Abstract
Background
Licorice (Glycyrrhizae radix et rhizome, GRR) has long been used as an ingredient in Korean traditional medicinal herbal formulas for various metabolic and reproductive diseases. Polycystic ovary syndrome (PCOS) is a common endocrine disorder in premenopausal women. In the present study, we examined the effects of GRR extract on PCOS-like symptoms in female rats.
Methods
Symptoms of PCOS were induced by Letrozole treatment for 4 weeks in 6-week-old female SD rats, after which the effects of GRR extract on recovery of normal hormonal levels and polycystic ovaries were assessed. Serum levels of luteinizing hormone (LH), follicular-stimulating hormone (FSH), LH/FSH ratio, and follicular cysts were evaluated, followed by the expression levels of known follicular phase markers such as Kitl, Cyp11a1, and Ptgs2.
Results
The serum level of FSH was reduced only in the Lestrozole treatment group (PCOS), whereas significant recovery of FSH level was observed in the Letrozole and GRR co-treatment group (PCOS + GRR). Serum LH levels were not altered in any of the groups. Furthermore, the LH/FSH ratio (known biomarker for PCOS) was elevated only in the Letrozole treatment group (PCOS), whereas it was significantly reduced in the Letrozole and GRR co-treatment group (PCOS + GRR). For histological changes, follicular cysts, antral follicles, and increased thickness of the theca- and granulosa layers were observed in the PCOS group, whereas these alterations were remarkably reversed by GRR treatment.
Conclusion
These results suggest that GRR extract inhibits the symptoms of PCOS by regulating imbalanced hormonal levels and irregular ovarian follicles.
Keywords: Glycyrrhizae radix et rhizoma, Letrozole, LH/FSH ratio, Licorice, Polycystic ovary syndrome
1. Introduction
Polycystic ovary syndrome (PCOS) is one of the most common causes of hyperandrogenism, infertility, and anovulation, and in women, which affects at least 5–10% of women of reproductive age.1 PCOS is characterized by irregular menses, miscarriage, dysfunction of follicular maturation, hyperandrogenism and dysregulation of hormones such as luteinizing hormone (LH) and follicular-stimulating hormone (FSH), resulting in acne and hirsutism.1, 2 At present, significant advancements in understanding the pathogenesis of PCOS have been made in addition to treatment modalities for PCOS symptoms.3
Licorice (Glycyrrhizae radix et rhizome, GRR) is prescribed in many Korean medicinal formulas for its potential effects in the regulation of inflammation, immune response, hepatic failure, spasms, and women's metabolic disorder.4, 5 This herbal remedy has long been used as a medicine in ancient documents such as Dongeuibogam as well as in Oriental traditional medicine. GRR is usually harvested during spring and autumn, although autumn is typically preferred. After harvesting, its fibrous roots are removed, dried under the sun, sliced thickly, and stored in a cool place prior to use. In Korean medicine, GRR is indispensable in many medicinal herbal formulas since it is able to harmonize with as well as moderate the characteristics of other medicinal herbs.6, 7
To date, rodents are the most widely used animal to study PCOS due to their benefits, including smaller size, short life-span, high reproduction rate, and variety of genetic strains.8 PCOS studies using experimental rat models have focused on neonatal androgenization, injection of steroidal analogs, and endocrine-disrupting chemicals such as aromatase inhibitor.3 Although there are numerous animal models available for the development of PCOS-like symptoms, no model has yet been established due to difficulties in testing the efficacies of variable substances. Androgens have been used to induce acute PCOS conditions in rodents through daily injection or s.c. implantation.9 Hence, re-establishment of normal reproductive and/or ovarian cycling occurs after withdrawal of androgens.10 Subcutaneous implantation includes the rat PCOS model induced by Letrozole, a non-steroidal aromatase inhibitor that blocks the conversion of androgens into estrogen.11 Letrozole-induced PCOS rat models present many features of human PCOS.3, 11 The Letrozole-induced model is ideal for the study of aromatase deficiency-induced classic PCOS, and Letrozole may be effective in co-treated with natural or medicinal substances.
