Abstract
Background
Currently, there is no clearly established therapy to treat mild cognitive impairment (MCI); consequently, alternative therapies, such as acupuncture, have been attempted. In many clinical studies, the potential benefits of acupuncture for cognitive improvement have been identified in clinical outcomes; however, the mechanism remains unclear. Accordingly, this study aims to investigate the therapeutic mechanism of acupuncture therapy using functional near-infrared spectroscopy and its feasibility in treating individuals with impaired cognitive function.
Methods
This study is designed to be a prospective, two-arm, parallel clinical trial involving 24 participants. The patient group will be treated with acupuncture twice per week for 12 weeks; meanwhile, the healthy control group will not undergo acupuncture treatment. Functional near-infrared spectroscopy assessment and a working memory test will be performed at baseline and every 6 weeks to investigate the therapeutic mechanism of acupuncture. The primary outcome will be measured using the Korean version of the Montreal Cognitive Assessment. The secondary outcomes will be the Alzheimer's Disease Assessment Scale-cognitive subscale score, working memory task accuracy, response rate, response time, and hemodynamic response of the prefrontal lobe. The outcomes will be evaluated at baseline, and at 6 and 12 weeks after subject allocation.
Discussion
This clinical pilot trial is designed to determine the feasibility of acupuncture as an effective and safe treatment for improving cognitive function in patients with MCI. Results of this study may provide guidance for future larger-scale clinical trials.
Trial registration
Clinical Research Information Service (CRIS), Republic of Korea: KCT0002451. Registered September 5, 2017.
Keywords: Acupuncture, Functional near-infrared spectroscopy, Mild cognitive impairment
1. Background
Mild cognitive impairment (MCI) refers to a transitional state between normal aging and early Alzheimer's disease (AD) and other dementias.1, 2, 3 In population-based studies, the prevalence of MCI in elderly individuals (≥65 years of age) is 10% to 20%, and 5% to 10% of patients with MCI progress to AD each year thereafter.4, 5, 6, 7 Given that AD is an irreversible illness, prevention and early intervention become increasingly important.8 This has led to increased interest in the early diagnosis and management of MCI. One of the leading symptoms of MCI and early AD is undermined memory, particularly working memory (WM).9, 10 WM is a short-term memory system that coordinates the temporary storage, simultaneous processing, and manipulation of information necessary to successfully perform complex cognitive functions.9, 10 WM is managed by the prefrontal and parietal lobes of the brain. In particular, the prefrontal lobe manages the overall higher-level cognitive functions, such as logical thinking, emotional control, planning and decision-making, in addition to WM. As such, impaired function of the prefrontal lobe explains, in large part, many symptoms of MCI.10, 11
Presently, there is no clearly established treatment for MCI, although several clinical studies have used AD treatment medications in cases involving MCI. This, however, this did not result in a significant decrease in the proportion of cases in which MCI progressed to AD.3, 12, 13 For this reason, various non-pharmacological treatments, such as cognitive training,14 physical treatment,15 and acupuncture,16, 17, 18, 19 have been attempted in managing MCI. In particular, acupuncture is a method that has been used to treat various neurological disorders for several thousands of years, including those involving stroke,20 AD,21 MCI,16, 17, 18, 19 and vascular dementia.22, 23
In several clinical studies, acupuncture has been shown to improve clinical outcome in patients with impaired cognitive function, and has emerged as a treatment that can be used alone or in combination with other therapies.17, 18, 19, 20, 21, 23 The use of acupuncture for cognitive function therapy has been increasing; however, its therapeutic mechanism remains unclear. In recent years, neuroimaging studies have been actively undertaken to identify the neural mechanisms of acupuncture that affect cognitive function in patients with MCI and/or AD.24, 25, 26 Feng et al.24 found that MCI patients exhibited reduced correlation in the temporal region (hippocampus, thalamus, fusiform gyrus) and prefrontal cortex compared with healthy age-matched subjects in the resting state. They also found that MCI patients exhibit significant functional connectivity changes in these abnormal regions after acupuncture. Wang et al.25 found that, after acupuncture, several regions exhibited increased or decreased activity in subjects with MCI and AD compared with normal subjects, and most of regions involved the temporal and frontal lobes, which are closely related to memory and cognition.
