Abstract
Acetate is a potential low-cost carbon source that can be generated by biological and chemical processes. In this study, deletion of sdhAB encoding succinate dehydrogenase, iclR encoding the isocitrate lyase regulator, and maeB encoding the malic enzyme, and overexpression of acs encoding acetyl-CoA synthetase, gltA encoding citrate synthase, and acnB encoding aconitate hydratase in the wild-type Escherichia coli MG1655 strain yielded the recombinant E. coli strain WCY-7, which could synthesize succinate from acetate. After 48 h batch fermentation, this strain accumulated 11.23 mM succinate from 50 mM sodium acetate. This work indicates that microbial fermentation using acetate as the sole carbon source may be a suitable route to produce high yields of the valuable chemicals.
Keywords: Succinate, Acetate, Metabolic engineering, Escherichia coli
Introduction
Succinate is an important four-carbon platform compound that is used widely in the food, chemical, and pharmaceutical industries (Ahn et al. 2016). Reports show that approximately USD$15 billion worth of bulk chemicals can be synthesized from succinate (Zeikus et al. 1999; Huang et al. 2018). Succinate has also been identified as one of the top 12 building block chemicals by the US Department of Energy (Bozell and Petersen 2010).
Traditionally, succinate is produced by chemical synthesis from maleic acid, 1,4-butanediol, or ethylene glycol (Li et al. 2016). However, depletion of fossil feed stocks and environmental risks make chemical methods unsustainable. Accordingly, microbial fermentation from renewable carbohydrates has received much greater attention recently. In addition, CO2, a primary greenhouse gas, can be fixed during the biosynthesis of succinate. As natural succinate producers, Mannheimia succiniciproducens, Actinobacillus succinogenes, and Anaerobiospirillum succiniciproducens can accumulate succinate as a major fermentation product (Song et al. 2008; Li et al. 2010; Lee et al. 2001). However, complex nutritional conditions are indispensable for culturing these bacteria, thereby limiting their application. With clear genetic background, convenient engineering tools and fast growth in cheap media, Escherichia coli has been selected and engineered for succinate production by several groups (Li et al. 2013, 2017; Zhang et al. 2018). Glucose is mainly selected as the carbon source when batch or fed-batch fermentation is performed for succinate producers. In addition, glycerol, xylose, or mix-sugars can also be used, but these are rarely chosen.
Acetate is one of the most used organic acids, and is generated by chemical processes and bacterial fermentation (Pal and Nayak 2016). In addition, acetate can also be obtained by anaerobic fermentation of waste organic materials such as residual sludge of municipal wastewater and cellulosic biomass hydrolysate (Bhatia and Yang 2017; Wu et al. 2016). Because acetate is available in abundance and common industrial microbes including E. coli; Corynebacterium glutamicum and Saccharomyces cerevisiae can use acetate as a carbon source; many valuable chemical compounds, such as polyhydroxyalkanoates, mevalonate, and fatty acids, have been produced from acetate (Xiao et al. 2013; Chen et al. 2018; Xu et al. 2018).
In this study, to realize the production of succinate from acetate, the optimal concentration of acetate was initially explored. The biosynthetic pathway of succinate from acetate in the E. coli MG1655 strain was then modified, which involved the inhibition of the tricarboxylic acid (TCA) cycle, increasing the assimilation of acetate, activation of the glyoxylate cycle, and reducing the flux of the gluconeogenesis pathway. Finally, acetate utilization and succinate production from the resulting engineered WCY-7 strain were determined in batch fermentation.
Materials and methods
Bacterial strains and construction of plasmids
All strains, plasmids, and oligonucleotides used in this study are listed in Tables 1, 2 and 3, respectively. E. coli MG1655 was selected for the construction of succinate producing strains. E. coli DH5α was selected as a base strain for the construction of recombinant plasmids. The acs gene, encoding acetyl-CoA synthetase, was inserted into the BamHI/HindIII sites of plasmids pCL1920 and pTrc99a, respectively, to explore the optimal expression level of this synthetase. Primers acs-FF and acs-FR were used for ligating acs into pCL1920 to generate plasmid pW-1. Primers acs-RF and acs-RR were used for ligating acs into pTrc99a to yield plasmid pW-2. Subsequently, gltA encoding citrate synthase and acnB encoding conitate hydratase were amplified by primer pairs gltA-20ZF/gltA-20ZR and acnB-20ZF/acnB-20ZR, respectively, and these two PCR products with 30–50 homologous arms were assembled and inserted into the SmaI site of pW-1 to generate plasmid pW-3 by the enzymatic assembly strategy (Gibson et al. 2009). Similarly, to insert these two genes into the SmaI site of pW-2, primer pairs gltA-99ZF/gltA-99ZR and acnB-99ZF/acnB-99ZR were employed, and the same enzymatic assembly strategy was used to yield recombinant plasmid pW-4.
Table 1.
Strains used in this study
| Name | Relevant genotype | References |
|---|---|---|
| DH5α | F −, endA1, hsdR17 (rK−, mK+), supE44, thi-l, λ−, recA1, gyrA96, ΔlacU169 (Φ80lacZ ΔM15) | Lab stock |
| MG1655 | F −, λ−, rph-1 | Lab stock |
| WCY-1 | MG1655 (ΔiclR) | This study |
| WCY-2 | MG1655 (ΔiclRΔsdhAB) | This study |
| WCY-3 | MG1655 (ΔiclRΔsdhABΔmaeB) | This study |
| WCY-4 | WCY-3/pW-1 | This study |
| WCY-5 | WCY-3/pW-2 | This study |
| WCY-6 | WCY-3/pW-3 | This study |
| WCY-7 | WCY-3/pW-4 | This study |
| QZ1111 | MG1655(ΔsdhAΔptsGΔpoxBΔptaΔiclR::kan) | (Kang et al. 2010) |
| WQ-1 | BW25113(ΔsdhAB::kan) | Lab stock |
Table 2.
Plasmids used in this study
| Name | Relevant genotype | References |
|---|---|---|
| pKD3 | bla, FRT-cat-FRT | Datsenko and Wanner (2000) |
| pKD46 | bla, helper plasmid | Datsenko and Wanner (2000) |
| pCP20 | bla and cat, helper plasmid | Cherepanov and Wackernagel (1995) |
| pCL1920 | SpcR | Lerner and Inouye (1990) |
| pTrc99a | bla | Amann et al. (1988) |
| pW-1 | pCL1920-acs | This study |
| pW-2 | pTrc99a-acs | This study |
| pW-3 | pCL1920-acs-gltA-acnB | This study |
| pW-4 | pTrc99a-acs- gltA-acnB | This study |
Table 3.
Primers used in this study
| Name | Sequence (5′–3′) |
|---|---|
| acs-FF | CCCAAGCTTAAGGAGATATACCATGAGCCAAATTCACAAACACACCA |
| acs-FR | CGCGGATCCTTACGATGGCATCGCGATAGCCTGCTTCTCT |
| acs-RF | CGCGGATCCAAGGAGATATACCATGAGCCAAATTCACAAACACACCA |
| acs-RR | CCCAAGCTTTTACGATGGCATCGCGATAGCCTGCTTCTCT |
| gltA-99ZF | CACACAGGAAACAGACCATGGAATTCGAGCTCGGTACCCAAGGAGATATACATATGGCTGATACAAAAGCAAAACTC |
| gltA-99ZR | GGCACGCTCAGCTACGTGCTTACGGTATTCTTCTAGCACATGTATATCTCCTTTTAACGCTTGATATCGCTTTTAAAGT |
| acnB-99ZF | TATGAAAAACGCGACTTTAAAAGCGATATCAAGCGTTAAAAGGAGATATACATGTGCTAGAAGAATACCGTAAGCACGT |
| acnB-99ZR | CAAGCTTGCATGCCTGCAGGTCGACTCTAGAGGATCCCCTTAAACCGCAGTCTGGAAAATCACCCC |
| gltA-20ZF | CAAGCTTGCATGCCTGCAGGTCGACTCTAGAGGATCCCCAAGGAGATATACATATGGCTGATACAAAAGCAAAACTC |
| gltA-20ZR | GGCACGCTCAGCTACGTGCTTACGGTATTCTTCTAGCACATGTATATCTCCTTTTAACGCTTGATATCGCTTTTAAAGT |
| acnB-20ZF | CAAGCTTGCATGCCTGCAGGTCGACTCTAGAGGATCCCCAAGGAGATATACATATGGCTGATACAAAAGCAAAACTC |
| acnB-20ZR | GTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTACCCTTAAACCGCAGTCTGGAAAATCACCCC |
| iclR-F | AATGGTCGTGGAGTTGAAGGTGTTGGTTT |
| iclR-R | GAAAGAACTACGAGGAATACGAGTAATCAT |
| sdhAB-F | TTCACTTCTCGCAGGAGTCCTCGTATGGT |
| sdhAB -R | GCTGTTCTGCATCGTGATCCCTTAA |
| maeB-F | ATGGATGACCAGTTAAAACAAAGTGCACTTGATTTCCATGAATTTCCAGTTCCAGGGAA GTGTAGGCTGGAGCTGCTTC |
| maeB-R | TTACAGCGGTTGGGTTTGCGCTTCTACCACGGCCAGCGCCACCATGTTGACGATACGAC ATGGGAATTAGCCATGGTCC |
| maeB-JF | TGTTTGATGCCGTCTAACTCGT |
| maeB-JR | GATTTTCTTCGCCAGTTCCTCACCG |
Gene deletion
The three genes, iclR, sdhAB, and maeB, which encode the isocitrate lyase regulator, succinate dehydrogenase and malic enzyme, respectively, were knocked out in the MG1655 strain sequentially to obtain strains WCY-1, WCY-2, and WCY-3. The one-step inactivation method was used to remove the maeB gene (Datsenko and Wanner 2000). Primers maeB-F and maeB-R and the template plasmid pKD3 were used to obtain linearized DNA flanked by FLP recognition target sites and homologous sequences for maeB deletion. The other two genes, iclR and sdhAB, were knocked out by linearized DNA fragments with an extending homologous sequence (Li et al. 2012). Primer pairs iclR-F/iclR-R and sdhAB-F/sdhAB-R and chromosomal DNA of strains QZ1111 and WQ-1 were used to amplify the linearized DNA fragments of iclR and sdhAB, separately. The PCR products were purified and electroporated into electrocompetent strains containing the pKD46 plasmid. Transformant cells were selected in solid Luria–Bertani (LB) medium-containing chloramphenicol (17 mg/L) or kanamycin (25 mg/L). Candidate clones were screened by PCR using iclR-F/iclR-R, sdhAB-F/sdhAB-R and maeB-JF/maeB-JR, separately. The chloramphenicol or kanamycin cassette was removed with the helper plasmid pCP20. Strain WCY-3 was transformed with pW-1, pW-2, pW-3, and pW-4 to generate strains WCY-4, WCY-5, WCY-6, and WCY-7, respectively. All primers were synthesized by TSINGKE Biological Technology (Qingdao, China).
Growth conditions
Strains for cloning and inoculums were grown in LB medium (1% tryptone, 0.5% yeast extract, and 1% NaCl) at 37 °C for 8–12 h supplemented with appropriate antibiotics [ampicillin (100 mg/L), chloramphenicol (17 mg/L), kanamycin (25 mg/L), and spectinomycin (50 mg/L)] as required. For batch fermentation, SMAC medium was used, and 50 mM sodium acetate and 2 g/L of yeast extract were added (Li et al. 2016). A single clone was pre-cultured in 5 mL LB medium at 37 °C with 250 rpm shaking overnight. One milliliter of the overnight cells was inoculated into 50 mL SMAC medium for batch fermentation, and flasks were incubated at 37 °C with 250 rpm shaking. Isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to the culture at a final concentration of 0.2 mM.
Analytical methods
For analyzing succinate and acetate, high-performance liquid chromatography (Thermo Fisher Scientific, USA) equipped with a column of Aminex HPX-87H ion exclusion particles was employed (300 × 7.8 mm, Bio-Rad, Hercules, CA, USA). Samples were centrifuged at 12,000 rpm for 5 min and then filtrated with a 0.22 µm aqueous membrane. The mobile phase was 5 mM sulfuric acid (in Milli-Q water) with a flow rate of 0.6 mL/min and the column was maintained at 65 °C.
Results and discussion
Pathway engineering for succinate production in wild-type E. coli
In wild-type E. coli, succinate is an important intermediate involved in the TCA cycle and cannot be accumulated (Fig. 1). To overcome this feature, sdhAB encoding succinate dehydrogenase was initially deleted in MG1655 to generate WCY-1. As a result, the glyoxylate shunt probably became the main succinate biosynthesis pathway. The iclR gene was then deleted to release the repression of the aceBAK operon and this procedure yielded strain WCY-2. To further avoid carbon flux directed into gluconeogenesis, the gene maeB in strain WCY-2 was deleted to give strain WCY-3. As shown in Table 4, the succinate production of the recombinant strain increased to 4.62 mM with target genes knocking out, whereas no succinate was detected for the wild-type MG1655 strain.
Fig. 1.
Metabolic pathways related to acetate assimilation and succinate production
Table 4.
Development of constructed E. coli strains and their yield of succinate titer
| Strains | Succinate titer (mM) |
|---|---|
| MG1655 | Na |
| WCY-1 | 1.68 ± 0.13 |
| WCY-2 | 2.04 ± 0.23 |
| WCY-3 | 4.62 ± 0.34 |
| WCY-4 | 5.02 ± 0.28 |
| WCY-5 | 5.95 ± 0.34 |
| WCY-6 | 9.87 ± 1.43 |
| WCY-7 | 11.23 ± 1.23 |
The succinate titer was the final production obtained in batch fermentation using 50 mM sodium acetate as the sole carbon source
aNot detected
Optimization of acetate utilization
The acetate metabolism pathways of E. coli have been elucidated previously. When excess glucose is provided to E. coli, a metabolic imbalance of glycolysis and the TCA cycle occurs, and acetyl-coenzyme A is directed into the biosynthetic pathway of byproducts, including acetate (Han et al. 1992; Majewski and Domach 1990). Acetate can be used as a primary carbon source during glucose starvation (Lin et al. 2006). In E. coli, acetate is initially converted into acetyl-CoA via the acetyl-CoA synthetase (Acs) pathway or phosphotransacetylase/acetate kinase (Pta-AckA) pathway (Fig. 1). Acetyl-CoA can then be directed into the TCA cycle and glyoxylate cycle, providing energy and precursors for cell growth. In a previous report, the Acs pathway was found to be more effective than the Pta–AckA pathway (Xiao et al. 2013). Accordingly, to increase assimilation of acetate, the acs gene was ligated into two plasmids, pCL1920 and pTrc99a, with copy numbers of 5 and 15–20, respectively.
Exploration of the optimal acetate concentration
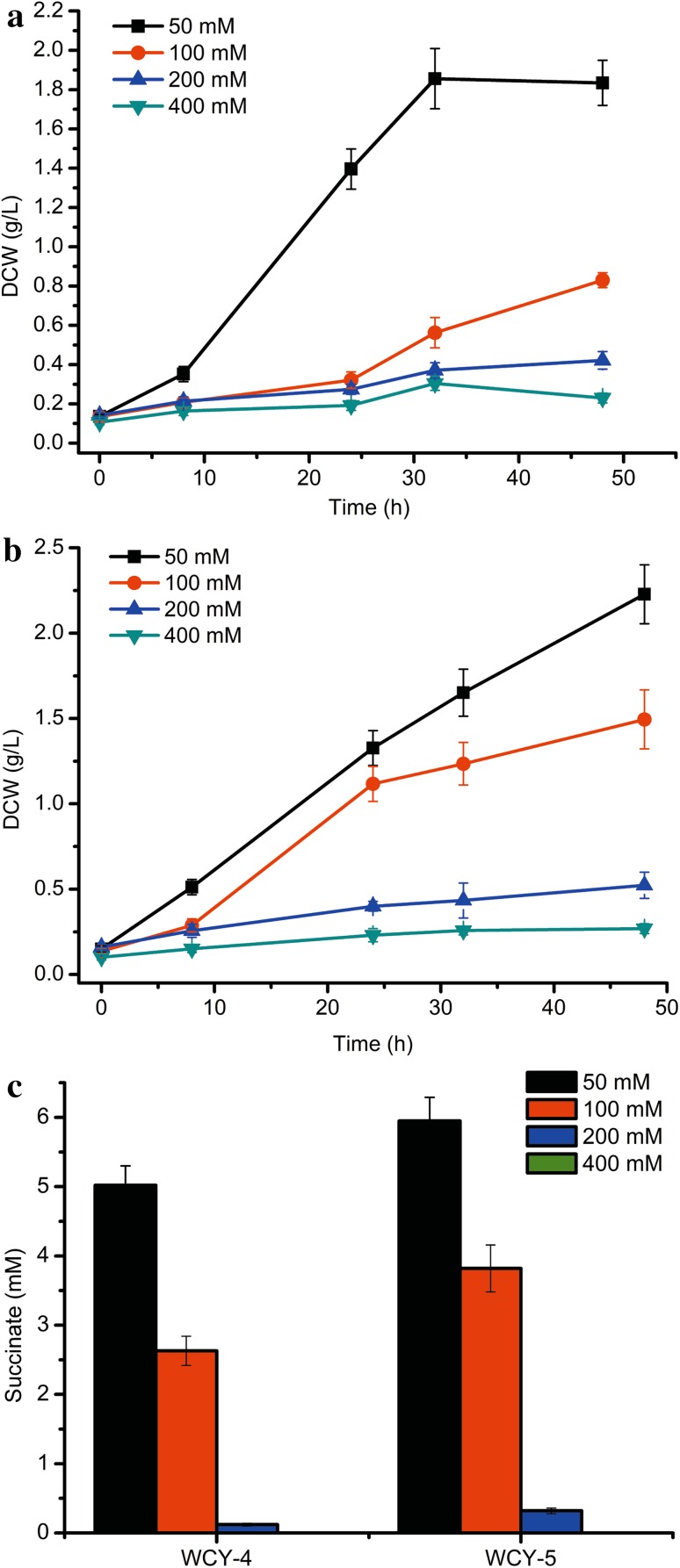
Acetate is toxic to E. coli and inhibits cell growth. Thus, the acetate concentration was optimized for strains WCY-4 and WCY-5, and the results are presented in Fig. 2. Both strains exhibited normal growth when 50 mM acetate was supplemented into the medium. For WCY-4, increasing the concentration of acetate to 100 mM inhibited cell growth as indicated by the longer lag phase and reduced dry cell weight (DCW 1.33 vs. 1.83 g/L). In contrast, no obvious lag period was observed for WCY-5 at 100 mM acetate, despite the DCW also decreasing (1.79 vs. 2.23 g/L) when compared with the results obtained at an acetate concentration of 50 mM. Because the difference between WCY-4 and WCY-5 was only the plasmid copy number, it is plausible that increasing the expression level of Acs is a benefit for strain survival at higher concentrations of acetate. Further increasing the acetate concentration to 200 and 400 mM almost completely inhibited the growth of both strains, and the final DCW values were below 0.6 g/L after 48 h batch fermentation. Nevertheless, the DCW values of WCY-5 were higher than those of WCY-4 at the same concentration of acetate. In addition, the accumulation of succinate by WCY-4 and WCY-5 at different acetate concentrations was also measured. For WCY-4, the succinate titer achieved the highest level of 5.02 mM when 50 mM acetate was supplemented. Increasing the acetate concentration further resulted in a clear decrease of succinate production. When 400 mM acetate was used, no succinate accumulation was detected. The trend for succinate production by WCY-5 was like that of WCY-4, except that WCY-5 exhibited a higher succinate titer when compared with that of WCY-4 at any concentration of acetate examined. As both strains showed the best combination of DCW and succinate titer at 50 mM, this concentration was selected for further experiments.
Fig. 2.
Growth and succinate titer of WCY-4 and WCY-5 at different concentrations of acetate. a DCW of WCY-4, b DCW of WCY-5, and c succinate titer of WCY-4 and WCY-5. The error bars represent standard deviations from three replicate fermentations
Increasing the carbon flux of the TCA cycle
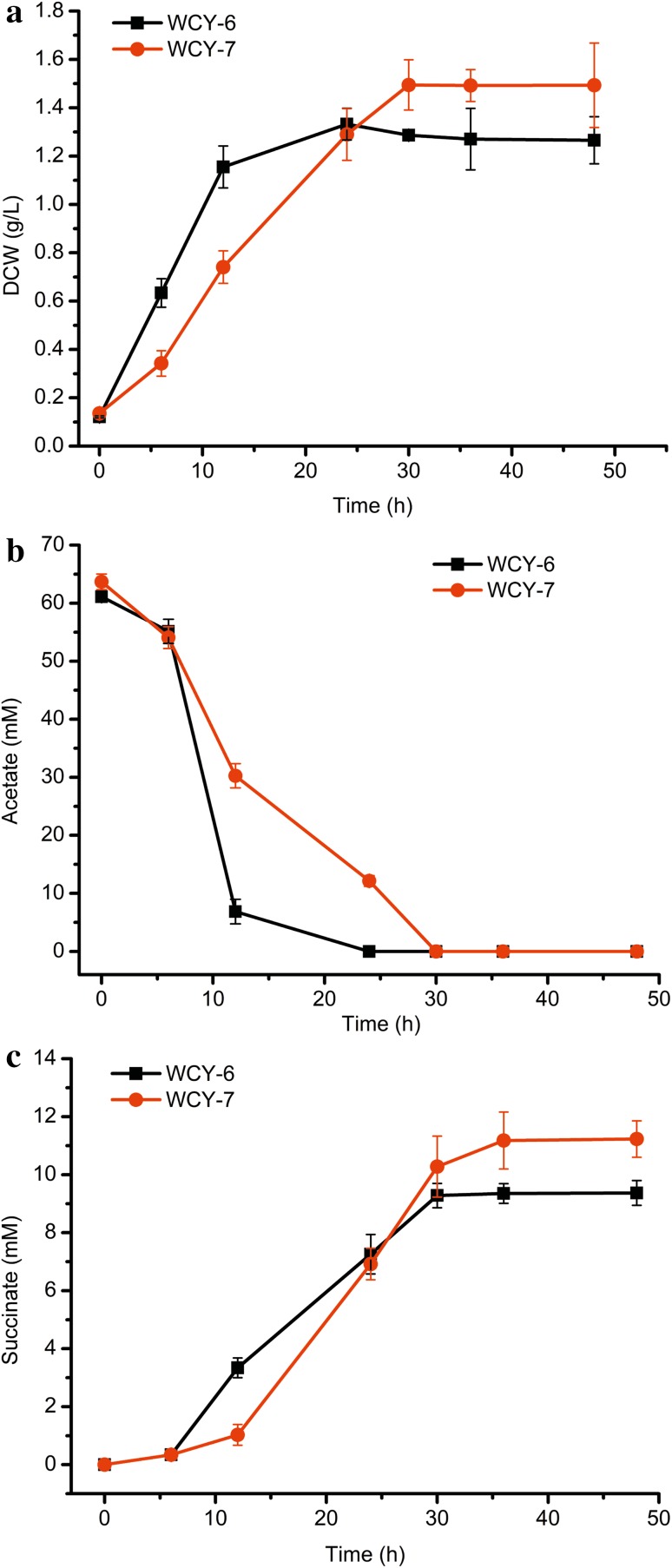
Considering succinate is an intermediate of the TCA cycle, increasing the total flux of the TCA cycle may be advantageous for the accumulation of succinate. Accordingly, the genes encoding citrate synthase (gltA) and aconitate hydratase (acnB) were cloned into pW-1 and pW-2, respectively, to generate pW-3 and pW-4. Batch fermentation of the resulting transformed strains, WCY-6 and WCY-7, was then performed. As shown in Fig. 3, WCY-6 exhibited faster growth than WCY-7 before 24 h. Since the copy number of pTrc99a is greater than that of pCL1920, the metabolic burden generated by the precursors and the energy supply for the recombinant plasmid were larger in WCY-7, which may lead to a longer lag period. However, the DCW at 48 h for WCY-7 was higher than that of WCY-6. Consistent with the growth curve, the acetate consumption of WCY-6 was faster than that observed for WCY-7 before 12 h. For both strains, 50 mM acetate was completely utilized after 30 h batch cultivation. In addition, succinate accumulation was clearly observed for both strains. WCY-6 produced 9.37 mM succinate after 48 h batch fermentation. Although WCY-7 exhibited slower succinate accumulation that correlated with the observed slower growth and acetate assimilation, the final titer of succinate reached 11.23 mM, which is 19.8% higher than that of WCY-6. In 2015, Li et al. constructed a recombinant E. coli MG03 strain that accumulated 16.45 mM succinate from 5 g/L sodium acetate (Li et al. 2016). The final succinate titer of MG03 was higher than that of WCY-7, but the total cultivation time was longer. Comparison of succinate production at 48 h reveals that WCY-7 produces slightly more succinate than the MG03 strain.
Fig. 3.
Batch fermentation results for strains WCY-6 and WCY-7. a Profiles of DCW, b acetate consumption, and c succinate production for WCY-6 and WCY-7 in batch fermentation. The error bars represent standard deviations from three replicate fermentations
When acetate is used as the carbon source, the energy produced may not be enough to maintain cell growth and product synthesis when compared with cell growth using glucose as the carbon source. Thus, ameliorating the potential energy supply shortfall represents a suitable strategy for the next modification to WCY-7. The glyoxylate cycle is preferred for succinate synthesis in WCY-7, because this cycle has a shorter synthetic pathway from isocitrate. In future efforts, deletion of icd encoding isocitrate dehydrogenase and overexpression of aceA encoding isocitrate lyase may further improve the succinate production of WCY-7. In addition, the relatively low titer of succinate resulted partly from an insufficient supply of acetate. However, as shown in Fig. 2, growth was seriously compromised when the acetate concentration was greater than 100 mM. By employing laboratory metabolic evolution, Sandoval et al. obtained an acetate tolerant E. coli strain that was able to grow and produce a high yield of ethanol in the presence of 40 g/L of sodium acetate (Fernandez-Sandoval et al. 2012). Using metabolic evolution in future efforts with the WCY-3 strain may be a route to enable the use of higher initial acetate concentrations in the culture medium.
Conclusions
In this study, a recombinant E. coli WCY-7 strain that produced 11.23 mM succinate from 50 mM sodium acetate was constructed. Although the current titer of succinate is below the practical level for commercial use, the WCY-7 strain provides a platform for future efforts to engineer an E. coli strain that has improved tolerance toward acetate and supply of energy. Because inexpensive acetate can be obtained readily and in high quantities from chemical industries and microbial fermentation, other high value-added chemicals can also be potentially produced using acetate as the carbon source.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31600066, 31100088), the Shandong Provincial Natural Science Foundation (ZR2016CB20, ZR2016CL02), State Key Laboratory of Microbial Technology Open Projects Fund (M2016-10), and the Shandong Province Science and Technology Development Plan (2013GSF12006).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Ahn JH, Jang YS, Lee SY. Production of succinic acid by metabolically engineered microorganisms. Curr Opin Biotechnol. 2016;42:54–66. doi: 10.1016/j.copbio.2016.02.034. [DOI] [PubMed] [Google Scholar]
- Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Yang YH. Microbial production of volatile fatty acids: current status and future perspectives. Rev Environ Sci Biotechnol. 2017;16(6):1–19. [Google Scholar]
- Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010;4:539–554. doi: 10.1039/b922014c. [DOI] [Google Scholar]
- Chen J, Li W, Zhang ZZ, Tan TW, Li ZJ. Metabolic engineering of Escherichia coli for the synthesis of polyhydroxyalkanoates using acetate as a main carbon source. Microb Cell Fact. 2018;17(1):102. doi: 10.1186/s12934-018-0949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158(1):9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sandoval MT, Huerta-Beristain G, Trujillo-Martinez B, Bustos P, Gonzalez V, Bolivar F, Gosset G, Martinez A. Laboratory metabolic evolution improves acetate tolerance and growth on acetate of ethanologenic Escherichia coli under non-aerated conditions in glucose-mineral medium. Appl Microbiol Biotechnol. 2012;96(5):1291–1300. doi: 10.1007/s00253-012-4177-y. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Han K, Lim HC, Hong J. Acetic acid formation in Escherichia coli fermentation. Biotechnol Bioeng. 1992;39(6):663–671. doi: 10.1002/bit.260390611. [DOI] [PubMed] [Google Scholar]
- Huang JF, Zhang B, Shen ZY, Liu ZQ, Zheng YG. Metabolic engineering of E. coli for the production of O-succinyl-l-homoserine with high yield. 3 Biotech. 2018;8(7):310. doi: 10.1007/s13205-018-1332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, Gao C, Wang Q, Liu H, Qi Q. A novel strategy for succinate and polyhydroxybutyrate co-production in Escherichia coli. Bioresour Technol. 2010;101(19):7675–7678. doi: 10.1016/j.biortech.2010.04.084. [DOI] [PubMed] [Google Scholar]
- Lee PC, Lee WG, Lee SY, Chang HN. Succinic acid production with reduced by-product formation in the fermentation of Anaerobiospirillum succiniciproducens using glycerol as a carbon source. Biotechnol Bioeng. 2001;72(1):41–48. doi: 10.1002/1097-0290(20010105)72:1<41::AID-BIT6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lerner CG, Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990;18(15):4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yang M, Wang D, Li W, Wu Y, Zhang Y, Xing J, Su Z. Efficient conversion of crop stalk wastes into succinic acid production by Actinobacillus succinogenes. Bioresour Technol. 2010;101(9):3292–3294. doi: 10.1016/j.biortech.2009.12.064. [DOI] [PubMed] [Google Scholar]
- Li M, Gu P, Kang J, Wang Y, Wang Q, Qi Q. Extending homologous sequence based on the single gene mutants by one-step PCR for efficient multiple gene knockouts. Folia Microbiol (Praha) 2012;57(3):209–214. doi: 10.1007/s12223-012-0111-z. [DOI] [PubMed] [Google Scholar]
- Li Y, Li M, Zhang X, Yang P, Liang Q, Qi Q. A novel whole-phase succinate fermentation strategy with high volumetric productivity in engineered Escherichia coli. Bioresour Technol. 2013;149:333–340. doi: 10.1016/j.biortech.2013.09.077. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang B, Wu H, Li Z, Ye Q, Zhang YP. Production of succinate from acetate by metabolically engineered Escherichia coli. ACS Synth Biol. 2016;5(11):1299–1307. doi: 10.1021/acssynbio.6b00052. [DOI] [PubMed] [Google Scholar]
- Li J, Li Y, Cui Z, Liang Q, Qi Q. Enhancement of succinate yield by manipulating NADH/NAD + ratio and ATP generation. Appl Microbiol Biotechnol. 2017;101(8):3153–3161. doi: 10.1007/s00253-017-8127-6. [DOI] [PubMed] [Google Scholar]
- Lin H, Castro NM, Bennett GN, San KY. Acetyl-CoA synthetase overexpression in Escherichia coli demonstrates more efficient acetate assimilation and lower acetate accumulation: a potential tool in metabolic engineering. Appl Microbiol Biotechnol. 2006;71(6):870–874. doi: 10.1007/s00253-005-0230-4. [DOI] [PubMed] [Google Scholar]
- Majewski RA, Domach MM. Simple constrained-optimization view of acetate overflow in E. coli. Biotechnol Bioeng. 1990;35(7):732–738. doi: 10.1002/bit.260350711. [DOI] [PubMed] [Google Scholar]
- Pal P, Nayak J. Acetic acid production and purification: critical review towards process intensification. Sep Purif Method. 2016;46(1):44–61. doi: 10.1080/15422119.2016.1185017. [DOI] [Google Scholar]
- Song H, Kim TY, Choi BK, Choi SJ, Nielsen LK, Chang HN, Lee SY. Development of chemically defined medium for Mannheimia succiniciproducens based on its genome sequence. Appl Microbiol Biotechnol. 2008;79(2):263–272. doi: 10.1007/s00253-008-1425-2. [DOI] [PubMed] [Google Scholar]
- Wu QL, Guo WQ, Zheng HS, Luo HC, Feng XC, Yin RL, Ren NQ. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: the mechanism and microbial community analyses. Bioresour Technol. 2016;216:653–660. doi: 10.1016/j.biortech.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Ruan Z, Liu Z, Wu SG, Varman AM, Liu Y, Tang YJ. Engineering Escherichia coli to convert acetic acid to free fatty acids. Biochem Eng J. 2013;76(28):60–69. doi: 10.1016/j.bej.2013.04.013. [DOI] [Google Scholar]
- Xu X, Xie M, Zhao Q, Xian M, Liu H. Microbial production of mevalonate by recombinant Escherichia coli using acetic acid as a carbon source. Bioengineered. 2018;9(1):116–123. doi: 10.1080/21655979.2017.1323592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus JG, Jain MK, Elankovan P. Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol. 1999;51(5):545–552. doi: 10.1007/s002530051431. [DOI] [Google Scholar]
- Zhang W, Zhu J, Zhu X, Song M, Zhang T, Xin F, Dong W, Ma J, Jiang M. Expression of global regulator IrrE for improved succinate production under high salt stress by Escherichia coli. Bioresour Technol. 2018;254:151–156. doi: 10.1016/j.biortech.2018.01.091. [DOI] [PubMed] [Google Scholar]