Recently, herbal remedies for PCOS have received attention as a form of lifestyle management in traditional medicine clinics, in which the menstrual cycle and normal serum hormones levels can be recovered.12 Herbal remedies are known to be effective in reducing testosterone as well as increasing FSH and 17β-estradiol levels,13, 14 and they have been shown to reduce polycystic ovaries and ovarian volume, improve insulin sensitivity, and normalize reproductive cycles.15, 16, 17 Additionally, clinical investigations have reported no adverse effects for herbal medicines.12, 18 However, conclusive evidence regarding absolute therapy could not be obtained in these clinical studies due to the absence of pre-clinical data explaining the mechanism of PCOS therapy.12, 13, 18
Natural substances are becoming more common in replacing established medications for the treatment of PCOS. Considering our interest in GRR extract as an alternative medicine and therapy for PCOS, we investigated whether or not GRR extract regulates hormonal imbalances, irregular follicular phase, and abnormal histologic changes in Letrozole-induced female PCOS rats. Additionally, genetic markers for follicular phase were examined using quantitative real-rime PCR in ovaries of PCOS rats.
2. Methods
2.1. Plant material and reagents
The GRR was purchased from Backjaedang, an Oriental medicine pharmacy in Daejeon, South Korea. The GRR extract was prepared by adding 10 times 70% (v/v) ethanol to the dried GRR (200 g), leaching at 80°C for 3 hours, and then obtaining a GRR ethanol extract. The GRR 70% ethanol extract was filtered through a 5um filter paper, concentrated by a vacuum rotary vacuum concentrator, and lyophilized using a freeze dryer. The final yield of GRR ethanol ext. was 22.41% w/w (44.85 g).
2.2. Animals
Sprague Dawley rats were obtained from Daehan Biolink (Eumseong, South Korea) and divided into three groups of six rats each. Rats were allowed to adapt to laboratory conditions (temperature: 20 ± 2°C, relative humidity: 45 ± 5%, light/dark cycle: 12 h) for 1 week. All animal experimental procedures were approved by the Ethics Committee of the Korea Institute of Oriental Medicine (approved no. 17-031). For the PCOS rat model, a 90-day release pellet (IRA; Innovative Research of America, OH, USA) containing Letrozole (1.8 mg/pellet) was implanted subcutaneously for 4 weeks under anesthesia [Zoletile (30 mg/kg)–Rompun (5 mg/kg)–saline mixture (2:1:2)] in 6-week-old female rats. For the acceleration of PCOS symptoms, a high dose of Letrozole (1 mg/kg) as prepared in 0.2% CMC (carboxymethyl cellulose) and administered by p.o. at 2 weeks after pellet implantation. After Letrozole (1 mg/kg) treatment, GRR extract (300 mg/kg) was prepared in 0.2% CMC and administered by p.o. treatment for 2 weeks. All rats were euthanized at 24 hours after the final injection.
2.3. Serum hormone analysis
Blood samples were collected directly from the inferior vena cava using a 1-mL syringe at the end of the experiment. Serum was obtained by centrifugation at 2000 × g for 10 min and stored at −70°C until use. Serum luteinizing hormone (LH) and follicular-stimulating hormone (FSH) were measured using ELISA kits (Cusabio Biotech, Wuhan, China). All hormone levels were measured according to the manufacturer's instructions.
2.4. Reverse transcription and real-time PCR
Total RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) following the manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA with a Thermo Scientific Revert Aid First Strand cDNA Synthesis Kit (Thermo, a Corporation, Massachusetts, USA) and amplified by RT-PCR using AmpliTaq Gold DNA polymerase and Quantitative real-time PCR using Premix ExTaq (TaKaRa, Shiga, Japan) with SYBR Green I (Molecular Probes, Eugene, OR, USA) and the Step One Plus system (Applied Biosystems). Primer was synthesized by Macrogen Inc. (Seoul, Korea). Actin expression was used as the control. The primers used for RT-PCR are shown in Table 2. All experiments were run in triplicate, and mRNA values were calculated based on the cycle threshold and monitored for an amplification curve.
Table 2.