Given these previous studies, acupuncture appears to be a clinically feasible treatment for cognitive dysfunction because acupuncture stimuli can activate parts of the brain associated with cognitive function, including the temporal and frontal lobes. To date, neuroimaging studies have largely focused on the evaluation of brain activity before, during, or immediately after stimuli from acupuncture. Studies investigating changes in the brain activity of MCI patients after long-term acupuncture treatment, however, are scarce. Accordingly, this study aims to examine changes in the hemodynamic response of the prefrontal cortex that appears during WM tasks, before and after 12 weeks of acupuncture treatment using functional near-infrared spectroscopy (fNIRS) to identify potential improvement in cognitive function and the therapeutic mechanisms of acupuncture. Our hypothesis is that, after long-term acupuncture therapy (12 weeks, 24 sessions), normal and MCI patients exhibit differences in prefrontal lobe activity in fNIRS measurements during a WM test, which can lead to improvement in cognitive function and changes in prefrontal blood flow of patients with MCI.
2. Methods
2.1. Objectives
The specific objectives of the present study are as follows: to evaluate hemodynamic response in patients with MCI after long-term acupuncture treatment; to assess whether long-term acupuncture treatment can improve cognitive function in patients with MCI compared with healthy controls not undergoing acupuncture treatment; and to evaluate the feasibility of acupuncture treatment, as well as provide guidance for future larger-scale clinical trials.
2.2. Design
A prospective, two-arm, parallel designed clinical trial will be performed at the Dunsan Korean Medicine Hospital of Daejeon University (Daejeon, South Korea). (Recruitment for the study began in September 2017 and continued until approximately December 2017. Patients were followed up until approximately March 2018. Data management, including quality control, is ongoing).
Subjects who voluntarily sign the consent form to participate in the clinical research will be assigned to a patient group or normal control group after assessment as to whether they meet the inclusion criteria. The patient group will be treated with acupuncture twice per week for 12 weeks; no treatment will be administered to the healthy control group. fNIRS measurements, and WM and neuropsychological tests will be performed at baseline, and at 6 and 12 weeks after subject allocation to a particular group. Additionally, every time subjects visit the clinic, vital signs, abnormal reactions, and medication history will be reviewed. A flow diagram of the proposed trial is shown in Fig. 1 and Table 1.
Fig. 1.

Study flow diagram. MCI, mild cognitive impairment.
Table 1.
Schedule for Enrolment, Intervenstion, and Assessments
| Timepoint |
Study period |
|||||||
|---|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-allocation |
||||||
| Week 0 | Week 1 | Week 1 (baseline) | Week 1–6 | Week 6 | Week 7–12 | Week 12 (close-out) | ||
| Patient group | Visit 1 | Visits 2–11 | Visit12 | Visits 13–23 | Visit 24 | |||
| Control group | Visit 1 | – | Visit 2 | – | Visit 3 | |||
| Enrolment | Eligibility screen (inclusion/exclusion criteria) | × | ||||||
| Informed consent | × | |||||||
| Allocation | × | |||||||
| Interventions | Acupuncture | Patient group | × | × | × | × | × | |
| Control group | ||||||||
| Assessments | Laboratory test | × | × | |||||
| MoCA-K | × | × | × | |||||
| CDR | × | |||||||
| GDS | × | |||||||
| Hachinski ischemic score | × | |||||||
| ADAS-cog | × | × | × | |||||
| Working memory task | × | × | × | |||||
| fNIRS assessment | × | × | × | |||||
| Others | Guidance for visitation | × | × | × | × | × | ||
MoCA-K, Korean translation of the Montreal Cognitive Assessment; CDR, Clinical Dementia Rating; GDS, Global Deterioration Scale; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; fNIRS, functional near-infrared spectroscopy.
2.3. Inclusion and exclusion criteria
2.3.1. Inclusion criteria
The common inclusion criteria for the patient and healthy control groups are individuals 40 to 80 years of age, and those who understand the purpose of the study and consent to participate. Inclusion criteria for the patient group are as follows: individuals who meet the Peterson diagnostic criteria for MCI, with memory problems for at least 3 months; at least 6 years of education; Hachinski ischemic score ≤6; Korean version of the Montreal Cognitive Assessment (MoCA-K) score ≤22; and Clinical Dementia Rating (CDR) score 0.5 AND global deterioration scale (GDS) score 2–3. The inclusion criterion for individuals allocated to the healthy control group is an MoCA-K score ≥23.
