Abstract
Gastric adenocarcinoma is one of the most common types of cancer worldwide, with an incidence of a million new cases annually. In addition to having a high mortality rate due to often delayed detection and its poor response to cancer therapy, it also spreads aggressively. Inflammation has been shown to play a role in carcinogenesis. Consequently, macrophages are important in phagocytosis, antigen presenting and producing cytokines and growth factors. As a response to microenvironmental signals, they may polarize into tumor resisting M1 or tumor promoting M2 macrophages. Recently, studies have indicated that M2-type macrophage resembling tumor-associated macrophages (TAMs) might be used as an independent prognostic factor for gastric cancer. This review will discuss the possible use of TAMs as prognostic tools for gastric cancer and whether they are suitable for use in clinical environment.
Keywords: Gastric cancer, Tumor-associated macrophages, Biomarkers, Prognosis, Diagnosis
Background
Worldwide, gastric cancer is one of the most commonly diagnosed types of malignancy. Even though the incidence of the disease has decreased in recent years, it is still the second most common cause of all cancer-related deaths.1, 2 Each year approximately 990,000 new cases are diagnosed and 378,000 of these patients will die because of gastric cancer.3 Gastric cancer is approximately 50% more common among males than females and the highest incidence rates can be found in Eastern Asia, South America and Central and Eastern Europe.4
The classification of tumors is based on clinical and pathological findings. Due to wide morphological variation, there are several ways to classify gastric adenocarcinomas. The most common classification tumor-node-metastasis (TNM) divides them to either a superficial (T1) or an advanced type (T2–T4).5 Classification can also be made by morphological characteristics including mucus production to the cytoplasm and glandular vs. tubular differentiation rate. Despite the fact that all gastric adenocarcinomas originate from the glandular epithelium of the stomach, the types vary morphologically depending on the location of the affected glands (pyloric, fundic, cardiac), the surface area and possible metastasized lesions of the cancer.6 A histopathological classification method, Lauren's criteria that divide it into intestinal, diffuse and mixed types, is widely used to describe gastric adenocarcinoma.7 It has been noted that diffuse-type adenocarcinoma is more commonly diagnosed in females and young patients while intestinal type is associated with H. Pylori infection and intestinal metaplasia.8, 9
Gastric cancer may be treated with radio- and/or chemotherapy, although surgical resection forms the cornerstone of the treatment. Despite the development of modern medicine, surgical or endoscopic intervention remains the only cure for the disease since the sensitivity to the oncologic treatment varies among the patients. This creates a real challenge for physicians to determine the optimal individually tailored treatment plan for each patient.9 Adjuvant chemo- and radiotherapy has shown no evidence of increasing the overall survival (OS) rate following resection if used alone. However, chemotherapy may enhance the quality of life when compared to best supportive care.10
In the microenvironment of solid tumors, macrophages are the most abundant immune cells. Their presence correlates with worse prognosis in several cancers. Tumor-associated macrophages (TAMs) promote tumor progression, invasion and angiogenesis by secreting inflammatory substrates, cytokines, growth factors and proteolytic enzymes. In addition, they suppress the host immune response and engage in the activation of growth enhancing signaling pathways in tumor cells.11
Macrophages and cancer
A crucial part of our immune system is maintained by tissue macrophages which arise from monocytes circulating in the blood. Connective tissue is rich in macrophages, especially the mucosal layer of the gastrointestinal tract. Macrophages have an important effect on apoptotic cell destruction, vessel formation and inflammation development.12
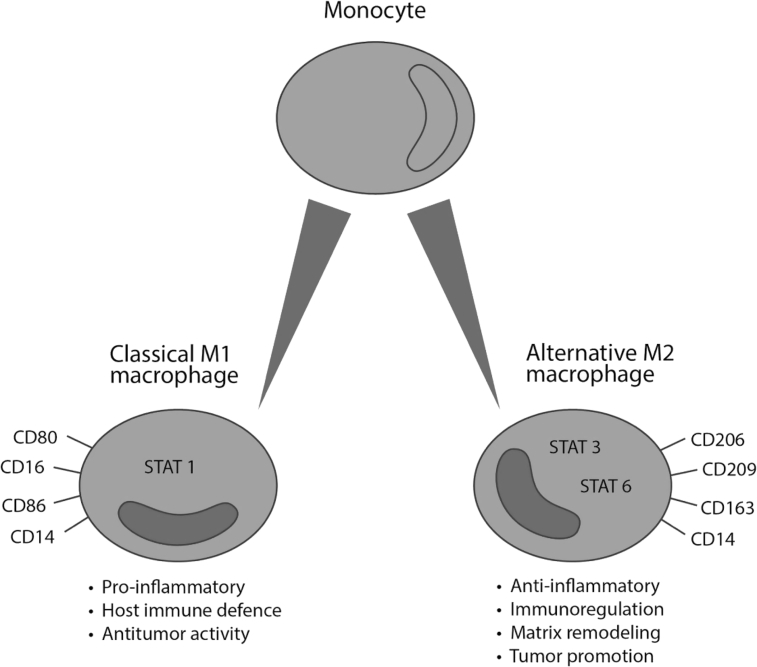
In response to environmental signals, such as interferon gamma (IFN-γ) and lipopolysaccharide (LPS), macrophages may polarize into two main types: M1 and M2 macrophages (Fig. 1). M1-type macrophages have an important role in antitumoral immunity and inflammatory response. They inhibit tumor growth by producing pro-inflammatory cytokines such as IL-6, IL-12, IL-8 and tumor necrosis factor alpha (TNF-α).13 In addition, M1 macrophages express major histocompatibility class one and two complexes that are required in presenting tumor-specific antigens.14 M2-type macrophages act as anti-inflammatory agents by suppressing host immune response. They also have an impact in tumor matrix remodeling which promotes tumor proliferation and invasion. In addition, M2-type macrophages advance the tumor progression by producing anti-inflammatory agents such as IL-10, IL-4 and IL-13.15 In contrast to M1-type macrophages, TAMs are referred to as type M2 macrophages that are maturated in tumor-tissue environment after leaving the blood circulation as monocytes. They may have an effect on the inactivation of T-cells, which crucially decreases the body's ability to resist cancer development and progression.16 It has been shown that the patients with a large number of TAMs in cancer tissue have worse surgical outcome compared to the patients with a lower number of TAMs. TAMs also promote tumor angiogenesis, enhance metastatic spreading and contribute to invasion by producing cytokines such as IL-6, IL-17, IL-23 and inhibiting cytotoxic T lymphocyte responses.17
Fig. 1.
Macrophage polarization. CD: cluster of differentiation; STAT: signal transducer and activator of transcription.
Infiltration of TAMs correlates with tumor invasion and metastasis. Tumor cells secrete the colony stimulating factor 1 (CSF-1), while TAMs secrete epithelial growth factor (EGF). They have inducing effect in co-migration and invasion of both cell types towards blood vessels.18 In vitro, distant metastasis macrophages increase the invasion rate compared to non-metastatic cells. The migration rate increases significantly when macrophage-containing cell line is cultured under hypoxic conditions. Hypoxia increased the expression of disintegrin and metalloproteinase domain-containing protein 8 & 9 coding genes (ADAM8 & ADAM9), while it decreased expression of matrix metallopeptidase 9 (MMP9) and tissue inhibitor of metalloproteinase 3 (TIMP3). ADAMs and TIMP3 gene together with MMP9 may contribute to the TAMs role in gastric cancer's fast and aggressive invasion.19
Based on previous studies, the development of new cancer treatments targeting tumor promoting macrophages is possible. Researchers are optimistic that by therapeutic manipulation, the M2 macrophages can be reprogrammed into M1 macrophages that inhibit the tumor growth and proliferation.20 Manipulation of M2 macrophages is done by interfering TAM specific signaling pathway that controls the shift between tumor-promoting and tumor-preventing macrophages.21 There are also potential treatment possibilities that inhibit the TAM accumulation in tumor stroma and, thus, have an inhibiting influence in tumor progression.22
TAM as a potential biomarker for gastric cancer
TAMs have shown a great potential as diagnostic biomarkers in multiple myeloma, breast cancer, prostate cancer and pancreatic cancer. Further, as a prognostic biomarker, TAMs have shown potential in lung cancer, esophageal squamous cell carcinoma and bladder cancer.23
Leukocytes infiltrate commonly solid tumors, indicating that the tumor has triggered the host immune response. This is mediated by tumor antigens that distinguish tumor cells from normal healthy cells.24 These leukocytes include a variety of different subsets and the activation and the complexity of these cells vary by location and by the malignancy stage of the tumor. Usually, TAMs express cluster of differentiation (CD) 163, CD204 or CD206 receptors on their surface, are nonspecific for different types of cancer and could possibly be used as a screening method for several advanced cancers in clinical practice.25 Current understanding on TAMs as a potential biomarker for gastric cancer is summarized in Table 1.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44
Table 1.