The List and Sequence of Primers Used for RT-PCR Analysis
| No. | Gene | Forward primer (5′–3′) | Forward primer (3′–5′) | Annealing temperature (°C) |
|---|---|---|---|---|
| 1 | Kitl | GGTAGCCAGGAGTTTGTTCT | TTGTGTGGCATAAGGGCT | 58 |
| 2 | Cyp11a1 | TCCTCAAAGCCAGCATCA | ATCTCGACCCATGGCAAA | 58 |
| 3 | Ptgs2 | ACCTCTCTGAACTATGGTGT | TGCAGTCTGCTTTATGCG | 58 |
| 4 | Actin | TACGTCGCCCTGGATTTT | ATGAAAGAGGGCTGGAAGAG | 60 |
2.5. Histological analysis
Ovary tissues were fixed in 10% buffered formalin for 48 hours and paraffin-embedded. Paraffin-embedded tissue sections were de-waxed, re-hydrated, and stained with hematoxylin and eosin (H&E). The stained slides were examined using the Pannoramic DESK Digital Slide Scanning System (3d Hstech, Budapest, Hungary). The section with the largest area was chosen for analysis to count the numbers of follicular cysts and antral follicles, and the thicknesses of the antral follicles theca- and granulosa layers were measured. Historical changes in ovaries were confirmed to the criteria of Wu et al.19
2.6. Statistical analysis
Results for the hormonal levels of LH, FSH, LH/FSH ratio, number of follicular cysts, and transcriptional levels of follicular phase markers are presented ±SEM. Differences between means were obtained by conducting Student ct-test, which was performed using Graph Pad Software (GraphPad Inc., San Diego, CA, USA).
3. Results
3.1. Anima condition and he serum hormone level(s) of PCOS rats
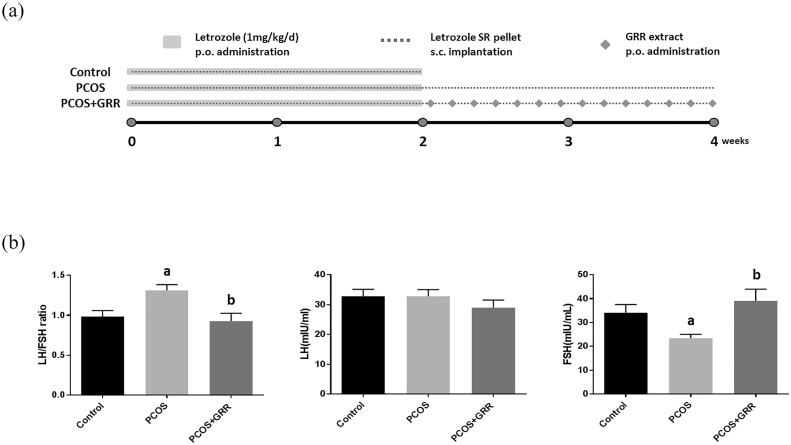
Although PCOS is a serious condition that can lead to anovulation and infertility, its underlying mechanism and precise cause are not fully understood. In the present study, we used PCOS rats to mimic human PCOS conditions and determine whether or not GRR (300 mg/kg) treatment could improve hormonal imbalance, irregular follicular phase, and polycysts. Letrozole-induced PCOS rats were induced by both sustained release of Letrozole (1.8 mg/pellet) and p.o. administration. Bodyweights and daily food intake were not significantly altered during the experiment, whereas uterine weight was significantly reduced in both the PCOS and PCOS + GRR groups (Table 1). Ovarian weight was slightly elevated in the PCOS group, but not significantly. Treatment with Letrozole, an aromatase inhibitor, resulted in marked reduction in the serum FSH level, which was significantly recovered by Letrozole and GRR extract co-treatment compared with the control group (Fig. 1b). Additionally, the serum level of LH was not significantly different among the groups (Fig. 1b). Since elevation of the serum LH/FSH ratio is a key symptom of PCOS,20 we calculated it based on the levels of LH and FSH. The LH/FSH ratio was significantly elevated in the PCOS group but was effectively regulated in the control group (Fig. 1b).
Table 1.