2.3.2. Exclusion criteria
Exclusion criteria include the following: diagnosed with dementia according to the Diagnostic and Statistical Manual of Mental Disorders-IV; history of neurological disorder(s) that can cause cognitive decline (e.g., Parkinson's disease, stroke, cerebral hemorrhage, tumors, normal pressure hydrocephalus); received any treatment for MCI during the previous 4 weeks; involved in other clinical trials within the previous 4 weeks; experienced hypersensitivity reactions after acupuncture treatment, or those who exhibit unusual difficulty with acupuncture treatment; individuals likely to exhibit a non-cooperative attitude or judged by the researchers to be unable to proceed with the study; and, finally, individuals who cannot undergo fNIRS measurements for any reason.
2.4. Recruitment
Participants will be recruited through advertisements in local newspapers and leaflets, as well as banners displayed in the hospital. The healthy control group will be selected by matching the patient group with age, sex, years of education. A control group of healthy individuals will be recruited to match those in the patient group in 5-year age categories.
2.5. Intervention
The patient group will undergo 10 min of acupuncture treatment at 14 anatomical points, twice per week for 12 weeks, corresponding to 24 treatment sessions in total; the healthy control group will not undergo any acupuncture treatment. Details regarding the acupuncture treatment are listed in the revised Standards for Reporting Intervention in Clinical Trials of Acupuncture27 (Table 2).
Table 2.
Acupuncture Treatment Based on the Revised Standards for Reporting Intervention in Clinical Trials of Acupuncture (STRICTA)
| Item | Details | |
|---|---|---|
| 1. Acupuncture rationale | 1a) Style of acupuncture | Acupuncture based on traditional Korean medicine therapy |
| 1b) Reasoning for treatments provided, based on historical context, literature sources, and/or consensus methods, with references where appropriate | Selected traditional acupoints on 12 meridian system based on related articles,16, 28 clinical experiences | |
| 1c) Extent to which treatment was varied | Use only fixed acupoints | |
| 2. Details of needling | 2a) Number of needle insertions per subject per session | 14 acupoints |
| 2b) Names (or location if no standard name) of points used (uni/bilateral) | GV20, EX-HN1, CV12, HT7 (bilateral), ST36 (bilateral), SP6 (bilateral), KI3 (bilateral) | |
| 2c) Depth of insertion, based on a specified unit of measurement, or on a particular tissue level | The depth of insertion varies depending on thickness of skin and subcutaneous tissue at the acupoint site (it will be usually 0.5-1 cm) | |
| 2d) Response sought | None | |
| 2e) Needle stimulation | No stimulation after needle insertion | |
| 2f) Needle retention time | 10 min | |
| 2g) Needle type (diameter, length, and manufacturer or material) | 0.20 × 30 mm stainless steel needle (Dongbang Medical Co., Korea) | |
| 3. Treatment regimen | 3a) Number of treatment sessions | A total of 24 treatment sessions will be administered to the patient group, and no treatment will be administered to the healthy control group |
| 3b) Frequency and duration of treatment sessions | Patient group will be asked to attend treatment session twice weekly for 12 weeks (24 treatments) | |
| 4. Other components of treatment | 4a) Details of other interventions administered to the acupuncture group | No other intervention will be allowed during the clinical study period. |
| 4b) Setting and context of treatment, including instructions to practitioners, and information and explanations to patients | Participants will be informed about acupuncture in the study as follows: “The acupuncture treatment is based on traditional Korean medicine therapy and previous clinical trials” | |
| 5. Practitioner background | 5) Description of participating acupuncturists (qualification or professional affiliation, years in acupuncture practice, other relevant experience) | Korean medicine doctor with clinical experience > 2 years |
| 6. Control and comparator intervention | 6a) Rationale for the control or comparator in the context of the research question, with sources that justify this choice | The comparator group in this study is a healthy control group without intervention |
| 6b) Precise description of the control or comparator. If sham acupuncture or any other type of acupuncture-like control is used, provide details as for Items 1–3 above. | Acupuncture treatment for healthy control group will not be implemented | |
2.6. Concomitant treatment
Both groups will be prohibited to undergo any acupuncture treatments other than those provided during the period of clinical trial participation. Participants in either group, however, will be allowed to undergo any treatment for cognitive impairment, but will be required to report the treatment performed. All medications and non-medication treatment not specified in the protocol will be described in the case report form.
2.7. fNIRS scanning procedure
Hemodynamic responses in the prefrontal cortex will be recorded using fNIRS developed by NIRX (NIRScout, NIRx Medical Technologies, USA). The system uses near-infrared light of two wavelengths (760 nm and 850 nm) to determine changes in the concentration of hemoglobin. A sampling rate of 7.81 Hz will be used to acquire the fNIRS data. The prefrontal cortex of the brain will be thoroughly examined using 8 sources and 7 detectors. The detectors will be placed around the center of the pre-frontal cortex (Fpz region) in accordance with the International 10–20 electrode system. A total of 20 channels will be configured using a source-detector combination. fNIRS data from the brain will be acquired while subjects are performing a WM task.
2.8. fNIRS data processing
The modified Beer–Lambert law (MBLL) will be used to convert optical density signals into concentration changes of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) (ΔHbO and ΔHbR). The MBLL for two wavelengths is given as:
| (1) |
in which ΔA(t; λj) (j = 1,2) is the unit-less absorbance (optical density) variation of the light emitter of wavelength λj, αHbX(λj) is the extinction coefficient of HbX in µM−1 mm−1, d is the unit-less differential path length factor (DPF), and l is the distance (in mm) between emitter and detector. Because the data will likely be contaminated with physiological “noise”, a low-pass filter with a cutoff frequency of 0.2 Hz will be used to remove respiratory and cardiac signals. The low-frequency drift from the data will then be minimized using a high-pass filter with a cutoff frequency of 0.01 Hz. Data processing will be performed using NIRSLAB software (NIRx, USA).
2.9. Outcome measurement
A clinical research coordinator with a nursing license will measure outcomes.
2.9.1. Primary outcome
The primary outcome of this study will be the MoCA-K score, evaluated at baseline, and at 6 and 12 weeks after subject allocation. The MoCA-K is a tool developed by Nasreddine et al29 to identify MCI based on the Montreal Cognitive Assessment scale. It was translated into Korean and underwent a validity evaluation.30 Subscales of this tool include visuospatial ability, executive function, attention–concentration–working memory, language, short-term memory recall, and orientation to time and place for evaluation of overall cognitive function. Measured on a scale of 30 points, the cutoff point to identify MCI is ≤22 points.30 In this study, subjects with reduced cognitive functions without dementia (≤22 points for MoCA-K) will be classified as MCI. MoCA-K will be evaluated at the time of screening, and 6 and 12 weeks after being assigned to one of the two groups.
2.9.2. Secondary outcomes
2.9.2.1. AD Assessment Scale-cognitive subscale (ADAS-Cog)
This is a tool for evaluating comprehensive cognitive functions involving memory, language, praxis, and the function of the frontal lobe.31 In particular, this tool is known to be sensitive to the treatment responses of patients with MCI or early dementia, and has classified such patients with few false positives. It is used as a tool to judge the efficacy of various anti-dementia treatments.32, 33, 34 ADAS-Cog will be evaluated at baseline, and at 6 and 12 weeks after allocation.
2.9.2.2. WM task accuracy, response rate, response time
Subjects will undergo a WM test while lying down to receive acupuncture. The WM task is presented on a computer monitor that can be viewed by the subjects in a lying position. For the WM test, the components of the visuospatial sketchpad from the WM model described by Baddeley35 will be used. The test will be conducted as a 2-back test, and based on the experimental models described by Crone et al,36 Klingberg et al,37 and van Ewijk et al.38
The visuospatial WM test consists of remembering the location information of red circles allocated within 2 × 2 (low WM load) and 4 × 4 (high WM load) grids. To distinguish between the probe and stimulation, the probe will be marked as a white circle with a red outline, while the stimulation is marked as a fully colored red circle (Fig. 2).
Fig. 2.
Working memory cue and probe for stimulus presentation and illustration for fixation.
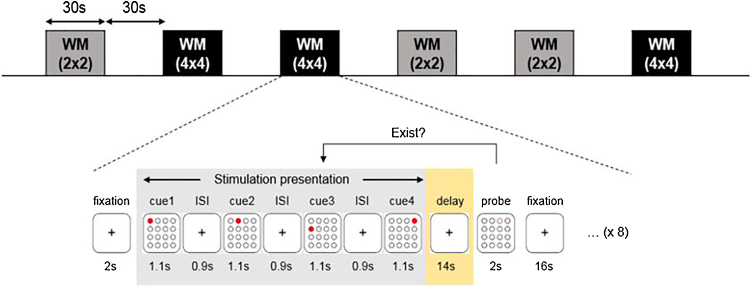
The WM task is composed in a block design using a delayed match to sample (Fig. 3). A delayed match to sample WM test presents visual stimuli with a series of location information and determines whether the location of the probe exists in the previously presented visual stimuli. Stimulation with location information will be presented for 1.1 s at intervals of 0.9 s. Three to eight location information stimuli will be randomly selected for the composition. After the stimulation presentation, the probe will be presented for 2 s, and the subject will be asked to report an answer using a button. A WM task of 30 s and a rest period of 30 s will be repeated six times for approximately 6 min. The six WM tasks will each have three 2 × 2 tasks and 4 × 4 tasks in random order.
Fig. 3.
4×4 working memory task procedure.
Subjects will answer “Yes” or “No” using a response button in the WM task. The input signal will be saved on the computer through an external signal entry device to calculate accuracy, response rate, and response time, which will serve as indices to assess WM tasks. Evaluation will be conducted at baseline, and 6 and 12 weeks after allocation.
2.9.2.3. Hemodynamic responses of the prefrontal lobe
The hemodynamic responses (Oxy-Hb, deOxy-Hb) of the prefrontal lobe during the WM task will be evaluated at baseline, and 6 and 12 weeks after allocation, using fNIRS.
2.10. Sample size calculation
For each group, there will be 10 subjects (a total of 20 participants) in the pilot study. Anticipating a 15% drop-out rate, a total of 24 subjects, with 12 subjects in each group, will be recruited.
2.11. Statistical analysis
Individuals who undergo >70% of entire acupuncture treatment (i.e., attend >17 of the 24 treatment sessions) will be considered to have satisfied the requirements for treatment completion; those who attended ≤17 sessions will be excluded from the per-protocol analysis.
All statistical analysis will be based on two-tailed tests, and the significance level will be set to 5%. Statistical analysis will be performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). In case of missing values, the study will use multiple imputations for processing. Series data will be expressed as mean and standard deviation, and the independent t-test or Wilcoxon rank sum test will be used for comparisons. Categorical data will be expressed as frequency and percent, and the chi-squared or Fisher's exact tests used for comparative analysis.
As for the effectiveness assessment variable analysis, the analysis of covariance setting fixed factor, baseline value and stratification factor as covariate was used for analysis. To compare differences in measured values before and after treatment within each group, the series variables will be analyzed using the paired t-test or Wilcoxon signed rank test, and categorical variables will be analyzed using the chi-squared or Fisher's exact tests. To compare differences regarding changes in visiting trends in each group, repeated measures analysis of variance will be used. To evaluate safety, the frequency of adverse events (AEs) and severe AEs associated with treatment will be analyzed.
2.12. Safety
To verify the safety of acupuncture treatment, those with severe illness during the study period will be identified. Before each acupuncture treatment, vital signs will be measured.
At each visit, subjects will be examined for any adverse reactions, and will be trained to voluntarily report the occurrence of AEs. The test conductor will verify whether there are any AEs by interviewing the subjects. If there are AEs, the date the event occurred and disappeared, the severity, measures taken, causal relation to the procedures, name of medication or treatments suspected of having caused the reaction, and treatment to address the AE, will be recorded in detail and the appropriate treatment will be immediately provided to the participant.
If an AE is suspected of causing harm to a subject at any time during the clinical trial, the principal investigator may intermittently intervene on the subject's behalf. If continuation of the intervention is judged to be harmful to the subject, based on evaluation of the causal relationship between the intervention and the progress of the AEs, the principal investigator may discontinue the intervention.
2.13. Quality control
To improve data quality, regular monitoring will be conducted. The clinical research associate will regularly monitor the study file, case report form, informed consent forms, and whether the clinical trial is being conducted in accordance with the approved protocol.
3. Discussion
Recently, there has been increasing interest in the use of acupuncture treatment for patients with MCI. In many clinical studies, the potential benefits of acupuncture for cognitive improvement have been identified in clinical outcomes,17, 18, 19, 21, 23 and neuroimaging studies have been conducted to determine the therapeutic mechanism.24, 25, 26
To our knowledge, this study will be the first clinical trial to examine the influence of long-term acupuncture treatment on the prefrontal activity of patients with MCI assessed using fNIRS. fNIRS is a recently developed optical imaging technology that measures changes in oxy-Hb and deoxy-Hb from the brain surface. It is a non-invasive and low-cost method to verify hemodynamic response at the cortical surface elicited by brain activity.11, 26, 39 Compared with fMRI, fNIRS is easily portable and has high temporal resolution. Because it can assess brain activity, both in an active and resting state, it is often used to measure focal brain activity in the field of brain and cognitive science.39
This study will be conducted as a prospective, two-group, parallel designed, clinical trial. To determine differences in brain activities of the prefrontal cortex that appear during WM tasks between MCI patients and age-matched healthy controls, we will compare the brain activity patterns of two groups in the WM task process through fNIRS assessment at baseline and after 12 weeks acupuncture treatment using a two-sample t-test. We would like to study that after 12 weeks acupuncture treatment the brain activity pattern of MCI patients become similar to that of healthy controls. Since the repetitive WM task can be confounding factor, the control group will be repeatedly performed the fNIRS assessment during the WM task as the patient. The paired t-test will be performed to compare differences in cognitive function, and the hemodynamic response before and after treatment within each group.
The acupuncture process in this trial includes a detailed description of all acupoints, the type of acupuncture needle used and the procedure, which is in accordance with the revised STRICTA standards27 (Table 2).
This study, however, may have several limitations, the first of which is the small sample size, which may make it difficult to generalize the results. Second, there is no placebo control group; therefore, possible placebo effects of acupuncture treatment cannot be verified. As such, it can be difficult to judge whether the results of this study are due to the acupuncture treatment or to placebo effect. However, this study is only a pilot trial and, through additional randomized-controlled follow-up studies, the effects of acupuncture treatment on improved cognitive functions can be verified. Finally, neither the acupuncturist nor the study participants could be blinded, which may lead to the introduction of bias. In follow-up studies, however, all measurements will be recorded and managed by an independent researcher to minimize the risk for bias.
Ethics approval
Before subject recruitment, the Institutional Review Board of Dunsan Korean Medicine Hospital, Daejeon University, approved the protocol of the clinical trial, case report form, and all measures that will be used in the study (DJDSKH-17-BM-13). Before participation, the subjects will be provided with study-related information in the consent form by the investigator, which will also serve to confirm voluntary participation. The participants will be able to remove themselves from the clinical trial at any time during the study period.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This study was supported by the Korea Institute of Oriental Medicine [grant number K17051]; Korean Health Industry Development Institute, Republic of Korea. This trial has been designed, conducted, analyzed, interpreted, and reported independently of the funding agencies.
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 3.Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: a systematic review. J Alzheimers Dis. 2007;12:23–35. doi: 10.3233/jad-2007-12104. [DOI] [PubMed] [Google Scholar]
- 4.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia J, Zhou A, Wei C, Wang F, Li F, Wu X. The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese. Alzheimers Dement. 2014;10:439–447. doi: 10.1016/j.jalz.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Panza F, D’introno A, Colacicco AM, Capurso C, Del Parigi A, Caselli RJ. Current epidemiology of mild cognitive impairment and other predementia syndromes. Am J Geriatr Psychiatry. 2005;13:633–644. doi: 10.1176/appi.ajgp.13.8.633. [DOI] [PubMed] [Google Scholar]
- 7.Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJ. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82:317–325. doi: 10.1212/WNL.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selkoe DJ. Preventing Alzheimer's disease. Science. 2012;337:1488–1492. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- 9.Germano C, Kinsella GJ. Working memory and learning in early Alzheimer's disease. Neuropsychol Rev. 2005;15:1–10. doi: 10.1007/s11065-005-3583-7. [DOI] [PubMed] [Google Scholar]
- 10.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Natneuroscience. 2004;7:75. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 11.Niu HJ, Li X, Chen YJ, Ma C, Zhang JY, Zhang ZJ. Reduced frontal activation during a working memory task in mild cognitive impairment: a non-invasive near-infrared spectroscopy study. CNS Neurosci Ther. 2013;19:125–131. doi: 10.1111/cns.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013;185:1393–1401. doi: 10.1503/cmaj.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes DE, Yaffe K, Belfor N, Jagust WJ, DeCarli C, Reed BR. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord. 2009;23:205. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gates N, Singh MAF, Sachdev PS, Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psyc. 2013;21:1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Xu M, Li H, Liang J, Yin L, Liu X. Acupuncture at the Taixi (KI3) acpoint activates cerebral neurons in elderly patients with mild cognitive impairment. Neural Regen Res. 2014;9:1163–1168. doi: 10.4103/1673-5374.135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Yang H, Zhang J, Zhang B, Liu T, Gan L. Efficacy and safety assessment of acupuncture and nimodipine to treat mild cognitive impairment after cerebral infarction: a randomized controlled trial. BMC Complement Altern Med. 2016;16:361. doi: 10.1186/s12906-016-1337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Zhang YL, Cao HJ, Hu H. Treating vascular mild cognitive impairment by cupuncture: a systematic review of randomized controlled trials. Chin J Integr Med. 2013;33:1626–1630. [PubMed] [Google Scholar]
- 19.Deng M, Wang X-F. Acupuncture for amnestic mild cognitive impairment: a meta-analysis of randomised controlled trials. Acupunct Med. 2016 doi: 10.1136/acupmed-2015-010989. acupmed-2015-010989. [DOI] [PubMed] [Google Scholar]
- 20.Wu P, Mills E, Moher D, Seely D. Acupuncture in poststroke rehabilitation. Stroke. 2010;41:e171–e179. doi: 10.1161/STROKEAHA.109.573576. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Peng W, Xu M, Li W, Liu Z. The effectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2015;94 doi: 10.1097/MD.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng W, Wang Y, Zhang Y, Liang CM. Acupuncture for vascular dementia. Cochrane Libr. 2007 doi: 10.1002/14651858.CD004987.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Zhang X, Liu C, Meng Y, Han J. Effect of acupuncture treatment on vascular dementia. Neurol Res. 2006;28:97–103. doi: 10.1179/016164106X91951. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Bai L, Ren Y, Chen S, Wang H, Zhang W. FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients. Magn Reson Imaging. 2012;30:672–682. doi: 10.1016/j.mri.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Nie B, Li D, Zhao Z, Han Y, Song H. Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS One. 2012;7:e42730. doi: 10.1371/journal.pone.0042730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai H, Takano M, Miyakawa K, Ota T, Takahashi T, Asaka H. A quantitative near-infrared spectroscopy study: a decrease in cerebral hemoglobin oxygenation in Alzheimer's disease and mild cognitive impairment. Brain Cogn. 2006;61:189–194. doi: 10.1016/j.bandc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 27.MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. J Evid Based Med. 2010;3:140–155. doi: 10.1111/j.1756-5391.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Jia J. Effect of acupuncture given at the HT 7, ST 36, ST 40 and KI3 acupoints on various parts of the brains of Alzheimer's disease patients. Acupunct Electrother Res. 2008;33:9–17. [PubMed] [Google Scholar]
- 29.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee J-Y, Lee DW, Cho S-J, Na DL, Jeon Hong Jin, Kim SK. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21:104–110. doi: 10.1177/0891988708316855. [DOI] [PubMed] [Google Scholar]
- 31.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 32.Cano SJ, Posner HB, Moline ML, Hurt SW, Swartz J, