1. Introduction
The benefits of cardiac resynchronisation therapy (CRT) on left ventricular (LV) function and clinical outcomes in adult patients with heart failure have encouraged the use of this therapy. Two large prospective randomized trials, the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial and the Cardiac Resynchronisation in Heart Failure (CARE-HF) trial, have shown that compared with optimal pharmacological therapy, CRT in advanced heart failure patients results in reductions in all-cause, cardiac and heart failure hospitalization rates.
However, evidence supporting its use in adolescents remains lacking. We describe our experience of CRT in the treatment of two adolescents with pacing induced cardiomyopathy (PiCMP).
2. Case 1
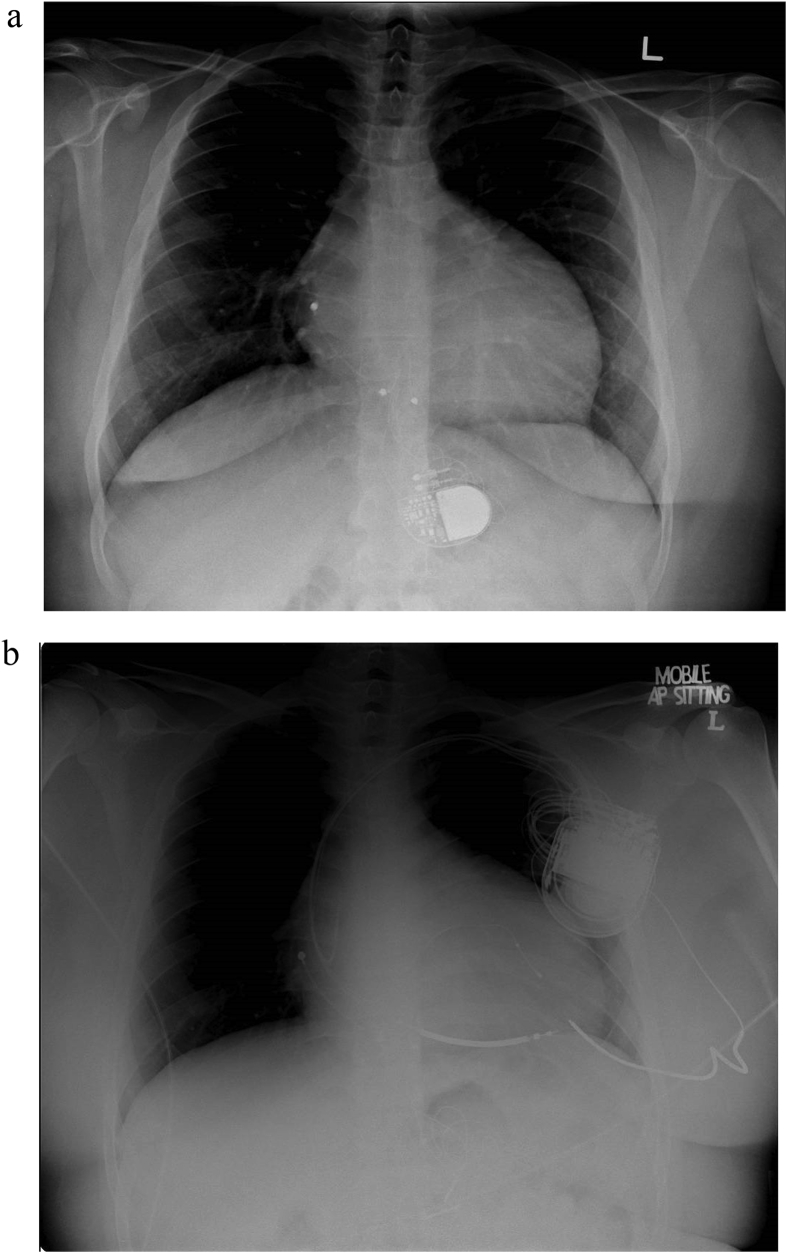
A male patient was diagnosed with congenital complete heart block (CCHB) at age 7. He underwent implantation of a dual chamber epicardial pacemaker at age 10, after developing symptoms of bradycardia. The epicardial atrial and ventricular leads were placed on the right atrial (RA) appendage and basal inferior segment of right ventricular (RV), respectively (Fig. 1A). Echocardiography then showed normal LV ejection fraction (LVEF).
Fig. 1.
CXR demonstrating (a) dual chamber epicardial pacemaker (b) CRT-D.
He was referred to our centre for follow up at age 18 with NYHA Class III heart failure symptoms. Echocardiogram showed severe LV systolic dysfunction with LVEF of 15%, a dilated LV measuring 8.1cm in end-diastole and 7cm in end-systole. Pacemaker interrogation showed 100% ventricular pacing (99.8% atrial sensing ventricular pacing, 0.2% dual chamber pacing).
A diagnosis of PiCMP was made given the chronicity of RV pacing and in the absence of other common causes of dilated cardiomyopathy (DCM) in a young adult such as infection or toxins. In view of symptomatic severe LV dysfunction, he underwent CRT-defibrillator (CRT-D) implantation with new transvenous leads positioned over RA appendage, RV septum, and coronary sinus (CS) (Fig. 1B). Echocardiogram 10 months after CRT showed improvement in LVEF to 45% with reduction in LV end-diastolic diameter to 6.5cm and end-systolic diameter to 5cm. He also reported a significant improvement in exercise tolerance with NYHA Class I symptoms, which was maintained at 5 year follow up post CRT-D implantation. There was minimal change with regards to electrical dyssynchrony post CRT in this case.
3. Case 2
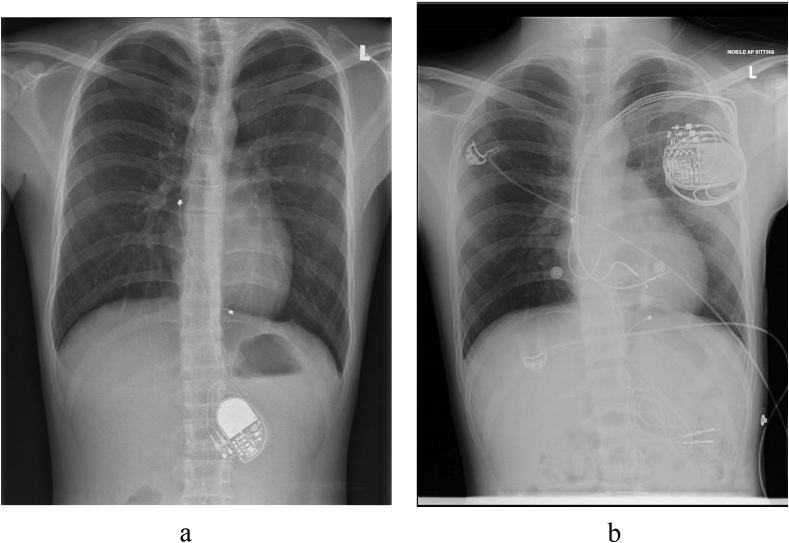
A male patient developed high-grade atrioventricular (AV) block secondary to viral myocarditis at age 8, for which he underwent implantation of a dual chamber epicardial pacemaker with RV lead positioned over mid-inferior RV (Fig. 2A). He was referred to us for follow up at age 17 with NYHA Class II symptoms. Echocardiography revealed a severely impaired LVEF of 35%. Electrocardiogram showed paced wide QRS complex of 166msec with left bundle branch block (LBBB) pattern morphology. Pacemaker interrogation revealed 100% ventricular pacing (99.8% atrial sensing with ventricular pacing and 0.2% dual chamber pacing).
Fig. 2.
CXR demonstrating (a) dual chamber epicardial pacemaker (b) CRT-P.
In view of his severely depressed LVEF and total dependence on ventricular pacing, a diagnosis of PiCMP was made, in the absence of other common causes of DCM. His device was upgraded to a CRT-pacemaker (CRT-P) with placement of transvenous RA lead over interatrial septum, RV lead over mid RV septum, and LV lead over the posterolateral CS (Fig. 2B). At 4 months post implantation, he reported significant clinical improvement with NYHA Class I symptoms, which was maintained at 3 years post-implantation. Echocardiography 2 years after CRT-P implantation showed a normalisation in LVEF to 50%. Electrocardiogram showed atrial-sensing and ventricular-pacing QRS complex of 106msec. There was minimal change with regards to LV dimension post CRT in this case.
4. Discussion
The prevalence of PiCMP secondary to RV pacing in adult patients has been reported at 9% 1 year following implantation, and 15% at long term follow up [4]. PiCMP is clinically more significant in paediatric patients, owing to the extended duration of their pacing dependence. Long term RV pacing may have a deleterious effect on LV function, resulting in adverse LV remodelling and interventricular dyssynchrony, leading to PiCMP. These have been attributed to the dyssynchronous electrical and mechanical activation between the interventricular septum and posterior/lateral wall of LV. The LV septal walls will exhibit rapid early systolic shortening due to its proximity with RV pacing site, while the lateral LV walls remain in pre-stretch/relaxed state. Similarly, delayed systolic shortening of the LV lateral walls will impose systolic stretch to the LV septal walls during it's premature relaxation. This abnormal contraction pattern of different LV segments result in a redistribution of myocardial strain and work, and subsequent less effective contraction [1].
This is associated with significant reduction in functional capacity, chronotropic incompetence and increased adverse outcomes such as heart failure and atrial fibrillation in patients with cumulative RV pacing in both DDD (mean 90% RV pacing burden) and VVI pacing (mean 50% RV pacing burden) groups. Chronic RV pacing has been associated with a significant reduction in functional capacity accompanied by chronotropic incompetence in paediatric patients as demonstrated by Motonaga et al. in a 5-year prospective study [13].
Currently, CRT is a Class I indication in adult patients with New York Heart Association (NYHA) Class II/III/IV symptoms, LVEF ≤ 35%, and QRS > 150ms, who are receiving optimal medical therapy [4]. Specific to the paediatric population, an international multi-centre retrospective review of CRT implantation in children with heart failure showed that CRT was associated with improvement in haemodynamic status, increase in mean LVEF of 12–14%, and corresponding functional improvement in NYHA grade by 1 class. Multiple case reports also describe significant narrowing of QRS duration, reduction in LV echocardiographic dimensions, improvement in LV synchrony, and reverse remodelling in patients as early as 1 month following upgrade from RV pacing to biventricular pacing or CRT [2,3]. Results from several small controlled and non-controlled studies also appear to indicate that CRT reverses LV remodelling, decreases LV end systolic and end diastolic volumes and increases LVEF in patients with PiCMP [5].
It is unclear if current evidence can be extrapolated to the paediatric population. Additionally, experience with CRT in children has been limited to retrospective studies and reviews, with the studied cohort comprising a diverse and heterogeneous population of children, including those with cardiomyopathy, congenital heart block, and congenital heart disease [[6], [7], [8], [9], [10], [11], [12]]. This heterogeneity in anatomical substrates and aetiologies of heart failure, and the variations in age and size make firm guidelines on CRT treatment difficult to establish.
5. Conclusion
Our case series suggests favourable functional and haemodynamic outcomes with CRT in a small unique population of young adults with PiCMP following chronic RV pacing. Early CRT is suggested to manage PiCMP prior to listing for cardiac transplantation.
Declaration of interest
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Tops L.F., Schalij M.J., Bax J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol. 2009;54(9):764–776. doi: 10.1016/j.jacc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Janousek J., Gebauer R.A., Abdul-Khaliq H. Cardiac resynchronisation therapy in paediatric and congenital heart disease: differential effects in various anatomical and functional substrates. Heart. 2009;95(14):1165–1171. doi: 10.1136/hrt.2008.160465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janousek J., Tomek V., Chaloupecky V., Gebauer R.A. Dilated cardiomyopathy associated with dual-chamber pacing in infants: improvement through either left ventricular cardiac resynchronization or programming the pacemaker off allowing intrinsic normal conduction. J Cardiovasc Electrophysiol. 2004;15(4):470–474. doi: 10.1046/j.1540-8167.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- 4.Vardas Panos E., Auricchio Angelo, Blanc Jean-Jacques. Guidelines for cardiac pacing and cardiac resynchronization therapy. Europace. Oct 2007;9(10):959–998. doi: 10.1093/europace/eum189. [DOI] [PubMed] [Google Scholar]
- 5.Fröhlich Georg, Steffel Jan, Hürlimann David. Upgrading to resynchronization therapy after chronic right ventricular pacing improves left ventricular remodelling. Eur Heart J. Jun 2010;31(12):1477–1485. doi: 10.1093/eurheartj/ehq065. [DOI] [PubMed] [Google Scholar]
- 6.Sirén M.K., Julkunen H., Kaaja R. The increasing incidence of isolated congenital heart block in Finland. J Rheumatol. 1998;25(9):1862–1864. [PubMed] [Google Scholar]
- 7.Michaëlsson M., Engle M.A. Congenital complete heart block: an international study of the natural history. Cardiovasc Clin. 1972;4(3):85–101. [PubMed] [Google Scholar]
- 8.Buyon J.P., Hiebert R., Copel J. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31(7):1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 9.Villain E., Coastedoat-Chalumeau N., Marijon E., Boudjemline Y., Piette J.-C., Bonnet D. Presentation and prognosis of complete atrioventricular block in childhood, according to maternal antibody status. J Am Coll Cardiol. 2006;48(8):1682–1687. doi: 10.1016/j.jacc.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Eronen M., Sirèn M.K., Ekblad H., Tikanoja T., Julkunen H., Paavilainen T. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Paediatrics. 2000;106(1 Pt 1):86–91. doi: 10.1542/peds.106.1.86. [DOI] [PubMed] [Google Scholar]
- 11.Bateau A., Pass R.H., Thambo J. Congenital and childhood atrioventricular blocks: pathophysiology and contemporary management. Eur J Pediatr. 2016 Jun;28 doi: 10.1007/s00431-016-2748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews R.E., Fenton M.J., Ridout D.A., Burch M. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United Kingdom and Ireland. Circulation. 2008;117(1):79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 13.Motonaga K.S., Punn R., Axelrod D.M. Diminished exercise capacity and chronotropic incompetence in paediatric patients with congenital complete heart block and chronic right ventricular pacing. Heart Rhythm. 2015 Mar;12(3):560–565. doi: 10.1016/j.hrthm.2014.11.036. [DOI] [PubMed] [Google Scholar]