Body Weight and Daily Food Intake of PCOS Rats
| Groups | Body weight (g) |
Daily food intake (g) | Ovarian weight (mg) | Uterine weight (mg) | |
|---|---|---|---|---|---|
| Baseline | After 28 days | ||||
| Control | 110.29 ± 2.06 | 193.87 ± 1.97 | 13.50 ± 2.58 | 123.70 ± 45.63 | 282.90 ± 80.47 |
| PCOS | 109.69 ± 2.62 | 196.46 ± 4.87 | 13.34 ± 1.75 | 134.44 ± 30.72 | 141.67 ± 57.00a |
| PCOS + GRR | 106.72 ± 2.20 | 199.66 ± 5.06 | 13.28 ± 1.04 | 127.75 ± 21.43 | 104.38 ± 40.19a |
p < 0.05 vs. Control.
Fig. 1.
Effect of GRR extract treatment on serum hormone level(s). After 2 weeks from experiment initiation, GRR extract was exposed into rat by P.O. treatment every day for 2 weeks. Control was removed pellet after 2-weeks from implantation. (a) Animal experimental design. (b) Plasma steroid hormonal level such as FSH, LH and LH/FHS ratio were measured using a competitive ELISA kit. ap < 0.05 vs. Control group. bp < 0.05 vs. PCOS group.
3.2. Follicular phase transcriptional markers in the ovary of PCOS rats
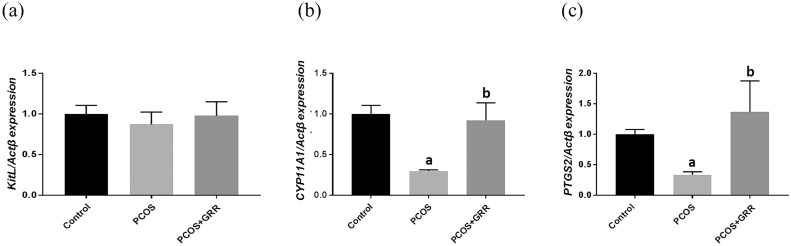
To investigate the effects of GRR extract, mRNA expression levels of ovarian Kitl, Cyp11a1, and Ptgs2, which are associated with follicle development and serve as biomarkers to diagnose follicular stage in ovaries (primary (Kitl), early antral (Cyp11a1), and late antral (Ptgs2) ovarian stages), were measured.21 The transcriptional level of Kitl was not significantly different between the groups, whereas mRNA levels of Cyp11a1 and Ptgs2 were significantly up-regulated in the GRR treatment group as compared with the PCOS group (Fig. 2). These results assume that induction of follicular phase markers, Cyp11a1 and Ptgs2, may be involved in triggering arrested follicular phase in Letrozole-induced PCOR rats during the ovarian cycle.
Fig. 2.
Effects of GRR extract on the mRNA expression of follicular phase marker genes in the ovary of rats. Comparison of folliculogenesis related gene mRNA expression levels between three groups (Control, PCOS and PCOS + GRR). (a) Kitl, (b) Cyp11a1 and (c) Ptgs2 mRNA expression were tested using RT-PCR in ovary tissue from rats of each experimental group. Experiments were carried out in triplicates and data expressed are means ± SEM. ap < 0.05 vs. Control group. bp < 0.05 vs. PCOS group.
3.3. Changes of ovarian morphological parameters in PCOS rats
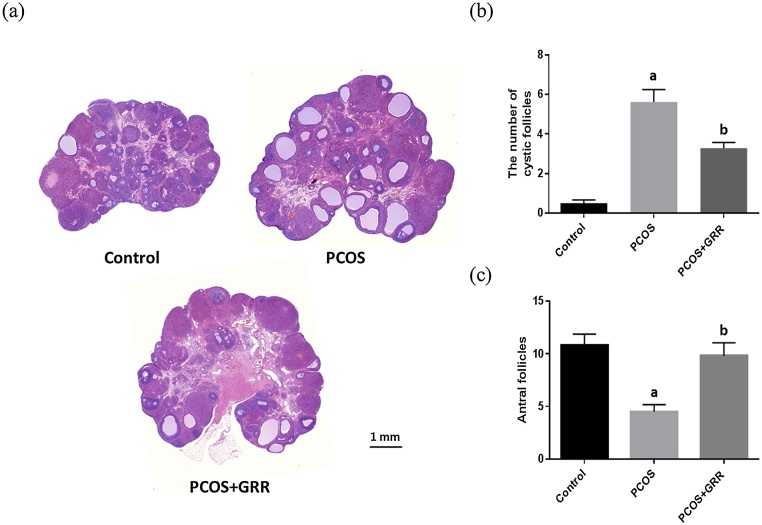
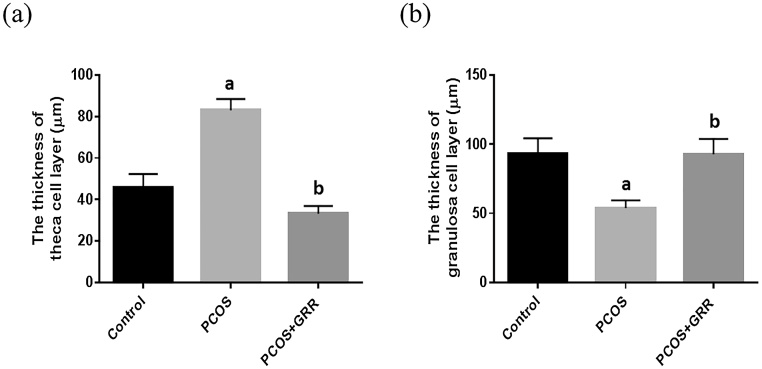
For observation of ovarian histological changes in PCOS rats, induction of PCOS caused by Letrozole treatment was observed by hematoxylin and eosin staining. The results obtained from changes in ovarian weight show that ovarian weight slightly increased after induction of PCOS, but not significantly (Table 1). Treatment with the aromatase inhibitor Letrozole resulted in an increased number of follicular cysts as well as increased thickness of the antral follicle theca layer compared to the control group, whereas the number of follicular cysts and theca layer thickness were reduced in GRR extract-treated rats (Fig. 3, Fig. 4). Moreover, the number of antral follicles and granulosa cell layer thickness were evaluated under a light microscope. The number of antral follicles and granulosa cell layer thickness were elevated by GRR extract compared to the control group (Fig. 3, Fig. 4).
Fig. 3.
Effects of GRR extract on the number of follicular cyst(s) in the ovary of rats. (a) H&E stained ovary, magnification (10×). (b) The number of follicular cyst(s). (c) The number of antral follicle(s) in the ovary (bottom). ap < 0.05 vs. Control group. bp < 0.05 vs. PCOS group.
Fig. 4.
Effects of GRR extract on the thickness of antral follicle theca- and granulosa cell layer(s) of PCOS in the ovary of rats. (a) The thickness of antral follicle theca cell layer (μm). (b) The thickness of antral follicle theca cell layer (μm). ap < 0.05 vs. Control group. bp < 0.05 vs. PCOS group.
4. Discussion
PCOS is a common diagnosis in women presenting with anovulatory infertility, and it affects 5–10% of women of reproductive age.1 Symptoms of PCOS related to ovulation manifest as amenorrhea or oligomenorrhea.22 Polycystic ovaries are enlarged and contain a large number of immature follicles. There are also metabolic disorder associated with PCOS such as insulin resistance and hyperinsulinemia in women.23, 24 During the follicle development reduces progesterone and estrogen levels due to regression of the corpus luteum.25 Consequently, release from negative feedback suppression allows a small but steady increase in FSH and LH levels, which stimulates the growth phase of ovarian follicles.25, 26 Hormonal contraceptives, selective estrogen receptor modulator (SERM), insulin sensitizers, gonadotropins, and ovarian surgery have been shown to be useful for improving PCOS symptoms in women.22, 27, 28 These treatment approaches address a variety of PCOS symptoms, although current PCOS treatments are not successful in all phases of irregular ovarian function.22, 27, 28
Herbal medicine usage by women has increased over the past decade. Herbal remedies are known to contain pharmacologically active constituents with physiological effects on female endocrinology and have been shown to be positively associated with reduced incidence of breast, bone, and cardiovascular diseases.29, 30 Previously, clinical studies have examined the androgen-lowering effects of Glycyrrhiza glabra, including reduction of testosterone levels in healthy women during menstrual cycles.31 In another clinical study, Cinnamomum cassia was treated to overweight women to alleviate oligo-/amenorrhoea and PCOS symptoms,32 and consumption of green tea improved PCOS symptoms such as overweight, hyperinsulinemia, and hyperandrogenism in a women's study.18 Selection of herbal medicine for treatment of PCOS often includes a combined prescription, which was shown to be more robust than single herbal treatment in several clinical trials.13, 15, 33 Based on consistent laboratory and clinical outcomes, herbal medicines were found to be useful for improving PCOS symptoms. However, limitations include inadequate PCOS animal models and small sample sizes.
The androgen-lowering effects of Glycyrrhiza uralensis have been demonstrated in an animal study examining hormone concentration in female rats, which reported significantly reduced free and total testosterone levels.34 However, no such results have been reported in a PCOS model. In the present study, we induced aromatase inhibition in 6-week-old female SD rats in order to induce conditions similar to those of PCOS in women. We then examined hormonal concentrations and historical changes in the ovaries as well as transcriptional markers for follicular phase in Letrozole-induced PCOS rats. In this study, the LH/FSH ratio significantly decreased following treatment with GRR extract. Administration of GRR extract resulted in successful elimination of PCOS-associated symptoms, including thickening of the theca layer, thinning of the granulosa layer of antral follicles, reduction of number of antral follicles, and induction of number of follicular cysts. The thickened granulosa layer supports the progression of follicular growth and maturation, and sufficient circulating levels of estradiol can be produced by aromatization of androgen in matured granulosa cells.35, 36 The granulosa-theca cell interaction is essential for the maintenance of normal follicular structure and function, however, the hyperplasia of the theca interna is represented the major source of ovarian dysfunction in PCOS.36
Although the distribution of the types of follicles is not clear, genes can serve as biomarkers to distinguish follicular stage or follicle type, such as Kitl, Cyp11a1, and Ptgs2 in the ovaries. Kitl is produced in granulosa cells and is expressed in the early stage follicular genesis in primary follicles.37 The transcriptional and/or translational expression of Cyp11a1 is regulated by FSH secretion.38 A previous study showed that Cyp11a1 and Ptgs2 expression could serve as a valuable marker for pre-ovulatory follicles such as antral follicles.39, 40 The present study investigated the expression of Kitl, Cyp11a1, and Ptgs2 in a Letrozole-induced rat model of PCOS in vivo. The transcription levels of Cyp11a1 and Ptgs2 were significantly elevated in GRR extract-treated rats, whereas the mRNA level of Kitl did not change in any of the groups.
In conclusion, we designed a rat model of PCOS by p.o. administration of a sustained release pellet (Letrozole) in order to obtain a model with clinical similarities to PCOS in women. We further demonstrated the anti-PCOS effects of GRR extract using an in vivo model of PCOS. Based on the results of the present study, it appears that GRR extract may be a potent therapeutic for the treatment of PCOS by controlling levels of serum FSH, LH/FSH ratio, irregular follicular phase, and abnormal historical changes in ovaries of PCOS rats.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by a grant from the Korea Institute of Oriental Medicine (grant no. K18292).
References
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi M.O., Dumesic D.A., Chazenbalk G., Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 3.Walters K.A., Allan C.M., Handelsman D.J. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86:149. doi: 10.1095/biolreprod.111.097808. 1–12. [DOI] [PubMed] [Google Scholar]
- 4.Park I., Ryuk J., Lee H., Go H., Ko B. In vitro and in vivo effects of ethanol extract combined with Curcumae Radix and Glycyrrhizae Radix et Rhizoma on menopausal metabolic disturbances. Int J Clin Exp Med. 2015;8:15076–15086. [PMC free article] [PubMed] [Google Scholar]
- 5.Xie W., Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. 2011;13:289–301. doi: 10.1111/j.1463-1326.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 6.Gong H., Zhang B.K., Yan M., Fang P.F., Li H.D., Hu C.P. A protective mechanism of licorice (Glycyrrhiza uralensis): isoliquiritigenin stimulates detoxification system via Nrf2 activation. J Ethnopharmacol. 2015;162:134–139. doi: 10.1016/j.jep.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 7.Su B., Li X.B. [Advance in studies on effect of Glycyrrhizae Radix et Rhizoma in relieving purgative activity of Rhei Radix et Rhizoma] Zhongguo Zhong Yao Za Zhi. 2015;40:577–581. [PubMed] [Google Scholar]
- 8.Singh K.B. Persistent estrus rat models of polycystic ovary disease: an update. Fertil Steril. 2005;84(Suppl 2):1228–1234. doi: 10.1016/j.fertnstert.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Parker C.R., Jr., Mahesh V.B. Hormonal events surrounding the natural onset of puberty in female rats. Biol Reprod. 1976;14:347–353. doi: 10.1095/biolreprod14.3.347. [DOI] [PubMed] [Google Scholar]
- 10.Shi D., Vine D.F. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 2012;98:185–193. doi: 10.1016/j.fertnstert.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Daneasa A., Cucolas C., Lenghel L.M., Olteanu D., Orasan R., Filip G.A. Letrozole vs estradiol valerate induced PCOS in rats: glycemic, oxidative and inflammatory status assessment. Reproduction. 2016;151:401–409. doi: 10.1530/REP-15-0352. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.H., Jo J. Successful treatment with Korean herbal medicine and lifestyle management in an obese woman with polycystic ovarian syndrome. Integr Med Res. 2017;6:325–328. doi: 10.1016/j.imr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akdogan M., Tamer M.N., Cure E., Cure M.C., Koroglu B.K., Delibas N. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother Res. 2007;21:444–447. doi: 10.1002/ptr.2074. [DOI] [PubMed] [Google Scholar]
- 14.Grant P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother Res. 2010;24:186–188. doi: 10.1002/ptr.2900. [DOI] [PubMed] [Google Scholar]
- 15.Kudolo G.B., Wang W., Javors M., Blodgett J. The effect of the ingestion of Ginkgo biloba extract (EGb 761) on the pharmacokinetics of metformin in non-diabetic and type 2 diabetic subjects – a double blind placebo-controlled, crossover study. Clin Nutr. 2006;25:606–616. doi: 10.1016/j.clnu.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Phipps W.R., Martini M.C., Lampe J.W., Slavin J.L., Kurzer M.S. Effect of flax seed ingestion on the menstrual cycle. J Clin Endocrinol Metab. 1993;77:1215–1219. doi: 10.1210/jcem.77.5.8077314. [DOI] [PubMed] [Google Scholar]
- 17.Frische E.J., Hutchins A.M., Martini M.C., Thomas W., Slavin J.L. Effect of flaxseed and wheat bran on serum hormones and lignan excretion in premenopausal women. J Am Coll Nutr. 2003;22:550–554. doi: 10.1080/07315724.2003.10719335. [DOI] [PubMed] [Google Scholar]
- 18.Tehrani H.G., Allahdadian M., Zarre F., Ranjbar H., Allahdadian F. Effect of green tea on metabolic and hormonal aspect of polycystic ovarian syndrome in overweight and obese women suffering from polycystic ovarian syndrome: a clinical trial. J Educ Health Promot. 2017;6:36. doi: 10.4103/jehp.jehp_67_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C., Lin F., Qiu S., Jiang Z. The characterization of obese polycystic ovary syndrome rat model suitable for exercise intervention. PLoS One. 2014;9:e99155. doi: 10.1371/journal.pone.0099155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banaszewska B., Spaczynski R.Z., Pelesz M., Pawelczyk L. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemia. Rocz Akad Med Bialymst. 2003;48:131–134. [PubMed] [Google Scholar]