Potential biomarkers for gastric cancer prognosis.
| Marker | Reference | Source | Elevated amount predicts |
|---|---|---|---|
| TAM infiltration (CD163+) | 35 | Tumor tissue | Poor prognosis |
| TAM density in solid tumors | 36,40 | Tumor tissue | Poor prognosis |
| Macrophage M2 infiltration | 40 | Tumor tissue | Poor prognosis |
| Macrophage M1 infiltration | 40 | Tumor tissue | Improved prognosis |
| TP | 26, 27, 28 | Tumor stroma | Poor prognosis |
| Diametrically polarized tumor-associated macrophages (protumoral M2 macrophages) | 38 | Tumor tissue | Poor prognosis |
| Tumor infiltrating antigen CD11+ | 37 | Tumor tissue | Poor prognosis |
| Serum macrophage MIF + CEA | 41, 42, 43 | Serum/tumor tissue | Poor prognosis |
| OPN | 29 | Tumor stroma | Poor prognosis |
| KRS | 34 | Tumor tissue/tumor-associated inflammatory cells | Poor prognosis |
| MR | 31 | Tumor tissue | Poor prognosis |
| CCL5/RANTES | 32, 33 | Tumor cells, macrophages, T cells | Increased tumor invasion Increased incidence of lymph node metastasis |
| NF-κB CCL2/CCR2 chemokines |
44 | Gene allele rs230510 and CCL2 rs4586 | A allele of rs230510 associated with improved OS; T allele of rs4586 associated with poor OS |
| Gastric adenoma, CD204 positive TAMs | 39 | Tissue stroma | Risk-factor for developing gastric adenocarcinoma |
| Tim-3 | 30 | Macrophages | Increased tumor invasion; increased incidence of lymph node metastasis & advanced clinical stage |
TAM: tumor-associated macrophage; CD: cluster of differentiation; TP: thymidine phosphorylase; MIF: macrophage migration-inhibitory factor; CEA: carcinoembryonic antigen; OPN: osteopontin; KRS: lysyl-tRNA synthetase; MR: mannose receptor; CCL: chemokine (C—C motif) ligand; RANTES: regulated upon activation, normal T-cell expressed and presumably secreted; NF-κB: nuclear factor-kappa B; CCR: C–C chemokine receptor; OS: overall survival; Tim-3: T-cell immunoglobulin and mucin domain-containing molecule-3.
Tumor cell/tissue protein markers
Thymidine phosphorylase (TP) is an enzyme that promotes angiogenesis, metastasis and invasion of various tumors. TP persuades oxidative stress and inflammation signals, taking part in producing reactive oxygen species by 2-deoxy-D-ribose. They influence vascular endothelial cell growth and cytokine production.26 Angiogenesis, invasion and metastasis of gastric cancers are correlated with production of TP in tumor stroma. Highly differentiated gastric adenocarcinomas expressed higher level of TP than poorly differentiated cancers.27 TP is found to be expressed in both tumor cells and macrophages in the tumor stroma of the gastric cancer. Microvessel density correlated with expression of TP in infiltrating macrophages/TAMs in both intestinal and diffuse type gastric cancer. This suggests that TP might be a potential biomarker for detecting macrophage infiltration in gastric cancer as well as predicting the possible lymph node and liver metastases in both diffuse and intestinal types of gastric adenocarcinoma. There is a significant association between macrophage infiltration in patients with intestinal type of gastric cancer and the poorer OS rate. Increased tumor-infiltration of TP expressing macrophages suggests increased tumor angiogenesis leading to poorer prognosis. In addition, the macrophage infiltration rate correlates jointly with TP expression and angiogenesis of the tumor.28
Lin et al29 studied osteopontin (OPN) and its role in macrophage recruitment in gastric cancer. Osteopontin is an extracellular matrix protein which is involved in many normal physiological processes. It promotes cell-mediated immune responses, acts as a cytokine and controls cell migration. Information was collected from 170 gastric cancer specimens and amounts of OPNs and TAMs were measured. This study indicated that OPNs skew macrophages to M2-TAM form. Co-expression of OPNs and CD204 in TAMs correlated with disease progression and poor 5-year survival (48.90%, P = 0.0131). This suggests the possible use of OPN and TAM amounts as biomarkers in gastric cancer.
New data regarding gastric cancer progression have also emerged from T-cell immunoglobulin and mucin domain-containing molecule-3 (Tim-3) expressed in macrophages, and its effect on the cancer immune system. The function of Tim-3 in macrophages and monocytes remains unclear. The gastric cancer patients showed upregulation of Tim-3 in monocytes compared to healthy controls. Upregulated Tim-3 is associated with lymph node metastasis, depth of tumor invasion and advanced clinical stages which suggest that Tim-3 might have an important effect in gastric cancer progression.30
Mannose receptor (MR) can be primarily found on the surface of macrophages where it acts as an immune adhesion molecule. It has an important function in phagocytosis and endocytosis. Tumor microenvironment expresses noticeably high amounts of MRs. Elevated amount of MR in gastric cancer cells correlated with tumor size, T-stage and N-stage. It was also noted that an elevated number of MRs were associated with shorter survival of the patients compared to patients with a lower number of MRs.31
Chemokine (C–C motif) ligand 5/regulated upon activation, normal T-cell expressed and presumably secreted (CCL5/RANTES) is a protein belonging to the chemokine family and is mainly expressed in T cells, macrophages and some tumor cells. It plays an important role in recruiting other leukocytes to the site of inflammation and together with cytokines induces proliferation and activation of natural killer cells (NK-cells) to form chemokine-activated killer (CHAK) cells.32 Gastric cancer tissues expressed highly elevated values of CCL5. Further, high values were associated with depth of tumor invasion, lymph node metastasis, advanced TNM stage and poor tumor differentiation. Serum CCL5 values of gastric cancer patients were also elevated in comparison to healthy individuals. CCL5 secreted by TAMs may have a promoting effect in tumor invasion, proliferation and metastasis of gastric cancer cells. Correlation studies suggested that CCL5 could be an important molecular marker for gastric cancer staging and disease progression.33
Lysyl-tRNA synthetase (KRS) is an aminoacyl-tRNA synthetase essential for protein synthesis. An abnormal amount of KRS is associated with many types of cancer. 43.3% of studied gastric carcinomas expressed high amounts of KRSs. Tumor-associated inflammatory cells (including macrophages and monocytes) expressed high amounts of KRSs in 37.2% of cases. There is an association between expression of KRSs in tumor or tumor-associated inflammatory cells and known clinicopathological parameters for prognosis of gastric cancer and therefore KRS may be an independent prognostic marker for gastric cancer.34
TAM-cell infiltration
Infiltration of TAMs (CD163+) differs essentially when tumor tissue is compared to normal one. In a study of 178 gastric cancer patients, the tumor tissue expressed high CD163+ infiltration rates in 52.8% of cases. Normal tissue expressed high amounts of CD163+ only in 21.3% of cases. Patients with high expression of CD163+ had a shorter OS time (28.00 ± 2.20 months) compared to the ones with low expression of CD163+ (46.00 ± 3.05 months, P < 0.001). Study suggests that the high expression of CD163+ together with transforming growth factor beta (TGF-β) is associated with aggressive features of the cancer leading to poor prognosis, and therefore, could be used as independent prognostic factors in gastric cancer.35 A recent meta-analysis showed that the OS was significantly decreased with solid tumor patients who had a high density of TAMs (RR = 1.64, 95% CI: 1.24–2.16). This suggests that TAM infiltration is associated with worsened prognosis in gastric cancer patients. It was also noted that the increased numbers of M1 macrophages may be associated with better OS among gastric cancer patients.36
One possible independent prognostic factor is tumor-infiltrating CD11b+ antigen (glycoprotein) presenting cells that include TAMs and dendritic cells. They act as important factors in antitumor immune response. Factors including a massive size of a tumor, venous invasion and lymph node metastases correlated with intratumoral infiltration of CD11b+ cells, where gastric cancer patients with a high amount of CD11b+ cells had a poor survival rate compared to the patients with a low amount of CD11b+ cells.37
Diametrically polarized TAMs in this case protumoral M2 macrophages, have been proven to have an impact on OS of surgically resected gastric cancer patients. It has been proposed that they influence tumor phenotype alteration, which supports the fact that tumor microenvironment has a crucial impact in tumor biology. Lymph node metastases correlated with infiltration rate of polarized TAMs in gastric cancer. TAMs could be combined with the TNM stage to refine a risk stratification system for prognosis in patients with gastric cancer.38
Recently there have been findings on the role of gastric adenomas in the development of gastric cancer. A higher amount of CD204 positive TAMs in stroma was found to be a risk factor for an adenoma developing into a gastric adenocarcinoma. This suggests that there might be a potential way to screen high-risk patients who may develop gastric adenocarcinoma from gastric adenoma.39
In a study of 143 cases of microsatellite instability-high (MSI-high) advanced gastric cancer, the low density of CD68+ and CD163+ TAMs suggested intestinal type tumor in Lauren's classification. CD163+ receptor was used to distinguish M2 type macrophages from other subsets. Infiltration of other lymphocytes also correlated with density of CD163+ TAMs. Additionally, the poor disease-free survival was associated with low amounts of CD163+ TAMs. There is an interaction between TAMs and tumor infiltrating lymphocytes (TILs). Prognosis can be assessed based on the balance of these two.45
Another meta-analysis about the significance of TAMs in gastric cancer was made based on twelve studies that included all together 1388 patients. Shorter OS correlated with total increase of TAM infiltration (HR = 1.70; 95% CI: 1.39–2.09; P < 0.001). Similar effect was observed concerning M2 macrophages infiltration (HR = 1.71; 95% CI: 1.19–2.45; P = 0.004). Moreover, an elevated amount of M1 macrophages was associated with better OS (HR = 0.46; 95% CI: 0.33–0.65; P < 0.001). This suggests that the total TAMs and infiltrating M2 macrophages might be a negative prognostic factor for patients with gastric cancer while increased density of M1 macrophages predicted better OS. Analysis also showed a correlation between high TAM amount and decreased risk for lymph node metastasis in gastric and ovary cancers. This indicated that patients with high density of TAMs have lower probability to have lymph node metastasis. Even so, a significant negative impact was seen in OS. To clarify this ambivalence, more studies are still needed.40
Serum enzyme marker
Serum macrophage migration-inhibitory factor (MIF) has shown a great potential in diagnosing gastric cancer in patients with dyspepsia. MIF is a proinflammatory cytokine that takes part in innate immune response by influencing macrophage.41 Serum MIF level increased with advancing gastric pathologies (P < 0.001). High serum MIF values (above 6600 pg/ml) indicated lower 5-year survival rate among gastric cancer patients. High level of carcinoembryonic antigen (CEA) also predicted worse survival and by combining MIF and CEA it was possible to make more exact prediction of the 5-year survival.42 Under lipopolysaccharide (LPS) stimulation, MIF, CD74 and toll-like receptor 4 (TLR4) could form a complex that significantly stimulates cell proliferation. Upregulation of these three molecules is associated with increasing clinical stage in gastric cancer.43 Expression of MIF was higher in gastric cancer tissues compared to adjacent non-cancer normal tissues (P < 0.001). A high amount of MIF is significantly associated with lymph node metastasis, poor tumor differentiation, advanced tumor stage and poor survival (P < 0.05 for all).41
Gene related markers
One interesting point in gastric cancer research is the importance of genes coding for macrophage function. Nuclear factor-kappa B (NF-κB) and CCL2/C–C chemokine receptor 2 (CCR2) chemokines have a role in tumor progression, angiogenesis, invasion and migration. The nuclear factor-κB p50 (NFKB1) allele rs230510 and CCL2 rs4586 were significantly associated with clinical outcome in patients with locoregional gastric cancer. This suggests that the genetic propensity of the host has an important impact in determining the auto-immune component of the tumor for the progression of gastric cancer.44
Summary
Being one of the most lethal types of cancer, gastric cancer has also shown to have the capability to be very resistant to several oncologic treatments. Early-stage cancers show no symptoms and the diagnosis is usually made at an advanced stage when the tumor starts to cause symptoms. At an advanced stage, spreading of the tumor is fast and the effect of the cancer therapy is limited. Therefore, it is important to find biomarkers for early diagnosis enabling better treatment planning, decreasing the mortality and aiming physicians to make more specific prognosis for the patient.46
TAMs have shown great potential as biomarkers for evaluating the gastric cancer staging and progression. In addition to gastric adenocarcinoma, it may also be a useful biomarker in several other cancers, such as lung cancer, esophageal squamous cell carcinoma and bladder cancer. Some potential gastric cancer-biomarkers have already been identified including TP, OPN and MR. Studies have shown that the amount of TAMs in tumor stroma predicts the size, stage and the metastasis of the gastric tumor. This ensures more accurate prognosis and individual treatment planning for patients with gastric cancer. Patients with higher amounts of TAMs have shorter OS rate compared to those with lower amounts of TAMs. TAMs may, thus, provide a work tool for risk-patient screening, early diagnosis and prognosis formation.
Perspectives and future directions
According to some studies, the division between M1/M2 macrophages is excessively simplistic for TAM classification since it does not take into account many factors affecting TAMs function including the location of the tumor microenvironment, type of the cancer and stage of the tumor. TAMs have also been noticed to express features from both macrophage types, and because of these overlapping features the division seems old-fashioned. It is challenging to identify different subgroups of macrophages due to functional and phenotypical heterogeneity. This suggests that more precise classification is needed in order to tell apart different subtypes of TAMs.47
Several reviews do not recognize macrophages as clinically significant biomarkers for gastric cancer due to the fact that many recently studied biomarkers tend to show low sensitivity when tested on a large population. Many biomarkers, especially genetic markers, have been tested by very controlled parameters, and since in gastric cancer the environmental and ethnic factors have a significant influence, it is a real challenge to find both a very specific and an unbiased marker.48 Nonetheless, many studies have shown that the TAMs are associated with worse prognosis among gastric cancer patients.
There is still a lot to learn about the macrophage metabolism and how they react to the signals they are getting from the tumor microenvironment. TAMs have shown a great potential in formation of more individual prognosis for gastric adenocarcinoma patients. In addition, they have also shown promising results in prediction of tumor size, depth of invasion and lymph node metastasis. More studies are still needed in order to evaluate whether the TAM could be used as a biomarker for gastric cancer in clinical use.
Conflicts of interest
The authors declare no conflict of interest.
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Kelley J.R., Duggan J.M. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 2.Ferro A., Peleteiro B., Malvezzi M. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Karimi P., Islami F., Anandasabapathy S., Freedman N.D., Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomark Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 6.Goseki N., Takizawa T., Koike M. Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut. 1992;33:606–612. doi: 10.1136/gut.33.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J., Shen H., Kapesa L., Zeng S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11:2959–2964. doi: 10.3892/ol.2016.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu B., El Hajj N., Sittler S., Lammert N., Barnes R., Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Alberts S.R., Cervantes A., van de Velde C.J. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(suppl 2):31–36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen S.R., Schmid M.C. Macrophages as key drivers of cancer progression and metastasis. Mediat Inflamm. 2017;2017:9624760. doi: 10.1155/2017/9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon S., Martinez-Pomares L. Physiological roles of macrophages. Pflügers Archiv. 2017;469:365–374. doi: 10.1007/s00424-017-1945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Través P.G., Luque A., Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediat Inflamm. 2012;2012:568783. doi: 10.1155/2012/568783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paolino M., Penninger J.M. The role of TAM family receptors in immune cell function: implications for cancer therapy. Cancers (Basel) 2016;8 doi: 10.3390/cancers8100097. pii: E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dwyer A.R., Greenland E.L., Pixley F.J. Promotion of tumor invasion by tumor-associated macrophages: the role of CSF-1-activated phosphatidylinositol 3 kinase and Src family kinase motility signaling. Cancers (Basel) 2017;9 doi: 10.3390/cancers9060068. pii: E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishigami S., Natsugoe S., Tokuda K. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–4083. [PubMed] [Google Scholar]
- 18.Heusinkveld M., van der Burg S.H. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Z., Kauttu T., Seppänen H. Both macrophages and hypoxia play critical role in regulating invasion of gastric cancer in vitro. Acta Oncol. 2013;52:852–860. doi: 10.3109/0284186X.2012.718444. [DOI] [PubMed] [Google Scholar]
- 20.Sarode P., Zheng X., Weigert A. LSC-2017-reprogramming of tumor associated macrophages by modulating Wnt/ß-catenin signaling in lung cancer. Eur Respir J. 2017;50:OA4859. [Google Scholar]
- 21.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gooden M.J., de Bock G.H., Leffers N., Daemen T., Nijman H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L., Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y.C., Zou X.B., Chai Y.F., Yao Y.M. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–529. doi: 10.7150/ijbs.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elamin Y.Y., Rafee S., Osman N., O'Byrne K.J., Gately K. Thymidine phosphorylase in cancer; enemy or friend? Cancer Microenviron. 2016;9:33–43. doi: 10.1007/s12307-015-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimaoka S., Matsushita S., Nitanda T. The role of thymidine phosphorylase expression in the invasiveness of gastric carcinoma. Cancer. 2000;88:2220–2227. [PubMed] [Google Scholar]
- 28.Kawahara A., Hattori S., Akiba J. Infiltration of thymidine phosphorylase-positive macrophages is closely associated with tumor angiogenesis and survival in intestinal type gastric cancer. Oncol Rep. 2010;24:405–415. doi: 10.3892/or_00000873. [DOI] [PubMed] [Google Scholar]
- 29.Lin C.N., Wang C.J., Chao Y.J., Lai M.D., Shan Y.S. The significance of the co-existence of osteopontin and tumor-associated macrophages in gastric cancer progression. BMC Cancer. 2015;15:128. doi: 10.1186/s12885-015-1114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z., Yin N., Zhang Z., Zhang Y., Zhang G., Chen W. Upregulation of T-cell immunoglobulin and mucin-domain containing-3 (Tim-3) in monocytes/macrophages associates with gastric cancer progression. Immunol Invest. 2017;46:134–148. doi: 10.1080/08820139.2016.1229790. [DOI] [PubMed] [Google Scholar]
- 31.Liu D.R., Guan Q.L., Gao M.T., Jiang L., Kang H.X. Mannose receptor as a potential biomarker for gastric cancer: a pilot study. Int J Biol Markers. 2017;32:e278–e283. doi: 10.5301/jbm.5000244. [DOI] [PubMed] [Google Scholar]
- 32.Maghazachi A.A., Al-Aoukaty A., Schall T.J. CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol. 1996;26:315–319. doi: 10.1002/eji.1830260207. [DOI] [PubMed] [Google Scholar]
- 33.Ding H., Zhao L., Dai S., Li L., Wang F., Shan B. CCL5 secreted by tumor associated macrophages may be a new target in treatment of gastric cancer. Biomed Pharmacother. 2016;77:142–149. doi: 10.1016/j.biopha.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Kim B.H., Jung W.Y., Lee H. Lysyl-tRNA synthetase (KRS) expression in gastric carcinoma and tumor-associated inflammation. Ann Surg Oncol. 2014;21:2020–2027. doi: 10.1245/s10434-014-3522-z. [DOI] [PubMed] [Google Scholar]
- 35.Yan Y., Zhang J., Li J.H. High tumor-associated macrophages infiltration is associated with poor prognosis and may contribute to the phenomenon of epithelial-mesenchymal transition in gastric cancer. Oncotargets Ther. 2016;9:3975–3983. doi: 10.2147/OTT.S103112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X.L., Jiang J.T., Wu C.P. Prognostic significance of tumor-associated macrophage infiltration in gastric cancer: a meta-analysis. Genet Mol Res. 2016;15 doi: 10.4238/gmr15049040. [DOI] [PubMed] [Google Scholar]
- 37.Okita Y., Tanaka H., Ohira M. Role of tumor-infiltrating CD11b+ antigen-presenting cells in the progression of gastric cancer. J Surg Res. 2014;186:192–200. doi: 10.1016/j.jss.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H., Wang X., Shen Z., Xu J., Qin J., Sun Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer. 2015;18:740–750. doi: 10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 39.Taniyama D., Taniyama K., Kuraoka K. Long-term follow-up study of gastric adenoma; tumor-associated macrophages are associated to carcinoma development in gastric adenoma. Gastric Cancer. 2017;20:929–939. doi: 10.1007/s10120-017-0713-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q.W., Liu L., Gong C.Y. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He L.J., Xie D., Hu P.J. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2015;21:9916–9926. doi: 10.3748/wjg.v21.i34.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia H.H., Yang Y., Chu K.M. Serum macrophage migration-inhibitory factor as a diagnostic and prognostic biomarker for gastric cancer. Cancer. 2009;115:5441–5449. doi: 10.1002/cncr.24609. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y.X., Yang M., Rong T.T. CD74 and macrophage migration inhibitory factor as therapeutic targets in gastric cancer. World J Gastroenterol. 2012;18:2253–2261. doi: 10.3748/wjg.v18.i18.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunakawa Y., Stremitzer S., Cao S. Association of variants in genes encoding for macrophage-related functions with clinical outcome in patients with locoregional gastric cancer. Ann Oncol. 2015;26:332–339. doi: 10.1093/annonc/mdu542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim K.J., Wen X.Y., Yang H.K., Kim W.H., Kang G.H. Prognostic implication of M2 macrophages are determined by the proportional balance of tumor associated macrophages and tumor infiltrating lymphocytes in microsatellite-unstable gastric carcinoma. PLoS One. 2015;10:e0144192. doi: 10.1371/journal.pone.0144192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin L.L., Huang H.C., Juan H.F. Discovery of biomarkers for gastric cancer: a proteomics approach. J Proteomics. 2012;75:3081–3097. doi: 10.1016/j.jprot.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 47.Petty A.J., Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9:289–302. doi: 10.2217/imt-2016-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beeharry M.K., Liu W.T., Yan M., Zhu Z.G. New blood markers detection technology: a leap in the diagnosis of gastric cancer. World J Gastroenterol. 2016;22:1202–1212. doi: 10.3748/wjg.v22.i3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]