Abstract
Background:
Left atrium (LA) physiology is influenced by changes in left ventricular (LV) performance and load.
Objectives:
To define the impact of acute changes in LV loading conditions on LA physiology in subacute myocardial infarction (MI).
Methods:
MI was percutaneously induced in 19 Yorkshire pigs. One to 2 weeks after MI, 14 pigs underwent acute LV unloading using a percutaneous LV assist device, Impella. The remaining 5 pigs underwent acute LV loading by percutaneous induction of aortic regurgitation. A pressure-volume catheter was inserted into the LA using a percutaneous trans-septal approach, and LA pressure-volume loops were continuously monitored. Atrial arrhythmia inducibility was examined by burst-pacing of the right atrium. NADPH oxidase (NOX) levels and ryanodine receptor phosphorylation were examined in LA tissues to study the potential impact of stretch-dependent oxidative stress.
Results:
MI resulted in reduced LV ejection fraction and increased LV end-diastolic pressure (EDP) with concomitant increase in LA pressure and volumes. Acute LV unloading resulted in a reduction of LVEDP, which led to proportional decreases in mean LA pressure and maximum LA volume. LA pressure-volume loops exhibited a flow-dependent left-downward shift with the device. This was associated with reduced LA passive stiffness suggesting the alleviation of the LA stretch that was present after MI. Prior to acute unloading of the LV, 71% of the pigs were arrhythmia-inducible; LV unloading reduced this to 29% (p = 0.02). Time to spontaneous termination of atrial arrhythmias was decreased from 55 sec (median, range: 5–300) to 3 sec (range: 0–59). In contrast, LV loading increased LA pressure without significant impacts on arrhythmogenicity. Molecular analysis of LA tissue revealed that NOX2 expression was increased after MI, whereas LV unloading reduced NOX2 levels and diminished ryanodine receptor phosphorylation.
Conclusions:
Acute LV unloading relieves LA stretch and reduces atrial arrhythmogenicity in subacute MI.
Keywords: LA load, percutaneous, LA P-V loop, atrial arrhythmia, stretch
Condensed Abstract:
Impact of acute changes in LV loading condition on LA physiology was examined in a pig model of subacute MI. Closed-chest LA pressure-volume assessment revealed a left-downward shift of LA pressure-volume loops with LV unloading using a percutaneous cardiac assist device. LA passive stiffness was reduced, suggesting an alleviation of LA stretch that was present after MI. LV unloading also reduced arrhythmia inducibility and maintenance. LA tissue analysis exhibited reduced NOX2 expression after LV unloading suggesting an inhibition of stretch-dependent oxidative stress as a possible mechanism. Our data demonstrate that acute LV unloading significantly improves LA physiology in a pig subacute MI model.
Introduction
The negative consequences of acute myocardial infarction (MI) include decreased left ventricular (LV) function, pulmonary congestion, and increased arrhythmogenesis. Located upstream of the LV, the left atrium (LA) has unique roles including as a reservoir, a pump, and supra-ventricular electrical conduction. Acute MI leads to hemodynamic derangements that result in pressure-volume overload of not only the LV, but also the LA leading to myocardial stretch of both chambers. LA stretch makes it difficult to expand further, and can lead to congestion of the lung through impaired reservoir function. Furthermore, stretch provides an arrhythmogenic substrate in the setting of ischemia (1). Atrial tachycardia and fibrillation (AT and AF respectively) are commonly observed in patients after acute MI and are associated with increased morbidity and mortality. Indeed, recent data shows new onset AF after acute coronary syndrome is associated with a 4.4-fold increase in in-hospital mortality (2).
Recently, percutaneous LV assist devices (pLVADs) have emerged as powerful options for managing post-MI patients (3,4), particularly those with cardiogenic shock. Clinical and preclinical studies show superior hemodynamic improvements over the intra-aortic balloon pump in this patient population (5–7), which has been the gold standard for nearly half a century in managing MI patients who require hemodynamic support. Acute LV unloading by pLVADs is well-known to decrease wall stress by decreasing LV volume and pressure (8–11). Because the LA is hemodynamically linked with LV performance and load (12), unloading of the LV is expected to passively influence the LA, potentially decreasing its stretch and modifying arrhythmia propensity. However, no systematic studies exist that directly investigate this phenomenon, and little is known about the hemodynamics and physiological effects of changes in LV load on LA. Therefore, we investigated the effects of LV unloading on the hemodynamic status of the LA and the ability of LV unloading to modify stretch-dependent AF in the setting of ischemia. We hypothesized that LV unloading using an LV-to-aorta hemodynamic support device will modulate the LV-LA pressure gradient, reduce LA pressure, and inhibit LA arrhythmogenesis in the pathologically stretched LA in the post-MI setting. To inquire further into the role of LV load on the LA in subacute MI setting, we also examined the effects of increased LV load by percutaneous induction of aortic regurgitation. This is the first study to directly quantify LA pressure-volume relationships in vivo in a closed-chest setting and to investigate the impact of LV load in ischemic heart failure.
Methods
Experimental protocol
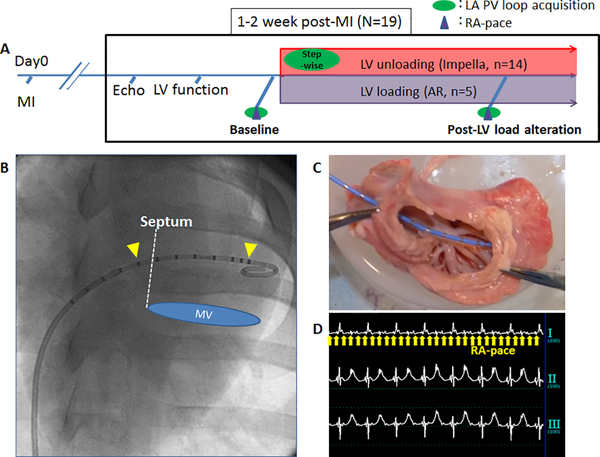
The experimental protocols involving animals complied with the Guide for the Care and Use of Laboratory Animals regulations and US regulatory agencies. Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee approved the study. A total of 19 Yorkshire pigs (41.9±4.54 Kg, 7 male and 12 female) were included in this study. One to two weeks after a percutaneous induction of MI (13), 14 pigs underwent acute LV unloading experiments using the Impella CP (Abiomed, Danvers, Massachusetts). Five pigs were induced aortic regurgitation (AR) to increase LV load. First, post-MI pigs underwent echocardiographic and hemodynamic measurements, followed by the pacing study for arrhythmia induction and LA pressure-volume assessment using percutaneously inserted high-fidelity catheter (Millar Instruments, Auckland, New Zealand). Under a continuous LA pressure-volume monitoring, the pLVAD was inserted in the LV for unloading group, whereas AR was induced for the loading group. The device flow was increased stepwise (P2, P4, P6, and P8), and the maximum achievable support level for each pig was used for all assessments. Moderate to severe AR was percutaneously induced by disrupting the aortic valve (Supplemental Video). Once hemodynamic stability was established, LA pressure-volume data were again obtained during a brief breath hold. Pacing studies were repeated to evaluate the impact of change in LV load on arrhythmia inducibility (Figure 1). Pigs were monitored for 2 hours after the initiation of the pLAVD or AR induction. NADPH oxidase (NOX) and ryanodine receptor phosphorylation in the LA tissue were examined by western blotting to investigate potential impact of stretch-dependent oxidative stress (14). More detailed protocol and methods are available in the supplemental materials.
Figure 1. Study protocol and methods for LA physiology evaluation.
A: Study protocol. Animals underwent LV unloading and loading experiments 1–2 weeks after MI and changes in the LA physiology were studied by evaluating PV loop and arrhythmia inducibility by rapid pacing of the RA. B: PV catheter (Millar) was inserted into the LA through the atrial septum via femoral vein. Yellow arrow heads indicate the excitation electrodes and those in between were used for volume measurements. C: Ex vivo simulation of the catheter location in the LA. Image is from the LV side looking up at the LA. D: Electrocardiogram during the burst-pacing of the RA to induce atrial arrhythmia. Yellow arrows show the pacing stimuli.
AR=aortic regurgitation, LA=left atrium, LV=left ventricle, MI=myocardial infarction, MV=mitral valve, PV=pressure-volume, RA=right atrium
Statistical analysis
Data are expressed as mean ± standard deviation. The Wilcoxon signed-rank test was used to compare the differences between the two time points of identical animals. The McNemar test was used to compare the incidence rates of arrhythmias before and after the pLVAD support. Correlation between two variables was examined using Spearman’s method. A p-value <0.05 was considered statistically significant.
Results
Impact of MI in pigs
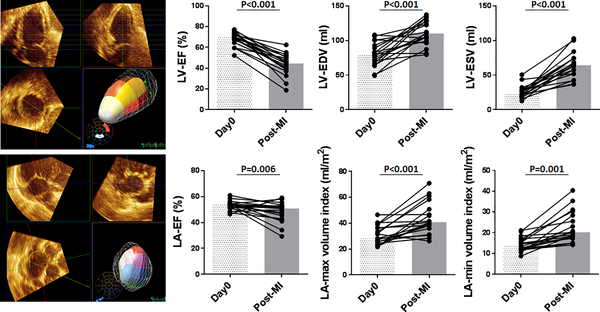
Induction of MI resulted in a decreased LV ejection fraction and increased LV volumes at the time of the LV unloading and loading experiments (Figure 2). LV end-diastolic pressure also significantly increased (Online Table 1). LA maximum and minimum volumes assessed by 3D echocardiography increased and there was a mild reduction in LA ejection fraction (Figure 2).
Figure 2. Echocardiographic LV and LA parameter changes associated with MI.
Left: Example images of 3D echocardiographic analysis of the LV (top) and the LA (Bottom). Pigs presented with reduced LV-EF, increased LV volumes, and LVEDP 1–2 weeks after MI. LA-EF was mildly reduced and the LA volumes were increased.
Bars represent medians.
EDP=end-diastolic pressure, EDV=end-diastolic volume, EF=ejection fraction, LA=left atrium, LV=left ventricle, MI=myocardial infarction
Acute LV unloading with a pLAVD
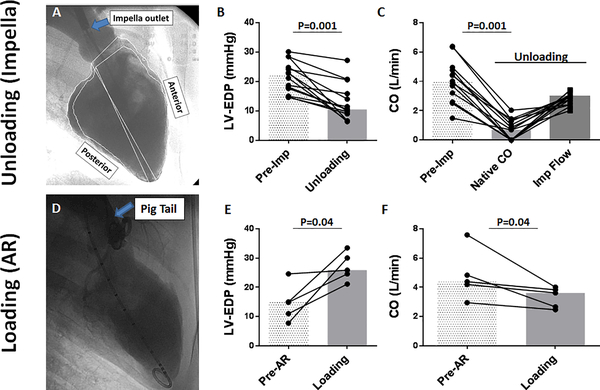
After baseline echocardiographic and hemodynamic assessment, the pLAVD was inserted to acutely unload the LV (Figure 3). Among the 14 studied animals, 12 pigs received P8 support (maximal support) throughout the study. The remaining pigs were supported with lower levels (P6 and P5 support), because of the RV failure that was unmasked when the LV was fully supported with P8. However, unloading of the LV was confirmed in all pigs by decreases in LV end-diastolic pressure and in native cardiac output (Figure 3). Consistent with our previous findings, LV end-diastolic dimension was decreased after LV unloading (Online Figure 1).
Figure 3. Impact of acute changes in the LV load with pLVAD and aortic regurgitation in pigs post-MI.
A: Left ventriculography during percutaneous LV support using pLVAD. Anterior wall was akinetic. The outlet cage of the Impella is indicated by blue arrow. pLVAD reduced LVEDP (B) and native CO (C). D: Aortography after AR induction. Pig tail catheter is indicated by the arrow. AR is noted by regurgitation of contrast into the LV. AR resulted in increased LVEDP (E) and reduced CO (F). Bars represent medians. Abbreviations: AR=aortic regurgitation, CO=cardiac output, EDP=end-diastolic pressure, LV=left ventricle
Impact of acute LV unloading on the LA pressure and volumes
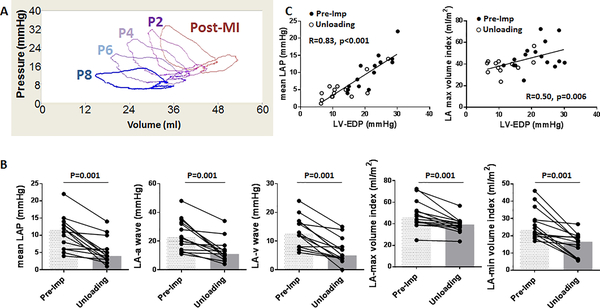
Continuous monitoring of the LA pressure-volume relationship during the stepwise increase in pLVAD flow exhibited a pump-flow dependent left-downward shift of the LA pressure-volume loops (Figure 4; Online Figure 2). Both LA pressure and volumes decreased significantly with the pLVAD -mediated LV unloading (Figure 4). A significant reduction in the LA pressure was observed throughout the cardiac cycle including, both a and v waves. Additionally, there were significant linear correlations between LV end-diastolic pressure and both mean LA pressure and maximum LA volume (Figure 4), indicating that the changes in LA parameters are due to improved LV-LA interaction.
Figure 4. Impact of acute LV unloading on the LA.
A: pLVAD pump flow was increased stepwise: P2 (31,000 rpm) – P4 (35,000 rpm) – P6 (39,000 rpm) –P8 (44,000 rpm) and LA pressure-volume loops were recorded for each step. Higher pump flow resulted in a more left-downward shift of the LA pressure-volume loop. Stepwise changes in pressure and volume parameters are shown in Supplemental Fig 2. B: LV unloading with Impella CP reduced mean as well as a and v waves of LA pressures. LA volumes also decreased significantly after percutaneous LV support. Bars represent medians. C: Plots of LA pressure (left) and volume (right) with LVEDP before (closed circles) and after (open circles) LV unloading. Both LA pressure and the volume showed linear correlations to LVEDP.
EDP=end-diastolic pressure, LA=left atrium, LAP=left atrial pressure, LV=left ventricle, MI= myocardial infarction
Reduced LA work after LV unloading using a pLVAD
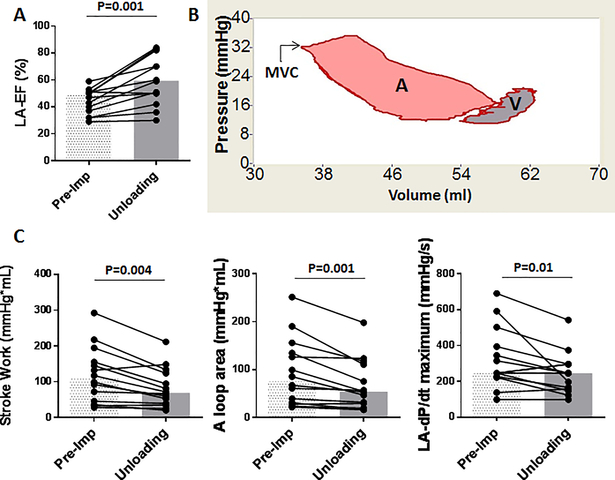
Reduction of LA maximum and minimum volumes was associated with an increased LA ejection fraction (Figure 5). In order to determine if this was associated with increased LA work, we analyzed the LA pressure-volume loops before and after LV unloading. Comparison of pressure-volume loop areas revealed significant reductions of both total and active LA stroke work (Figure 5), suggesting that the LA work was reduced despite increased LA ejection fraction. LA dP/dt maximum was also reduced consistent with the unloading of the LA.
Figure 5. LA unloading and improved LA-EF after LV unloading.
A: LA-EF assessed by pressure-volume loop increased after LV unloading. B: Representative LA pressure-volume loop. Similar to the LV, the area of the LA pressure-volume loop provides information on the LA work (42). Left side of the loop is associated with atrial contraction and the pink area (A) indicates the active LA work. Right side of the loop (V) is the passive part of the LA function and the loop rotates clockwise. Sum of the A and V loops are the LA stroke work. MVC indicates mitral valve closure. C: LA stroke work, A loop area (LA active work), and dP/dt maximum all reduced significantly after percutaneous LV support using a pLVAD. Bars represent medians. Abbreviations: EF=ejection fraction, LA=left atrium, SW=stroke work
Improved LA passive stiffness with acute LV unloading
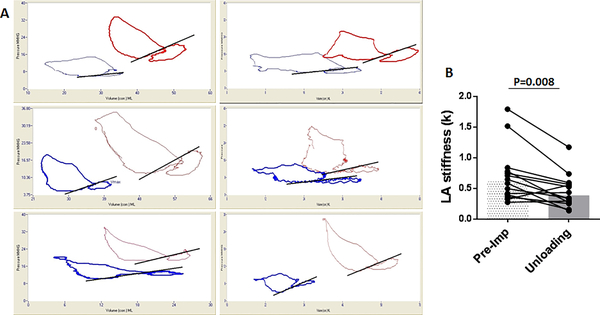
We examined the impact of acute LV unloading with the pLVAD on diastolic passive LA wall stiffness. As shown in Fig 6, the majority of pigs demonstrated a significant reduction in the LA stiffness constant k after acute LV unloading using the pLVAD. The observed reductions in LA pressure, volume, and passive stiffness with acute LV unloading strongly suggest that LV unloading attenuates the LA stretch that was present after MI in our subacute model of MI (Central Illustration).
Figure 6. Reduced LA stiffness after acute LV unloading.
A: Representative LA pressure volume loops before (red) and after (blue) acute LV unloading using Impella CP. B: LA stiffness constant, k, is reduced after acute LV unloading. See supplemental methods for stiffness constant. Bars represent medians.
LA=left atrium
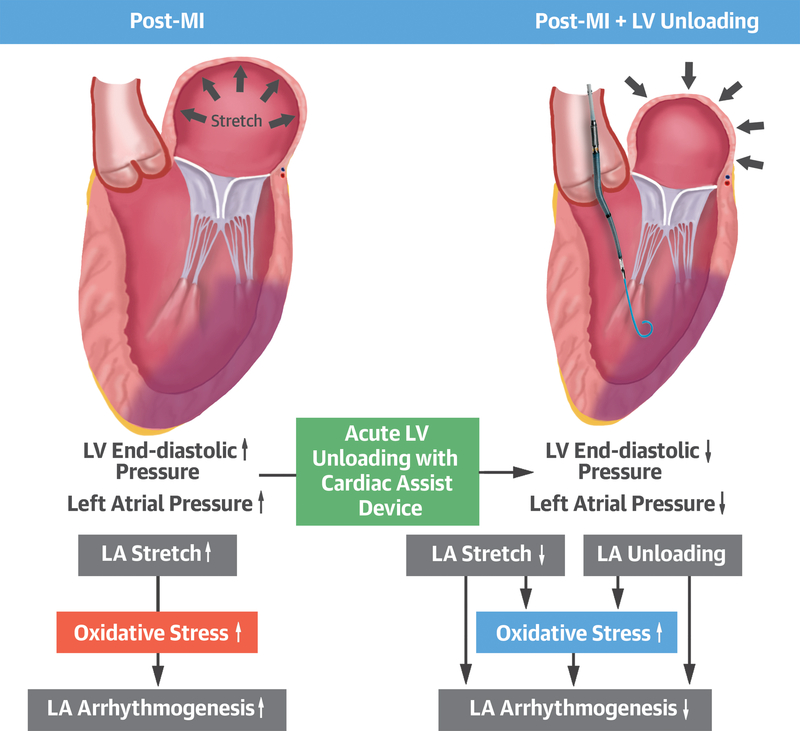
Central Illustration: LV Unloading Relieves LA Stretch and Inhibits Arrhythmias.
Increase in left atrial (LA) pressure after myocardial infarction stretches LA and promotes incidence of atrial arrhythmias. Left ventricular (LV) unloading relieves LA stretch by reducing the LA pressure along with the LV end-diastolic pressure. Inhibition of arrhythmia is associated with reduced stretch-dependent oxidative stress in the LA. Abbreviations: EDP = end-diastolic pressure, LAP = left atrial pressure, MI = myocardial infarction
Decreased arrhythmia incidence and maintenance with acute LV unloading
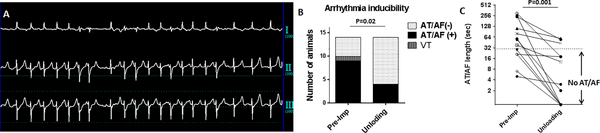
Since atrial stretch is a known promoter of atrial arrhythmias, we tested whether alleviating the LA stretch reduces the propensity to develop arrhythmias. Prior to the pLVAD insertion, pacing-induced AT/AF that was sustained >30 seconds were induced in 9 pigs while 1 additional pig developed a ventricular tachycardia after a short period of atrial fibrillation (71% induction). In contrast, after LV unloading, only 4 pigs developed AT/AF (29%), and none developed ventricular arrhythmias (Fig 7). Moreover, LV unloading reduced the time to spontaneous termination of atrial arrhythmias from 55 sec (median, range:5–300) to 3 sec (range:0–59). (Fig 7).
Figure 7. Reduced atrial arrhythmogenicity after acute LV unloading.
A: Representative tracing of induced AF. B: Rapid right atrial pacing induced AT/AF in 9 pigs before pLVAD initiation. One pig developed a VT after short AF. Arrhythmia inducibility was markedly reduced after acute LV unloading using a pLVAD (Atrial arrhythmias sustaining >30sec were defined positive as shown in C). C: Time to spontaneous termination of atrial arrhythmias also decreased significantly after percutaneous LV support using a pLVAD. One pig was converted to sinus rhythm with direct current shock after sustained atrial fibrillation >5 min.
AF=atrial fibrillation, AT=Atrial tachycardia, LV=left ventricle, VT=ventricular tachycardia
Impact of LV loading on the LA
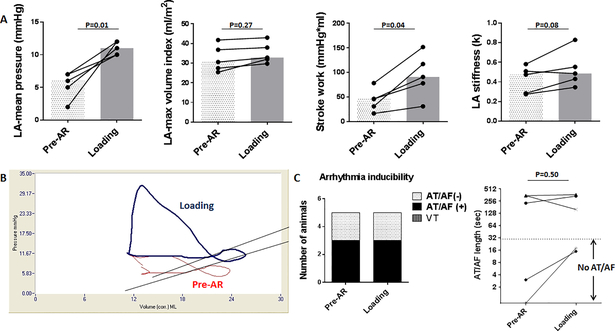
To further characterize the impact of changes in LV loading condition, we examined the effect of acutely increased LV load by percutaneously inducing AR (Fig 3 D-F). AR successfully increased LVEDP in pigs post-MI, and this resulted in an increased LA pressure. There was a trend toward increased LA volumes, but this failed to reach significance. This can be observed in the representative LA pressure-volume loop in Fig 8 in that the main shift upon loading was upward (pressure) and only slightly rightward (volume). LA stroke work was increased significantly, and stiffness showed increase without statistical significance. Interestingly, there were no significant changes in the arrhythmia inducibility or maintenance using the same induction protocol employed for the unloading group.
Figure 8. Impact of LV loading on LA.
A: Induction of AR resulted in significant elevation of mean LA pressure and stroke work. LA volume and stiffness increased without statistical significance. Bars represent medians. B: Representative change in LA PV loop before (red) and after AR (blue) induction. The PV loop shifted up and to the right, but the shift in volume was less prominent. C: There were no major impacts on arrhythmia inducibility and maintenance associated with LV loading.
AF=atrial fibrillation, AT=Atrial tachycardia, AR=aortic regurgitation, LA=left atrium, LV=left ventricle, VT=ventricular tachycardia
Reduced NOX2 level in the LA associated with acute LV unloading
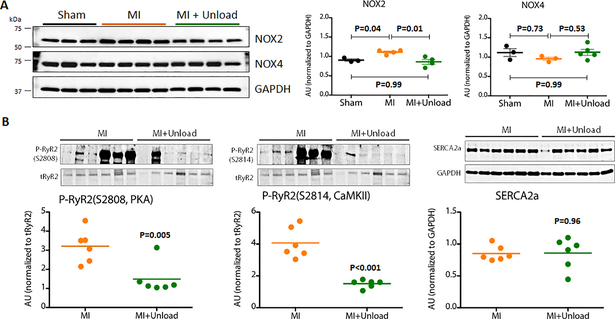
Because NOX2-dependent oxidative stress has been shown to be involved in cardiomyocyte stretch-induced arrhythmogenic properties, we examined NOX2 levels in LA tissues. Consistent with previously reported in vitro results, we found increased NOX2 levels in the LA after MI compared to the LA from naïve pigs (Figure 9). pLVAD-mediated LV unloading reduced NOX2 levels in the LA (Figure 9; Online Figure 3) and this was associated with decreased ryanodine receptor phosphorylation at both S2808 and S2814 sites. In contrast, LA levels of NOX4, another NOX isoform predominantly expressed in the heart, did not show significant differences between naïve, post-MI, and post-MI with unloading. These results suggest that the anti-arrhythmic effect was at least partly mediated by decreased LA oxidative stress and modulation of ryanodine receptor activity. In contrast, LV loading with AR did not result in significant changes in NOX levels (Online Figure 4).
Figure 9. NOX2 expression in the LA and its relation to ryanodine receptor phosphorylation.
A: Representative blots of NOX expression and their quantitation. NOX2 level was increased in the LA after MI, however it was reduced by LV unloading with a pLVAD. In contrast, NOX4 expression was not changed after MI of after LV unloading. B: LV unloading reduced phosphorylation of ryanodine receptor at both PKA dependent (S2808) and CAMKII dependent sites (S2814). There was no difference in LA SERCA2a expression associated with LV unloading.
CAMKII=Calcium/calmodulin-dependent protein kinases II, GAPDH= Glyceraldehyde-3-Phosphate Dehydrogenase, LA=left atrium, LV=left ventricle, MI=myocardial infarction, NOX=nicotinamide adenine dinucleotide phosphate oxidase, PKA= Protein kinase A, SERCA2a= sarcoplasmic reticulum Ca2+ ATPase, RyR2=ryanodine receptor2, Unload=unloading
Discussion
The central finding of our study is that acute unloading of the LV leads to passive reduction of LA pressure, volume, and work that result in the prevention of atrial arrhythmias in a subacute MI pig model. A unique method of percutaneous LA pressure-volume loop assessment in a closed chest setting was employed to characterize the impact of LV load on LA physiology. In addition to reduced LA volume, the LA passive stiffness index decreased by LV unloading. This suggests that LA stretch present after MI was effectively attenuated by LV unloading. Reduced atrial arrhythmogenicity was associated with reversal of increased LA NOX2 levels post-MI, along with a diminished ryanodine receptor phosphorylation. These results suggest a stretch-induced NOX2 dependent oxidative stress, in part, mediates atrial arrhythmogenesis in a post-MI setting, and this mechanism is reversible with LV unloading.
To our knowledge, our study provides the first detailed characterization of the acute impact of a continuous flow LV-to-aorta LVAD on LA physiology in a post-MI setting. Moreover, this is also the first in vivo study to demonstrate that alleviating pre-existing LA stretch can reduce atrial arrhythmogenicity in post-MI setting. Taken together, our study demonstrates that acute mechanical unloading of the LV using the pLVAD is capable of producing significant benefits on the hemodynamic stresses within in the LA that are associated with MI. While our findings need to be confirmed in data from patients supported with a pLVAD post-MI, our study indicates that LV unloading would be expected to facilitate the management of congestive heart failure by positively affecting the hemodynamics upstream of the LV.
Impact of pLVAD-mediated acute LV unloading on the LA
Understanding the impact of altered LV load on the LA in the post-MI setting is of paramount importance as it relates to the two prevalent sequelae of an MI, atrial arrhythmia and congestive heart failure. After a large MI, LV systolic function becomes decreased and LV operates under an increased LV end-diastolic pressure in order to maintain cardiac output via the Frank-Starling mechanism. However, increased LV end-diastolic pressure also leads to a requisite increase in LA pressure that stretches LA wall (Central Illustration). Increased LA pressure also increases lung capillary pressure that can cause lung congestion leading to congestive heart failure, whereas stretch of the LA wall increases the incidence of atrial arrhythmias (15–17).
In general, the impact of mechanical LV unloading on LA physiology and hemodynamics has not been well studied. This includes both pLVADs and surgically implanted LVADs. The majority of previous studies employed pulmonary capillary wedge pressure as a surrogate of LA pressure (5,18–21). While a good agreement between pulmonary capillary wedge pressure and LA pressure has been reported (22,23), the information available from right heart catheterization is limited to the pressure and it does not provide assessment of concurrent LA volume changes associated with LVAD support. By using a continuous direct LA pressure-volume monitoring by percutaneous catheterization, we demonstrate for the first time that LA volume decreases together with the pressure in a linearly correlated manner to the LV end-diastolic pressure during acute LV unloading. We also found that the LA passive stiffness index was decreased after LV unloading in subacute MI pigs. In contrast, increased LV load induced by AR significantly elevated LA pressure, but had lesser effects on volumes and the stiffness. These results suggest that the LA is already stretched in these post-MI animals, and the increase in pressure is not capable of further stretching the LA wall. Together, these data support that the observed LA stretch post-MI is due to compensatory increases in LV filling pressure, and this process is reversible by modifying LA hemodynamics through mechanical unloading the LV. Interestingly, while LA pressure and volume parameters showed a flow-dependent decrease, stiffness showed an initial reduction at low level of support (P2) and remained similar thereafter (Supplemental Fig 2). This result suggests that low-grade LV unloading may be sufficient to alleviate LA stretch in post-MI and supports the use of mechanical LV unloading for not only patients with cardiogenic shock, but for those with congestive heart failure with increased LA pressure.
Reduced atrial arrhythmias
Atrial arrhythmias are a common complication after an MI with a reported incidence as high as 37% (24). Various mechanisms have been proposed for this increased propensity to atrial arrhythmias including atrial stretch, atrial ischemia, inflammation, and autonomic nervous activation (24,25). Although evidence suggests that all these factors play roles in promoting atrial arrhythmia, whether it is possible to inhibit atrial arrhythmias by modulating these factors remains largely unexplored. Our results confirm that atrial stretch is indeed one of the most important mechanisms for both inducibility and sustainability of atrial arrhythmias. Meanwhile, circulating atrial natriuretic peptide levels showed only minor changes after LV load changes (Supplemental Fig 5), suggesting a lesser role at least in our study setting. Our data specifically highlights that alleviating stretch is both attainable and a viable approach to reducing atrial arrhythmogenicity in a recent MI.
The importance of increased LA pressure and associated stretch for atrial arrhythmia development has been implicated by several clinical studies (26–28) and ex vivo experiments (15,17,29). In a rabbit isolated Langendorff-perfused heart, Ravelli et al (15) elegantly demonstrated that an acute increase in atrial pressure leads to increased AF inducibility through shortening of atrial effective refractory period, whereas restoring the pressure promptly reverses the abnormal rhythm. Unfortunately, no studies have examined whether these findings are reproducible in vivo, most likely because of the difficulty in specifically manipulating LA pressures in the intact physiological environment. In vivo confirmation in our study at subacute phase of MI is particularly important because it is possible that a continuously stretched LA may have undergone electrical or structural remodeling that can provide the arrhythmogenic substrate post-MI. Limiting stretch in vivo is a direct means of testing whether structural and/or electrical remodeling would still be reversible. Using our unique experimental approach with mechanical LV unloading, we demonstrate that the findings of Ravelli et al. are indeed reproducible in an LA that has been exposed to continuously elevated pressure for at least 1 week in a clinically relevant animal model.
Our study provides additional insights into the mechanisms involved in development of post-MI AF. We found that LA NOX2 expression is increased after MI, whereas this was partly reversed by acute LV unloading. Prosser et al (14) reported that cardiomyocyte stretch induces arrhythmogenic Ca2+ sparks via activation of NOX2 in isolated ventricular myocytes in a rapid and reversible manner (14,30). This was associated with increased ryanodine receptor sensitivity, leading to arrhythmogenic Ca2+ release from the sarcoplasmic reticulum. They suggested that post-translational modifications of the ryanodine receptor induced by NOX2-dependent oxidative stress as the responsible mechanism. Our study is in line with their findings, while demonstrating that the same mechanism likely takes place in the LA. Our data suggest that decreased ryanodine receptor phosphorylation at both S2808 and S2814 sites, which are shown to be activated under oxidative stress (31,32), may be playing a role in prevention of the diastolic calcium leak through this channel (33). Several experimental and clinical studies have provided evidence for a relationship between NOX2-mediated oxidative stress and AF (34–36). Dynamic changes of NOX2 after MI and after LV unloading in our data are consistent with previous report that demonstrated its rapid transcription and degradation (37). Together with parallel changes in arrhythmia inducibility, our data suggest that NOX2 mediated oxidative stress may play key roles in increased arrhythmogenicity in the subacute phase of MI. Experiments with transgenic mice have not yet conclusively defined specific roles for NOX isoforms in AF formation (16). While transgenic mouse experiments are of central importance to advancing our understanding of specific proteins, interpretation of these data are not straightforward as the sustained loss/overexpression of a protein may have very different effects in a physiological system compared to transient changes (38).
Reduced LA work
Analysis of the LA pressure-volume loop relationship also revealed decreases in LA active work after pLVAD-mediated LV unloading. Together with decreased LA dP/dt maximum, these data suggest that the pLVAD reduces the active work of the LA in addition to the LV. It is possible that reduced LA work also contributes to the decrease in arrhythmia inducibility, because chronic beta-blocker treatment using carvedilol has been shown to reduce the incidence of AF in post-MI cohort from 5.4% to 2.35% (39). However, this data was acquired in patients during longitudinal follow-up. The contribution of reduced LA work on LA arrhythmogenicity in the acute setting needs to be validated in the future studies.
Closed chest LA pressure-volume assessment
To our knowledge, this is the first demonstration of LA pressure-volume loop assessment in a closed-chest setting. Several studies have used a surgical approach to insert a pressure-volume catheter (40) or sonomicrometry crystals (41) to evaluate LA physiology. Because the LA is a low-pressure, compliant chamber, an open-chest or an open-pericardium may have large influence on the physiological properties of the LA. Our experiments in their entirety were conducted in a closed-chest model including the model creation, thus avoid this potential impact. While catheter-based LA pressure-volume assessment is not as established as that in the LV, we found good agreement between changes in LA volumes measured by echocardiography and that measured by the pressure-volume catheter, suggesting that volume measurements using this technique were reliable.
Limitations
There are a number of limitations to our study that need to be considered. First, our study used a subacute MI pig model and whether our findings apply to more early or chronic time points remains to be studied. We would like to highlight, however, that our study provides important evidence that the plasticity of atrial stretch and associated arrhythmia is not only at the very acute phase of MI, but is also present at the subacute phase of MI. Second, it remains unclear if temporal inhibition of atrial arrhythmias with LV unloading can provide long-term benefit to post-MI patients. However, atrial arrhythmia incidence is highest in the peri-MI phase and there is established evidence that AF begets AF by promoting electrical remodeling. Therefore, inhibition of AF at the most susceptible period after MI may be able to provide longterm benefit. Third, the role of systemic neurological and inflammatory responses was not studied fully, and LV unloading may affect LA physiology through these mechanisms. Nevertheless, involvement of LA oxidative stress, which is a downstream of mechanical and regulatory effects, is likely to play important roles through other mechanisms as well. Finally, acute LV loading study included only 5 animals and may be underpowered to detect changes in arrhythmias.
Conclusion
We show that acute unloading of the LV using the pLVAD reduces LA stretch and unloads LA through improved LV-LA interaction in a subacute MI pig model. Reduced atrial arrhythmogenicity associated with reversal of LA stretch was demonstrated for the first time in non-acute in vivo setting using our unique experimental approach. Our data also suggest that modulation of stretch-induced mechano-transduction via NOX2 and downstream ryanodine receptor modulation may be associated with the inhibition of atrial arrhythmias during acute LV unloading.
CLINICAL PERSPECTIVES
Competency in Patient Care:
Left ventricular (LV) unloading with the percutaneous LV assist device (pLVAD) lowers left atrial pressure and stiffness and reduces the incidence of atrial arrhythmias in patients with recent myocardial infarction.
Translational Outlook:
Although pLVAD support in patients with acute coronary syndromes is currently limited to those with cardiogenic shock, additional studies may identify cases in which LV unloading could be used to treat heart failure even in the absence of cardiogenic shock.
Supplementary Material
Acknowledgments
Funding: This study was supported by AHA 17SDG33410873, NIH R01 HL139963 (K.I.), R01 HL119046, R01 HL117505, R01HL128099, R01 HL129814, R01HL131404, & R43HL108581 (R. J. H.), R00 HL116645 (C.K.), and a Transatlantic Leducq Foundation grant (R.J.H.). K.I is supported by a grant from American Heart Association 17SDG33410873. We would also like to acknowledge the Gene Therapy Resource Program (GTRP) of the National Heart, Lung, and Blood Institute, National Institutes of Health. O.B. was supported by the Deutsche Herzstiftung.
Abbreviation List
- AF
atrial fibrillation
- AR
aortic regurgitation
- AT
atrial tachycardia
- LA
left atrium
- LV
left ventricle
- MI
myocardial infarction
- NOX
nicotinamide adenine dinucleotide phosphate oxidase
- pLVADs
percutaneous left ventricular assist devices
Footnotes
Disclosures: K.I. receives a research grant from Abiomed. (Danvers, Massachusetts). The Impella device and console were supplied by the Abiomed. Other authors have no disclosures related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alasady M, Shipp NJ, Brooks AG et al. Myocardial infarction and atrial fibrillation: importance of atrial ischemia. Circ Arrhythm Electrophysiol 2013;6:738–45. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Pacheco H, Marquez MF, Arias-Mendoza A et al. Clinical features and in-hospital mortality associated with different types of atrial fibrillation in patients with acute coronary syndrome with and without ST elevation. J Cardiol 2015;66:148–54. [DOI] [PubMed] [Google Scholar]
- 3.Burkhoff D Device therapy: Where next in cardiogenic shock owing to myocardial infarction? Nat Rev Cardiol 2015;12:383–4. [DOI] [PubMed] [Google Scholar]
- 4.Rihal CS, Naidu SS, Givertz MM et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 2015;65:e7–e26. [DOI] [PubMed] [Google Scholar]
- 5.Seyfarth M, Sibbing D, Bauer I et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–8. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–83. [DOI] [PubMed] [Google Scholar]
- 7.Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW, TandemHeart Investigators G. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006;152:469 e1–8. [DOI] [PubMed] [Google Scholar]
- 8.Kapur NK, Qiao X, Paruchuri V et al. Mechanical Pre-Conditioning With Acute Circulatory Support Before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart failure 2015;3:873–82. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Li J, Zhao W et al. Early Assistance With Left Ventricular Assist Device Limits Left Ventricular Remodeling After Acute Myocardial Infarction in a Swine Model. Artificial organs 2016;40:243–51. [DOI] [PubMed] [Google Scholar]
- 10.Burkhoff DS G; Doshi D, Uriel N Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol 2015;66:2663–74. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe S, Fish K, Kovacic JC et al. Left Ventricular Unloading Using an Impella CP Improves Coronary Flow and Infarct Zone Perfusion in Ischemic Heart Failure. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkhoff D, Maurer MS, Joseph SM et al. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart failure 2015;3:275–82. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa K, Aguero J, Tilemann L et al. Characterizing preclinical models of ischemic heart failure: differences between LAD and LCx infarctions. Am J Physiol Heart Circ Physiol 2014;307:H1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 2011;333:1440–5. [DOI] [PubMed] [Google Scholar]
- 15.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation 1997;96:1686–95. [DOI] [PubMed] [Google Scholar]
- 16.Youn JY, Zhang J, Zhang Y et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol 2013;62:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eijsbouts SC, Majidi M, van Zandvoort M, Allessie MA. Effects of acute atrial dilation on heterogeneity in conduction in the isolated rabbit heart. J Cardiovasc Electrophysiol 2003;14:269–78. [DOI] [PubMed] [Google Scholar]
- 18.Klotz S, Deng MC, Stypmann J et al. Left ventricular pressure and volume unloading during pulsatile versus nonpulsatile left ventricular assist device support. Ann Thorac Surg 2004;77:143–9; discussion 149–50. [DOI] [PubMed] [Google Scholar]
- 19.Drakos SG, Kfoury AG, Hammond EH et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol 2010;56:382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegenthaler MP, Brehm K, Strecker T et al. The Impella Recover microaxial left ventricular assist device reduces mortality for postcardiotomy failure: a three-center experience. The Journal of thoracic and cardiovascular surgery 2004;127:812–22. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill WW, Schreiber T, Wohns DH et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol 2014;27:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy AI, Venkateshvaran A, Dash PK et al. The pulmonary capillary wedge pressure accurately reflects both normal and elevated left atrial pressure. Am Heart J. 2014;167:876–883. [DOI] [PubMed] [Google Scholar]
- 23.Vinayakumar D, Bijilesh U, Sajeev CG et al. Correlation of pulmonary capillary wedge pressure with left atrial pressure in patients with mitral stenosis undergoing balloon valvotomy. Indian Heart J 2016;68:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Yang YM, Zhu J. Mechanisms of new-onset atrial fibrillation complicating acute coronary syndrome. Herz 2015;40 Suppl 1:18–26. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J 2009;30:1038–45. [DOI] [PubMed] [Google Scholar]
- 26.Aronson D, Mutlak D, Bahouth F et al. Restrictive left ventricular filling pattern and risk of new-onset atrial fibrillation after acute myocardial infarction. Am J Cardiol 2011;107:1738–43. [DOI] [PubMed] [Google Scholar]
- 27.Solti F, Vecsey T, Kekesi V, Juhasz-Nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc Res 1989;23:882–6. [DOI] [PubMed] [Google Scholar]
- 28.Galvao Braga C, Ramos V, Vieira C et al. New-onset atrial fibrillation during acute coronary syndromes: predictors and prognosis. Rev Port Cardiol 2014;33:281–7. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Kakimoto Y, Toda K, Naruse K. Mechanobiology in cardiac physiology and diseases. J Cell Mol Med 2013;17:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prosser BL, Ward CW, Lederer WJ. X-ROS signalling is enhanced and graded by cyclic cardiomyocyte stretch. Cardiovasc Res 2013;98:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson JR, Joiner ML, Guan X et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008;133:462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan S, Spear J, Chandran K, Joseph J, Kalyanaraman B, Avadhani NG. Oxidative stress induced mitochondrial protein kinase A mediates cytochrome c oxidase dysfunction. PLoS One 2013;8:e77129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulindependent protein kinase II. J Mol Cell Cardiol 2010;49:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley SC, Jr., Hoch NE, McCann LA et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation 2005;112:1266–73. [DOI] [PubMed] [Google Scholar]
- 35.Kim YM, Guzik TJ, Zhang YH et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res 2005;97:629–36. [DOI] [PubMed] [Google Scholar]
- 36.Adam O, Frost G, Custodis F et al. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol 2007;50:359–67. [DOI] [PubMed] [Google Scholar]
- 37.Rolas L, Boussif A, Weiss E et al. NADPH oxidase depletion in neutrophils from patients with cirrhosis and restoration via toll-like receptor 7/8 activation. Gut 2017. [DOI] [PubMed] [Google Scholar]
- 38.Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Cautionary insights on knockout mouse studies: the gene or not the gene? Brain Behav Immun 2009;23:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMurray J, Kober L, Robertson M et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol 2005;45:525–30. [DOI] [PubMed] [Google Scholar]
- 40.Zakeri R, Moulay G, Chai Q et al. Left Atrial Remodeling and Atrioventricular Coupling in a Canine Model of Early Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gare M, Schwabe DA, Hettrick DA, Kersten JR, Warltier DC, Pagel PS. Desflurane, sevoflurane, and isoflurane affect left atrial active and passive mechanical properties and impair left atrial-left ventricular coupling in vivo: analysis using pressure-volume relations. Anesthesiol. 2001;95:689–98. [DOI] [PubMed] [Google Scholar]
- 42.Pagel PS, Kehl F, Gare M, Hettrick DA, Kersten JR, Warltier DC. Mechanical function of the left atrium: new insights based on analysis of pressure-volume relations and Doppler echocardiography. Anesthesiol. 2003;98:975–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.