- 21.Epifano O., Dean J. Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab. 2002;13:169–173. doi: 10.1016/s1043-2760(02)00576-3. [DOI] [PubMed] [Google Scholar]
- 22.Morgante G., Massaro M.G., Di Sabatino A., Cappelli V., De Leo V. Therapeutic approach for metabolic disorders and infertility in women with PCOS. Gynecol Endocrinol. 2018;34:4–9. doi: 10.1080/09513590.2017.1370644. [DOI] [PubMed] [Google Scholar]
- 23.Diaz R. Reproductive endocrinology: diabetes, puberty and pcoS risk. Nat Rev Endocrinol. 2011;7:3. doi: 10.1038/nrendo.2010.204. [DOI] [PubMed] [Google Scholar]
- 24.Gebel E. A syndrome of their own: PCOS and its links to diabetes in women. Diabetes Forecast. 2012;65:34. [PubMed] [Google Scholar]
- 25.Jonard S., Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10:107–117. doi: 10.1093/humupd/dmh010. [DOI] [PubMed] [Google Scholar]
- 26.Franks S., Stark J., Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 27.Artini P.G., Di Berardino O.M., Simi G., Papini F., Ruggiero M., Monteleone P. Best methods for identification and treatment of PCOS. Minerva Ginecol. 2010;62:33–48. [PubMed] [Google Scholar]
- 28.Kuang H., Jin S., Thomas T., Engmann L., Hansen K.R., Coutifaris C. Predictors of participant retention in infertility treatment trials. Fertil Steril. 2015;104:1236–1243. doi: 10.1016/j.fertnstert.2015.08.001. e1231-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith C.A., Bateson D.J., Weisberg E. A survey describing the use of complementary therapies and medicines by women attending a family planning clinic. BMC Complement Altern Med. 2013;13:224. doi: 10.1186/1472-6882-13-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stankiewicz M., Smith C., Alvino H., Norman R. The use of complementary medicine and therapies by patients attending a reproductive medicine unit in South Australia: a prospective survey. Aust N Z J Obstet Gynaecol. 2007;47:145–149. doi: 10.1111/j.1479-828X.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 31.Armanini D., Mattarello M.J., Fiore C., Bonanni G., Scaroni C., Sartorato P. Licorice reduces serum testosterone in healthy women. Steroids. 2004;69:763–766. doi: 10.1016/j.steroids.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Wang J.G., Anderson R.A., Graham G.M., III, Chu M.C., Sauer M.V., Guarnaccia M.M. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: a pilot study. Fertil Steril. 2007;88:240–243. doi: 10.1016/j.fertnstert.2006.11.082. [DOI] [PubMed] [Google Scholar]
- 33.Kao Y.H., Hiipakka R.A., Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141:980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi T., Nishii O., Okamura T., Yaginuma T. Effect of traditional herbal medicine, shakuyaku-kanzo-to on total and free serum testosterone levels. Am J Chin Med. 1989;17:35–44. doi: 10.1142/S0192415X89000073. [DOI] [PubMed] [Google Scholar]
- 35.Wood J.R., Nelson V.L., Ho C., Jansen E., Wang C.Y., Urbanek M. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem. 2003;278:26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 36.Bachler M., Menshykau D., De Geyter C., Iber D. Species-specific differences in follicular antral sizes result from diffusion-based limitations on the thickness of the granulosa cell layer. Mol Hum Reprod. 2014;20:208–221. doi: 10.1093/molehr/gat078. [DOI] [PubMed] [Google Scholar]
- 37.Albertini D.F., Barrett S.L. Oocyte-somatic cell communication. Reprod Suppl. 2003;61:49–54. [PubMed] [Google Scholar]
- 38.Wayne C.M., Fan H.Y., Cheng X., Richards J.S. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 39.Sirois J., Sayasith K., Brown K.A., Stock A.E., Bouchard N., Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10:373–385. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]
- 40.Hatzirodos N., Hummitzsch K., Irving-Rodgers H.F., Rodgers R.J. Transcriptome comparisons identify new cell markers for theca interna and granulosa cells from small and large antral ovarian follicles. PLoS One. 2015;10:e0119800. doi: 10.1371/journal.pone.0119800. [DOI] [PMC free article] [PubMed] [Google Scholar]