hyperacute rejection due to the presence of preformed antibodies directed largely to galactose-α1,3-galactos (Gal) antigens expressed on pig vascular endothelial cells [9, 32–35]
However, pigs are now available with several genetic modifications that protect their tissues from the primate immune response (and/or correct molecular incompatibilities between pigs and primates), including knockout of the gene encoding Gal (α1,3-galactosyltransferase gene-knockout [GTKO] pigs) [36] [37] (Table 2). As a result of this technological revolution, pig solid organ transplants in NHPs, especially kidneys and hearts, do not undergo hyperacute rejection, and prolonged organ survival is increasingly being reported, extending to many months or even years [35, 38–40] [41].
Table 2.
Identified antigens (and associated synthesizing enzymes) on pig vascular endothelial cells against which humans have anti-pig antibodies.
| Antigens | Enzymes |
|---|---|
| Galactose-α1,3-galactose (Gal) | α1,3-Galactosyltransferase (GT) |
| N-glycolylneuraminic acid (Neu5Gc) | Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) |
| Sda | β1,4 N-acetylgalactosaminyltransferase (β4GalNT2) |
Immunobiology of skin xenotransplantation
After pig skin xenotransplantation, the patient is exposed to antigens expressed on the vascular endothelial cells of the pig, (e.g., Gal), but which are not present in humans. Preformed xenoreactive antibodies in the human recipient bind to these antigens, activate complement, and result in rapid rejection. These antibodies develop during infancy, and are believed to be a response to colonization of the gastrointestinal tract by bacteria and viruses that express some of the same carbohydrate structures as pig cells [42].
To date, only three pig xenoantigens have been definitively identified, the most important of which is Gal [35] (Table 2). This glycan results from the activity of the enzyme, α1,3-galactosyltransferase. In contrast, humans, apes, and Old World monkeys are defective in this gene, and thus produce high levels of anti-Gal antibodies [43]. When a pig organ is immediately revascularized (by blood vessel surgical anastomosis), these antibodies can cause hyperacute rejection (within minutes) [35]. After pig skin transplantation, as a result of this antibody-antigen interaction, complement-mediated injury of the endothelium occurs, resulting in thrombosis. The graft does not become vascularized and fails as a ‘white’ graft [35, 43, 44].
To prevent hyperacute rejection of pig organ grafts in NHPs, pigs have been genetically-engineered by ‘knocking out’ the α1,3-galactosyltransferase gene. Skin grafts from these pigs show prolonged survival, which now equates to that of an allograft [9, 10, 35, 38, 45].
However, pigs express two other known antigens against which humans have preformed antibodies (‘nonGal’ antigens) (Table 2), which contribute to graft failure. N-glycolylneuraminic acid (Neu5Gc) is an important nonGal antigen that is expressed in pigs, apes, and Old World NHPs, but not in humans [30, 46–48]. Its expression is dependent on the cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene [30] [49], and knockout of this gene results in loss of expression [49]. Organ and skin grafts from these pigs cannot be tested in Old World monkeys because they also express Neu5Gc (and therefore do not make anti-Neu5Gc antibodies). Like humans, New World monkeys do not express Neu5Gc [50], and so could be a suitable experimental animal to study the effect of Neu5Gc expression on pigs.
The third pig antigen is Sda, the product of the enzyme β1,4-N-acetylgalactosaminyltransferase (β4GalNT2) (Table 2) [51]. However, no relevant studies in porcine skin xenotransplantation have been conducted with respect to Sda.
Scobie et al. reported the long-term immunologic effects after exposure to fresh pig skin in blood and serum samples collected from severe burn patients up to 34 years after temporary fresh pig skin transplantation, and compared their data with those from age-matched control burn patients who had not received pig skin grafts. They reported a persistent moderate increase in anti-Gal IgG (a response to porcine Gal antigens) and a high response of anti-Neu5Gc IgG (a response to porcine Neu5Gc antigens) that persisted for many years [30]. They did not test for the presence of anti-Sda antibodies.
In addition to being subjected to the human humoral response, a cellular response develops similar to that seen to an allograft. As in humans, pig skin is divided into three layers, i.e., epidermis, dermis, and hypodermis (or subcutis). Langerhans cells in both pig and human epidermis are mostly present in the lowermost epidermal layers. Pig Langerhans cells express swine leukocyte class II antigens (SLA-II), equivalent to human leukocyte class II antigens (HLA-II) [52]. Swine leukocyte antigens generate a rather stronger T cell response than do HLA [53], and this also needs to be suppressed.
Genetic modification of the pig
The great advantage of xenotransplantation is that, for the first time, it allows modification of the donor rather than simply treatment of the recipient of the transplant. Since the birth of the first cloned large mammal, Dolly the sheep [54], genetic engineering of large mammals, particularly pigs, has progressed rapidly [55] [56].
Resistance to the innate immune response to a pig xenograft
Today, there is an increasing availability of pigs with genetic modifications that protect the pig tissues from the primate immune response (and/or correct molecular incompatibilities between pigs and primates) (Table 3) [55]. These genetic manipulations take the form of (i) knockout of a pig gene that is responsible for the expression of an antigen against which the primate (human or NHP) has natural preformed antibodies that bind to these antigens and initiate complement- and/or coagulation-mediated destruction, or (ii) the insertion of a human transgene that provides protection against the human complement, coagulation, or inflammatory responses [55].
Table 3.
Selected genetically-modified pigs currently available for xenotransplantaion (excluding antigen-deletion).
| Complement regulation by human complement-regulatory gene expression: |
| CD46 (membrane cofactor protein) |
| CD55 (decay-accelerating factor) |
| CD59 (protectin or membrane inhibitor of reactive lysis) |
| Suppression of cellular immune response by gene expression or downregulation: |
| CIITA-DN (MHC class II transactivator knockdown, resulting in swine leukocyte antigen class II knockdown) |
| Class I MHC-knockout (MHC-I-KO) |
| HLA-E/human β2-microglobulin (inhibits human natural killer cell cytotoxicity) |
| Human FAS ligand (CD95L) |
| Human GnT-III (N-acetylglucosaminyltransferase III) gene |
| Porcine CTLA4-Ig (Cytotoxic T-Lymphocyte Antigen 4 or CD152) |
| Human TRAIL (tumor necrosis factor-alpha-related apoptosis-inducing ligand) |
| Anticoagulation and anti-inflammatory gene expression or deletion: |
| von Willebrand factor (vWF)-deficient (natural mutant) |
| Human tissue factor pathway inhibitor (TFPI) |
| Human thrombomodulin |
| Human endothelial protein C receptor (EPCR) |
| Human CD39 (ectonucleoside triphosphate diphosphohydrolase-1) |
| Anticoagulation, anti-inflammatory, and anti-apoptotic gene expression |
| Human A20 (tumor necrosis factor-α-induced protein 3) |
| Human heme oxygenase-1 (HO-1) |
| Human CD47 (species-specific interaction with SIRPα inhibits phagocytosis) |
| Porcine asialoglycoprotein receptor 1 gene-knockout (ASGR1-KO) (decreases platelet phagocytosis) |
| Human signal regulatory protein α (SIRPα) (decreases platelet phagocytosis by ‘self’ recognition) |
Reproduced with permission from Cooper DK, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation. 2016;23(2):83–105
All of the 3 known pig antigens have now been deleted from pigs by knockout of the underlying genes [38, 56]. When one or more of these pig antigens is not expressed, there is a significant reduction in the binding of primate antibodies to pig vascular endothelial cells (Figure 1) [38]. Further graft protection is provided by the expression of one or more human complement-regulatory proteins (e.g., CD46, CD55, CD59) and/or one or more human coagulation-regulatory proteins (e.g., thrombomodulin, tissue factor pathway inhibitor, endothelial protein C receptor, CD39) (Table 3).
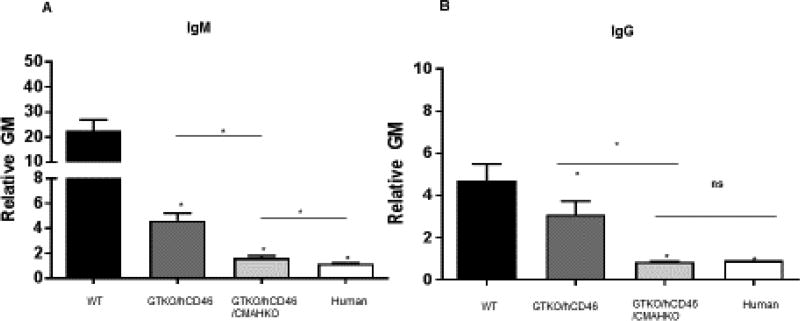
Figure 1. Human IgM and IgG antibody binding to pig and human aortic endothelial cells (AECs) by flow cytometry.
Human IgM (a) and IgG (b) binding to GTKO/hCD46 pig AECs was significantly decreased compared with wild-type (WT) pig AECs (*P<0.05), and was further decreased to GTKO/hCD46/NeuGcKO pig AECs (*P<0.05). There was significantly greater IgM binding to GTKO/hCD46/NeuGcKO pig AECs than human AECs (‡P<0.05), but there was no statistical significance of the extent of IgG binding between them. (MFI=mean fluorescence intensity; ns=not significant.) (Reproduced with permission from Wijkstrom M, et al. Kidney Int. 2017;91:790–796.)
As a result of these genetic manipulations (now including as many as 6 in a single pig), the transplantation of pig organs into NHPs is no longer limited by early antibody-mediated (hyperacute or acute vascular) rejection. Furthermore, some of the genetic manipulations carried out in pigs to reduce the effects of primate natural antibody and complement and coagulation activation (the humoral response), as well as the innate immune cellular response, have been found to reduce the effects of the T cell response (the adaptive cellular immune response), thus possibly reducing the need for exogenous immunosuppressive therapy [38] [55]. Nevertheless, exogenous biologic and/or pharmacologic immunosuppressive therapy is still required to prevent the cellular response to a pig xenograft.
Suppression of the adaptive immune response
In the absence of adequate immunosuppressive therapy, exposure to the pig xenograft elicits the production of xenoreactive antibodies in the human or NHP recipient to other antigens expressed in the pig, e.g., to SLA class I and II [35, 46, 57]. Currently, the experimental protocols for xenotransplantation in NHPs require administration of immunosuppressive agents that suppress immunity systemically. However, severe burn patients already have immune dysfunction, and thus, if administered immunosuppressive therapy, would be at greater risk for infection. Therefore, immunosuppressive protocols are less than ideal in the treatment of burns, making it necessary to explore novel protocols to prolong skin xenograft survival without the need for systemic immunosuppressive therapy.
The question that needs to be answered is “can the T cell-mediated cellular and elicited antibody responses be prevented by genetic modification of the source pig?” The human T cell response to pig cells is stronger than to allogeneic human cells, but it can certainly be reduced, if not yet eliminated, by several genetic approaches.
Deletion of major pig antigens
The T cell response to a pig graft that does not express Gal, Neu5Gc, and/or Sda is weaker than to a wild-type (genetically-unmodified) pig graft [58], and the absence of these antigens is associated with reduced human T cell proliferation (Figure 2) [59].
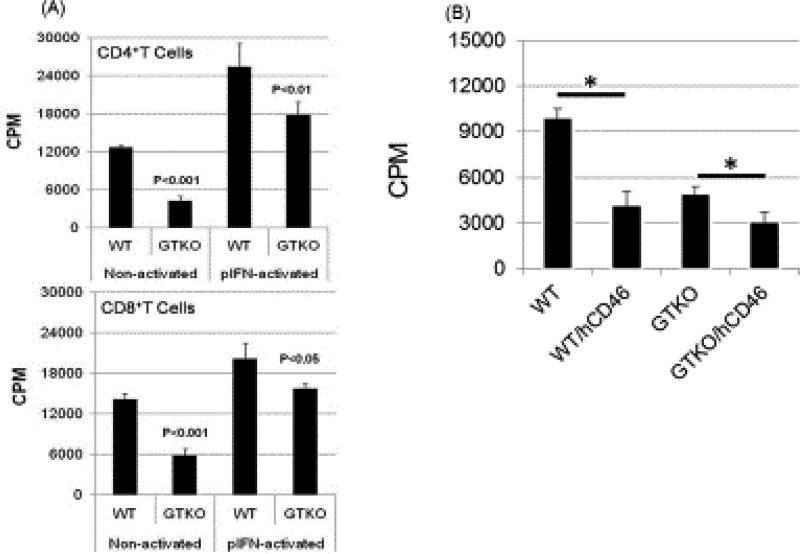
Figure 2. (A) Human CD4+T cells and CD8+Tcells responses to WT and GTKO porcine aortic endothelial cells (pAECs) before and after activation by pIFN-γ, and (B) human peripheral blood mononuclear cell (PBMC) responses to WT and GTKO pAECs expressing hCD46.
(A) The proliferative response of human CD4+T cells (n=3) and CD8+T cells (n=3) to WT and GTKO pAECs before and after activation by pIFN-γ. The response was significantly less to GTKO pAECs before (p<0.001) and after (p<0.05) activation. 3H incorporation values are presented as CPM. Data represent the mean (+/− SEM) and are representative of three different experiments.
(Reproduced with permission from Wilhite T, et al. Xenotransplantation. 2012;19:56–63.)
(B) Human cellular responses to pAECs expressing hCD46. Human PBMCs were co-cultured with pAECs. The mean of triplicate results was expressed as 3H-thymidine uptake. Data are representative of two different humans. PBMC proliferation is presented as counts per minute (CPM) for 3H incorporation. Data represent the mean (±SEM) and are shown. (*P<0.05). (Reproduced with permission from Ezzelarab M, et al. Xenotransplantation. 2015;22:487–489.)
Transgenic expression of human complement-regulatory proteins
Although the role of complement in organ transplantation has largely been limited to the humoral response to an ABO- or HLA-incompatible allograft and/or to a xenograft, complement may also be involved in the promotion of T cell activation and thus increase the T cell response [60] [61]. The expression of a human complement-regulatory protein, e.g., CD46, on pig cells reduces the proliferation of human T cells, thus reducing the T cell response [62]. Similarly, the proliferative response to GTKO pig cells expressing CD46 is significantly weaker than to GTKO cells [62] (Figure 2).
Transgenic expression of costimulation blockade molecules
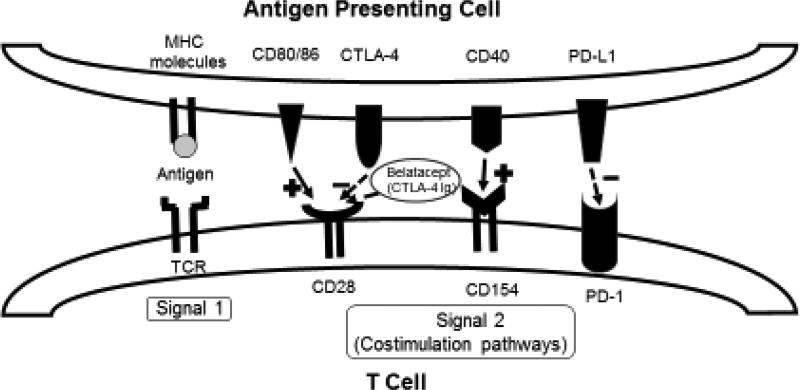
How can the T cell response be further reduced or inhibited by genetic engineering of the pig? Immunologically, CD4+T cells are initially stimulated through the T cell receptor, by the recognition of antigen presented with major histocompatibility complex class II (MHC-II) molecules (signal 1) [63]. But full T cell activation also requires binding of costimulatory molecules (signal 2) [63] (Figure 3). There are several costimulation pathways (listed in the legend to Figure 3) [64] [65]. The absence of signal 2, which can be achieved by several novel agents, leads to the development of T cell anergy [64], with the result that T cells are no longer activated to reject the graft.
Figure 3. Costimulation pathways in T cell regulation.
Upon MHC-antigen interaction with the TCR, costimulation pathways can augment or suppress the activation of the T cell. From left to right: (i) CD28 is activated by CD80/CD86, after which CTLA-4 is upregulated and, with higher affinity than CD80/CD86, binds to CD28, inhibiting the signal. CTLA-4Ig and belatacept work by taking advantage of their higher affinity to CD28 over CD80/CD86, and thereby block CD80/CD86 activation of CD28. (ii) The CD40/CD154 pathway is another potent activator of T cells. Monoclonal antibodies against either of these surface proteins have potential for application in transplant immunosuppression. (iii) PD-1 is expressed on T cells, and its interaction with PD-1 Ligand (PD-L1) induces a suppressive signal to the T cell.
Cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4-Ig) is a well-established agent (e.g., abatacept and belatacept - United States FDA-approved for treatment following kidney allotransplantation [66] [67] and in rheumatoid arthritis [68] that, when administered to patients with an allograft, is capable of down-regulating T cell activation by blocking one of the costimulation pathways and inducing T cell anergy [69]. The gene for CTLA4-Ig has been introduced into the pig [69, 70]. Skin grafts from pigs transgenic for human CTLA4-Ig survived for 13 days compared to those from wild-type pigs survived for 6 days [69]. The CD40/CD154 and PD-1/PD-L1 pathways are other costimulatory pathways that, when blocked, mediate a negative signal and contribute to the regulation of T cell activation [71] (Figure 3), but have not yet been incorporated into genetically-engineered pigs.
Reduction in expression of MHC (SLA) class II
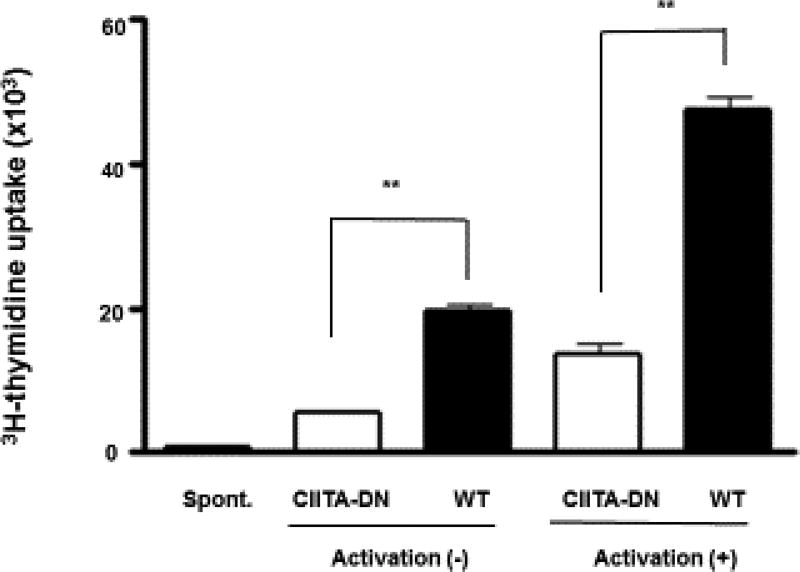
The MHC-II transactivator (CIITA) has been termed the master regulator for MHC class II. It enhances MHC gene expression, and thus stimulates a more vigorous T cell response [72]. High sequence homology between human and pig CIITA allows us to use a human CIITA mutant transgene to reduce swine MHC class II (SLA class II) expression, which results in reduced expression of SLA class II on the vascular endothelium of the pig’s organ and inhibits the T cell response [53, 55, 73] (Figure 4). It has been demonstrated to have a significant effect in vitro and a modest effect in vivo [73].
Figure 4. Significant reduction of the human CD4+T cell response to dominant-negative mutant class II transactivator (CIITA-DN) porcine aortic endothelial cells.
Human (h) CD4+T cells were co-cultured with pAECs for 7 days (n=6). The response of hCD4+T cells was evaluated by [3H] thymidine incorporation. As a negative control, hCD4+T cells were cultured with culture medium (spont). There was a significantly lower hCD4+T cell response to CIITA-DN pAECs compared with WT pAECs (**P<0.01). (Reproduced with permission from Hara H, et al. Immunology. 2013;140:39–46.)
Knockout of MHC (SLA) class I
After skin allotransplantation, untreated grafts become infiltrated with CD4+T cells, CD8+T cells, and macrophages [6]. Therefore, downregulation of activation of these cells in skin grafts may reduce graft rejection. Knockout of SLA class I expression in the pig has recently been achieved, and its effect is being explored [55, 74]. Moreover, over-expression of human CD47 (hCD47) in pigs may prevent macrophage activation and phagocytosis of pig cells [55, 75]. CD47 is also expressed on Langerhans cells [76]. CD47 engagement in vivo significantly inhibits the emigration of Langerhans cells from the epidermis into draining lymph nodes [76].
Inhibition of natural killer (NK) cell activity
NK cells may also play a role in skin xenograft (and allograft) rejection [55, 77, 78]. The observation that human NK cells are able to lyse porcine endothelial cells in vitro suggested that NK cells participate in the rejection of pig-to-human xenografts [79]. Expression of HLA-E on pig cells inhibits human NK cell cytotoxicity and, unlike classical MHC molecules, does not induce an allogeneic T cell response [80]. HLA-G is normally transcribed in fetal-derived cytotrophoblasts where it provides significant suppression of maternal immune responses to the fetus. It can inhibit various functions of NK and cytotoxic T cells [81]. Therefore, genetic engineering of the pig to express HLA-E and/or G may contribute to prolonging graft survival by reducing human NK cell-mediated rejection [55, 80, 82, 83].
These combined genetic engineering approaches would almost certainly inhibit acute rejection of a skin xenograft, and would hopefully allow a reduced intensity of systemic immunosuppressive therapy (or even negate the need for it) in a patient with a pig skin graft.
Genetically-modified pig skin grafting in NHPs – recent progress
To the best of our knowledge, there are seven studies of genetically-modified pig skin xenotransplantation in NHPs reported in the literature. Five of these studies related to viable pig skin-to-NHPs xenotransplantation models relevant to the treatment of burns [45] [9] [44] [10] [84] (Table 4). Two of the seven studies related to induction of immunological tolerance, and were not relevant for the treatment of patients with burns [85, 86].
Table 4.
Recent experience in genetically-modified pig skin transplantation In NHPs
| References (year) | Number | Donor | Immunosuppressive regimen | Survival (days) |
|---|---|---|---|---|
| Recipient : Baboon | Pig | |||
| Weiner J et al. (2010) | 2 | WT | CyA | 4,4 |
| 2 | GTKO | CyA | 11, 14 | |
| Alexander A et al. (2014) | 10 | GTKO | none | 7,10,11,11,12,12,12,13,13,13 |
| Leto Barone et al. (2015) | 8 | WT | CyA or none | 4,4,4,4,4,4,4,4 |
| 8 | GTKO | CyA or none | median 11 | |
| D.A. Leonard et al. (2017) | 17 | GTKO | none | median 11.8 |
| Recipient : Monkey | ||||
| Fujita T et al. (2004) | 2 | WT | none | 3, 2 |
| 3 | GnT III | none | 9,9,9 | |
| 1 | DAF(CD55) | none | 9 | |
| 1 | DAF/GnT III | none | 9 | |
| 1 | GnT III | TAC(14days after Tx) | 21 | |
| 3 | DAF/GnT III | TAC(14days after Tx) | 14,14,31 |
CyA = cyclosporine A, GnTIII = N-acethylglucosaminyltransferase III, GTKO = α1,3-galactosyltransferase gene-knockout, TAC = tacrolimus, WT = wild type
Fujita et al. took skin from pigs transgenic for (i) the human complement-regulatory protein, CD55, and (ii) N-acetylglucosaminyltransferase III (GnT-III), which diminishes Gal expression through suppression of the activity of α1,3-galactosyltransferase [87]. The grafts survived for a maximum of 31 days in cynomolgus monkeys administered a short course (2 weeks) of the conventional immunosuppressive agent, tacrolimus [44]. In a skin graft from a single transgenic pig (GnTIII), the early phase of rejection was prevented (because of GnTIII’s effect in downregulating xenoantigenicity). Expression of CD55 was effective in reducing or preventing the injury associated with complement activation, not only in the hyperacute rejection phase, but also in the acute (or delayed) antibody-mediated rejection phase. Therefore, the combination of CD55 expression and GnTIIII appeared to be effective in protecting the graft from early rejection [44].
Tena et al. reported that transgenic expression of human CD47 on hematopoietic cells of GTKO pigs substantially increased the duration of xenogeneic chimerism after pig hematopoietic cell transplantation in baboons, and prolonged survival of porcine skin grafts for 53 days in the absence of exogenous immunosuppressive therapy [86] which is the the longest reported to date. As previously mentioned, overexpression of human CD47 in pigs might prevent macrophage activation and phagocytosis of pig cells [55, 75]. This result possibly demonstrates the importance of inhibition of both the recipient`s donor-reactive T cells and of macrophage activation and phagocytosis in skin xenotransplantation.
Weiner et al. reported that GTKO pig skin survived longer (11–14 days) than wild-type (Gal-positive) pig skin (4 days), demonstrating that skin grafts from GTKO pigs survive as long as MHC-mismatched allogeneic skin grafts (9–11 days) [45]. Albritton et al. and Leto Barone et al. confirmed these results by demonstrating comparable survival of MHC-mismatched allogeneic (baboon-to-baboon) skin grafts and xenogeneic (pig-to-baboon) skin grafts [9, 10]. Leonard et al have recently reported that cryopreserved GTKO pig skin grafts survive as long as fresh GTKO grafts (mean 11.8 vs 11.8 days) [84].
Importantly, they evaluated sequential GTKO and allogeneic skin grafts to determine whether such serial grafts could extend the period of temporary wound coverage before definitive grafting with autologous skin was carried out [9, 10]. Rejection of an initial GTKO pig skin xenograft (after 11–13 days) did not induce an accelerated immune response to a subsequent skin allograft by cross-sensitization. Furthermore, rejection of a primary skin allograft did not induce accelerated rejection of a subsequent GTKO pig skin xenograft. The demonstration that serial grafting of GTKO and allogeneic skin is possible (in either order) could potentially double the temporary skin graft coverage time in severely-burned patients, providing an extended period for healing of autologous graft donor sites or the in vitro culture of suitable autologous skin for permanent coverage [9, 10]. Moreover, definitive closure of a burn wound with an autologous graft is not adversely affected by rejection of a preceding GTKO pig xenograft or an allograft [84].
There is one additional report that is of some relevance. In a pig-to-rat skin transplantation model (in which both species express Gal antigens), Wang et al. reported that hCTLA4-Ig-transgenic porcine skin grafts exhibited significantly prolonged survival compared to wild-type pig skin grafts derived from the same pig strain (13 vs 6 days, P<0.01) [69], suggesting that hCTLA4-Ig expression in the donor pig may be an effective and safe method of extending pig skin graft survival by suppressing the cellular response.
Discussion and prospects for future clinical application of pig skin xenotransplantation
Experimental organ transplantation from genetically-engineered pigs into NHPs has made considerable progress in recent years, but has necessitated the administration of long-term exogenous immunosuppressive therapy to the recipient [35] [39] [55], which would not be clinically-warranted for patients in need of skin grafts. In contrast, wild-type (i.e., genetically-unmodified) pig heart valves have been used in cardiac surgery for valve replacement for approximately 50 years, without the need for immunosuppressive therapy [88] [89].
In recent years, however, following bioprosthetic heart valve replacement (BPHV), there has been increasing evidence that, despite glutaraldeyde-fixation of the valve (which decreases the immunogenicity of the valve, but would not be applicable to pig skin xenotransplantation as it impairs the viability of the tissues), there is a significant immune reaction to the valve. This leads to calcification and valve failure. It occurs much more rapidly in young patients, e.g., teenagers, who have a more vigorous immune system compared to the elderly. Ironically, it is young patients who would benefit most from a BPHV because, unlike a mechanical prosthetic valve, the implantation of a BPHV does not require the patient to receive long-term anticoagulant therapy. The patient is therefore not at risk from the complications associated with the life-long anticoagulation required in patients with mechanical prosthetic valves. However, because of their more vigorous immune response and such factors as increased calcium metabolism, BPHVs in young patients develop rapid structural deterioration and calcification, often within a two or three years. In the elderly, this does not occur and the BPHV might function satisfactorily for well over a decade. There is little doubt that BPHVs taken from pigs genetically-engineered to protect their tissues from the human immune response would function, even in younger patients, for considerably longer than those taken from wild-type, unmodified pigs [90] [88] [89] [91].
There is, therefore, no pig tissue that can be transplanted without a risk of immune-related deterioration or rejection, and so the problems associated with pig skin transplantation are not unique. However, the immunological disadvantages of pig skin xenotransplantation (Table 1) can, to some extent, be overcome by use of skin from the genetically-modified pigs currently available (Table 3). For example, skin transplants in NHPs using GTKO/hCD46 (or/and hCD55 or/and hCD59) pigs as sources are resistant to hyperacute rejection [9, 44, 55], and graft survival is prolonged [9, 10, 44]. Our in vitro study [62] and the in vivo study of Fujita et al [44] support this conclusion.
For even longer survival of pig skin xenografts, however, other genetic modifications of the pigs, such as expression of a costimulation blockade molecule (Figure 3) [69–71], or expression of a mutant CIITA [53, 73], hCD47 [86], HLA-G [83] and/or HLA-E [82], or knockout of SLA-Class-I [74], may be necessary.
The achievement of immunological tolerance to a pig skin xenograft (i.e., a state in which the recipient does not attempt to reject the graft) would be the ideal outcome, but will be difficult. Two major strategies are being explored [92]. (i) The concomitant transplantation of donor thymus tissue (at the time of skin transplantation) to ‘reprogram’ the T cells to regard the pig antigens as ‘self’. (ii) The induction of mixed hematopoietic cell chimerism by bone marrow or mobilized hematopoietic cell transplantation. However, it may be impossible to utilize either of these approaches in patients with burns because (a) there may be insufficient time to prepare the patient, and (b) at present relatively intense immunosuppressive induction therapy is required, which the burn patient may be unable to tolerate.
Although achieving permanent graft survival may be difficult, grafts from pigs with multiple genetic modifications may (i) reduce graft immunogenicity and (ii) increase resistance to humoral and cellular rejection to the point where no or only minimal exogenous immunosuppressive therapy is required to achieve prolonged pig skin graft survival. Even if this were for only a matter of weeks or months, this would add greatly to the successful treatment of patients with severe burns.
Highlights.
A current research summarization of skin xenotransplantation for burns
Emphasis on developments in genetic engineering of pigs to protect the graft from immunological injury
Genetically-engineered pig skin grafts now survive as long as an allograft.
Rejection of a skin xenograft is not detrimental to a subsequent allograft.
Acknowledgments
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959.
Abbreviations
- BPHV
bioprosthetic heart valve
- CTLA4-Ig
cytotoxic T lymphocyte-associated antigen 4-immunoglobulin
- CIITA
major histocompatibility complex class II transactivator
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- HLA
human leukocyte antigens
- MHC
major histocompatibility complex
- Neu5Gc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- NK
natural killer
- pAEC
pig aortic endothelial cell
- PD-1
programmed death 1
- SLA
swine leukocyte antigens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors declare any competing interests.
References
- 1.Bloemsma GC, Dokter J, Boxma H, Oen IM. Mortality and causes of death in a burn centre. Burns. 2008;34:1103–7. doi: 10.1016/j.burns.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Gibran NS, Wiechman S, Meyer W, Edelman L, Fauerbach J, Gibbons L, et al. Summary of the 2012 ABA Burn Quality Consensus conference. Journal of burn care & research : official publication of the American Burn Association. 2013;34:361–85. doi: 10.1097/BCR.0b013e31828cb249. [DOI] [PubMed] [Google Scholar]
- 3.Renz EM, King BT, Chung KK, White CE, Lundy JB, Lairet KF, et al. The US Army burn center: professional service during 10 years of war. The journal of trauma and acute care surgery. 2012;73:S409–16. doi: 10.1097/TA.0b013e318275499f. [DOI] [PubMed] [Google Scholar]
- 4.Brusselaers N, Pirayesh A, Hoeksema H, Richters CD, Verbelen J, Beele H, et al. Skin replacement in burn wounds. J Trauma. 2010;68:490–501. doi: 10.1097/TA.0b013e3181c9c074. [DOI] [PubMed] [Google Scholar]
- 5.Chiu T, Burd A. "Xenograft" dressing in the treatment of burns. Clin Dermatol. 2005;23:419–23. doi: 10.1016/j.clindermatol.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Richters CD, Hoekstra MJ, du Pont JS, Kreis RW, Kamperdijk EW. Immunology of skin transplantation. Clin Dermatol. 2005;23:338–42. doi: 10.1016/j.clindermatol.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Hermans MH. Preservation methods of allografts and their (lack of) influence on clinical results in partial thickness burns. Burns. 2011;37:873–81. doi: 10.1016/j.burns.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, et al. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19:243. doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leto Barone AA, Mastroianni M, Farkash EA, Mallard C, Albritton A, Torabi R, et al. Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns. 2015;41:565–74. doi: 10.1016/j.burns.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Albritton A, Leonard DA, Leto Barone A, Keegan J, Mallard C, Sachs DH, et al. Lack of cross-sensitization between alpha-1,3-galactosyltransferase knockout porcine and allogeneic skin grafts permits serial grafting. Transplantation. 2014;97:1209–15. doi: 10.1097/TP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke JA. HIV transmission and skin grafts. Lancet (London, England) 1987;1:983. doi: 10.1016/s0140-6736(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 12.Walter RJ, Matsuda T, Reyes HM, Walter JM, Hanumadass M. Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burns. 1998;24:104–13. doi: 10.1016/s0305-4179(97)00110-1. [DOI] [PubMed] [Google Scholar]
- 13.Aberer W, Stingl L, Pogantsch S, Stingl G. Effect of glucocorticosteroids on epidermal cell-induced immune responses. J Immunol. 1984;133:792–7. [PubMed] [Google Scholar]
- 14.Burke JF, May JW, Jr, Albright N, Quinby WC, Russell PS. Temporary skin transplantation and immunosuppression for extensive burns. The New England journal of medicine. 1974;290:269–71. doi: 10.1056/NEJM197401312900509. [DOI] [PubMed] [Google Scholar]
- 15.Achauer BM, Hewitt CW, Black KS, Martinez SE, Waxman KS, Ott RA, et al. Long-term skin allograft survival after short-term cyclosporin treatment in a patient with massive burns. Lancet (London, England) 1986;1:14–5. doi: 10.1016/s0140-6736(86)91896-9. [DOI] [PubMed] [Google Scholar]
- 16.Phillips TJ. Cultured epidermal allografts--a temporary or permanent solution? Transplantation. 1991;51:937–41. doi: 10.1097/00007890-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Aubock J, Irschick E, Romani N, Kompatscher P, Hopfl R, Herold M, et al. Rejection, after a slightly prolonged survival time, of Langerhans cell-free allogeneic cultured epidermis used for wound coverage in humans. Transplantation. 1988;45:730–7. doi: 10.1097/00007890-198804000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33:946–57. doi: 10.1016/j.burns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Wessels Q. Engineered alternative skin for partial and full-thickness burns. Bioengineered. 2014;5:161–4. doi: 10.4161/bioe.28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wurzer P, Keil H, Branski LK, Parvizi D, Clayton RP, Finnerty CC, et al. The use of skin substitutes and burn care-a survey. J Surg Res. 2016;201:293–8. doi: 10.1016/j.jss.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 21.Busby SA, Robb A, Lang S, Takeuchi Y, Vesely P, Scobie L. Antibiotic susceptibility and resistance of Staphylococcus aureus isolated from fresh porcine skin xenografts: risk to recipients with thermal injury. Burns. 2014;40:288–94. doi: 10.1016/j.burns.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Hermans MH. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference? Burns. 2014;40:408–15. doi: 10.1016/j.burns.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Cooper DK. Xenografting - the early, early years. Brit Transplant Soc Newsletter. 1997:21–2. [Google Scholar]
- 24.Switzer WE, Moncrief JA, Mills W, Jr, Order SE, Lindberg RB. The use of canine heterografts in the therapy of thermal injury. J Trauma. 1966;6:391–8. doi: 10.1097/00005373-196605000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Ge L, Sun L, Chen J, Mao X, Kong Y, Xiong F, et al. The viability change of pigskin in vitro. Burns. 2010;36:533–8. doi: 10.1016/j.burns.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Silvetti AN, Cotton C, Byrne RJ, Berrian JH, Fernandez Menendez A. Preliminary experimental studies of bovine embryo skin grafts. Transplantation bulletin. 1957;4:25–6. [PubMed] [Google Scholar]
- 27.Weiss RA. Xenografts and retroviruses. Science (New York, NY) 1999;285:1221–2. doi: 10.1126/science.285.5431.1221. [DOI] [PubMed] [Google Scholar]
- 28.Boneva RS, Folks TM. Xenotransplantation and risks of zoonotic infections. Annals of medicine. 2004;36:504–17. doi: 10.1080/07853890410018826. [DOI] [PubMed] [Google Scholar]
- 29.Kimsa-Dudek M, Strzalka-Mrozik B, Kimsa MW, Blecharz I, Gola J, Skowronek B, et al. Screening pigs for xenotransplantation: expression of porcine endogenous retroviruses in transgenic pig skin. Transgenic Res. 2015;24:529–36. doi: 10.1007/s11248-015-9871-y. [DOI] [PubMed] [Google Scholar]
- 30.Scobie L, Padler-Karavani V, Le Bas-Bernardet S, Crossan C, Blaha J, Matouskova M, et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. 2013;191:2907–15. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabtree SJ, Robertson JL, Chung KK, Renz EM, Wolf SE, Hospenthal DR, et al. Clostridium difficile infections in patients with severe burns. Burns. 2011;37:42–8. doi: 10.1016/j.burns.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–62. [PubMed] [Google Scholar]
- 33.Cooper DK. Depletion of natural antibodies in non-human primates--a step towards successful discordant xenografting in humans. Clinical transplantation. 1992;6:178–83. [PubMed] [Google Scholar]
- 34.Cooper DK, Koren E, Oriol R. Genetically engineered pigs. Lancet (London, England) 1993;342:682–3. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 35.Cooper DK, Ezzelarab MB, Hara H, Iwase H, Lee W, Wijkstrom M, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation. 2016;23:83–105. doi: 10.1111/xen.12219. [DOI] [PubMed] [Google Scholar]
- 36.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science (New York, NY) 2003;299:411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7335–40. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wijkstrom M, Iwase H, Paris W, Hara H, Ezzelarab M, Cooper DK. Renal xenotransplantation: experimental progress and clinical prospects. Kidney international. 2017;91:790–6. doi: 10.1016/j.kint.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24 doi: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML, 3rd, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nature communications. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, 3rd, Larsen CP, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–30. doi: 10.1111/xen.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. The Journal of biological chemistry. 1988;263:17755–62. [PubMed] [Google Scholar]
- 43.Cooper DK, Koren E, Oriol R. Oligosaccharides and discordant xenotransplantation. Immunological reviews. 1994;141:31–58. doi: 10.1111/j.1600-065x.1994.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 44.Fujita T, Miyagawa S, Ezoe K, Saito T, Sato N, Takahagi Y, et al. Skin graft of double transgenic pigs of N-acetylglucosaminyltransferase III (GnT-III) and DAF (CD55) genes survived in cynomolgus monkey for 31 days. Transplant immunology. 2004;13:259–64. doi: 10.1016/j.trim.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Weiner J, Yamada K, Ishikawa Y, Moran S, Etter J, Shimizu A, et al. Prolonged survival of GalT-KO swine skin on baboons. Xenotransplantation. 2010;17:147–52. doi: 10.1111/j.1399-3089.2010.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miwa Y, Kobayashi T, Nagasaka T, Liu D, Yu M, Yokoyama I, et al. Are Nglycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11:247–53. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 47.Samraj AN, Laubli H, Varki N, Varki A. Involvement of a non-human sialic Acid in human cancer. Frontiers in oncology. 2014;4:33. doi: 10.3389/fonc.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225–35. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 50.Springer SA, Diaz SL, Gagneux P. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 2014;66:671–4. doi: 10.1007/s00251-014-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91:287–92. doi: 10.1097/TP.0b013e318203c27d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Debeer S, Le Luduec JB, Kaiserlian D, Laurent P, Nicolas JF, Dubois B, et al. Comparative histology and immunohistochemistry of porcine versus human skin. European journal of dermatology : EJD. 2013;23:456–66. doi: 10.1684/ejd.2013.2060. [DOI] [PubMed] [Google Scholar]
- 53.Hara H, Witt W, Crossley T, Long C, Isse K, Fan L, et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140:39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–6. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 55.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research. The Journal of pathology. 2016;238:288–99. doi: 10.1002/path.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galili U. Induced anti-non gal antibodies in human xenograft recipients. Transplantation. 2012;93:11–6. doi: 10.1097/TP.0b013e31823be870. [DOI] [PubMed] [Google Scholar]
- 58.Lin YJ, Hara H, Tai HC, Long C, Tokita D, Yeh P, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–62. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilhite T, Ezzelarab C, Hara H, Long C, Ayares D, Cooper DK, et al. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 2012;19:56–63. doi: 10.1111/j.1399-3089.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nature reviews Immunology. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 61.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ezzelarab MB, Ayares D, Cooper DK. Transgenic expression of human CD46: does it reduce the primate T-cell response to pig endothelial cells? Xenotransplantation. 2015;22:487–9. doi: 10.1111/xen.12209. [DOI] [PubMed] [Google Scholar]
- 63.Halloran PF. Immunosuppressive drugs for kidney transplantation. The New England journal of medicine. 2004;351:2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 64.McGrath MM, Najafian N. The role of coinhibitory signaling pathways in transplantation and tolerance. Frontiers in immunology. 2012;3:47. doi: 10.3389/fimmu.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malvezzi P, Jouve T, Rostaing L. Costimulation Blockade in Kidney Transplantation: An Update. Transplantation. 2016;100:2315–23. doi: 10.1097/TP.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Archdeacon P, Dixon C, Belen O, Albrecht R, Meyer J. Summary of the US FDA approval of belatacept. Am J Transplant. 2012;12:554–62. doi: 10.1111/j.1600-6143.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- 67.Arora S, Tangirala B, Osadchuk L, Sureshkumar KK. Belatacept : a new biological agent for maintenance immunosuppression in kidney transplantation. Expert opinion on biological therapy. 2012;12:965–79. doi: 10.1517/14712598.2012.683522. [DOI] [PubMed] [Google Scholar]
- 68.Rochman Y, Yukawa M, Kartashov AV, Barski A. Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig. PloS one. 2015;10:e0122198. doi: 10.1371/journal.pone.0122198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Yang HQ, Jiang W, Fan NN, Zhao BT, Ou-Yang Z, et al. Transgenic expression of human cytoxic T-lymphocyte associated antigen4-immunoglobulin (hCTLA4Ig) by porcine skin for xenogeneic skin grafting. Transgenic Res. 2015;24:199–211. doi: 10.1007/s11248-014-9833-9. [DOI] [PubMed] [Google Scholar]
- 70.Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–85. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 71.Plege-Fleck A, Lieke T, Romermann D, Duvel H, Hundrieser J, Buermann A, et al. Pig to rat cell transplantation: reduced cellular and antibody responses to xenografts overexpressing PD-L1. Xenotransplantation. 2014;21:533–42. doi: 10.1111/xen.12121. [DOI] [PubMed] [Google Scholar]
- 72.Devaiah BN, Singer DS. CIITA and Its Dual Roles in MHC Gene Transcription. Frontiers in immunology. 2013;4:476. doi: 10.3389/fimmu.2013.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwase H, Ekser B, Satyananda V, Zhou H, Hara H, Bajona P, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transplant immunology. 2015;32:99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reyes LM, Estrada JL, Wang ZY, Blosser RJ, Smith RF, Sidner RA, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193:5751–7. doi: 10.4049/jimmunol.1402059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tena A, Kurtz J, Leonard DA, Dobrinsky JR, Terlouw SL, Mtango N, et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am J Transplant. 2014;14:2713–22. doi: 10.1111/ajt.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu X, Fukunaga A, Nagai H, Oniki S, Honma N, Ichihashi M, et al. Engagement of CD47 inhibits the contact hypersensitivity response via the suppression of motility and B7 expression by Langerhans cells. The Journal of investigative dermatology. 2006;126:797–807. doi: 10.1038/sj.jid.5700176. [DOI] [PubMed] [Google Scholar]
- 77.Kennett SB, Porter CM, Horvath-Arcidiacono JA, Bloom ET. Characterization of baboon NK cells and their xenogeneic activity. Xenotransplantation. 2010;17:288–99. doi: 10.1111/j.1399-3089.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- 78.Ito A, Shimura H, Nitahara A, Tomiyama K, Ito M, Kanekura T, et al. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. International immunology. 2008;20:1343–9. doi: 10.1093/intimm/dxn092. [DOI] [PubMed] [Google Scholar]
- 79.Seebach JD, Comrack C, Germana S, LeGuern C, Sachs DH, DerSimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159:3655–61. [PubMed] [Google Scholar]
- 80.Crew MD. Play it in E or G: utilization of HLA-E and -G in xenotransplantation. Xenotransplantation. 2007;14:198–207. doi: 10.1111/j.1399-3089.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 81.Gonzalez A, Rebmann V, LeMaoult J, Horn PA, Carosella ED, Alegre E. The immunosuppressive molecule HLA-G and its clinical implications. Critical reviews in clinical laboratory sciences. 2012;49:63–84. doi: 10.3109/10408363.2012.677947. [DOI] [PubMed] [Google Scholar]
- 82.Weiss EH, Lilienfeld BG, Muller S, Muller E, Herbach N, Kessler B, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 83.Forte P, Pazmany L, Matter-Reissmann UB, Stussi G, Schneider MK, Seebach JD. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167:6002–8. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 84.Leonard DA, Mallard C, Albritton A, Torabi R, Mastroianni M, Sachs DH, et al. Skin grafts from genetically modified alpha-1,3-galactosyltransferase knockout miniature swine: A functional equivalent to allografts. Burns. 2017 doi: 10.1016/j.burns.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gojo S, Shimizu A, Ierino FL, Banerjee PT, Cooper DK, LeGuern C, et al. Xenogeneic and allogeneic skin grafting after retrovirus-mediated SLA class II DR gene transfer in baboons. Transplant Proc. 2000;32:289–90. doi: 10.1016/s0041-1345(99)00960-4. [DOI] [PubMed] [Google Scholar]
- 86.Tena AA, Sachs DH, Mallard C, Yang YG, Tasaki M, Farkash E, et al. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation. 2017;101:316–21. doi: 10.1097/TP.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cooper DK. Xenoantigens and xenoantibodies. Xenotransplantation. 1998;5:6–17. doi: 10.1111/j.1399-3089.1998.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 88.Manji RA, Ekser B, Menkis AH, Cooper DK. Bioprosthetic heart valves of the future. Xenotransplantation. 2014;21:1–10. doi: 10.1111/xen.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manji RA, Lee W, Cooper DKC. Xenograft bioprosthetic heart valves: Past, present and future. International journal of surgery (London, England) 2015;23:280–4. doi: 10.1016/j.ijsu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 90.Cooper DK. How important is the anti-Gal antibody response following the implantation of a porcine bioprosthesis? The Journal of heart valve disease. 2009;18:671–2. [PubMed] [Google Scholar]
- 91.Lee W, Long C, Ramsoondar J, Ayares D, Cooper DK, Manji RA, et al. Human antibody recognition of xenogeneic antigens (NeuGc and Gal) on porcine heart valves: could genetically modified pig heart valves reduce structural valve deterioration? Xenotransplantation. 2016;23:370–80. doi: 10.1111/xen.12254. [DOI] [PubMed] [Google Scholar]
- 92.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunological reviews. 2014;258:241–58. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]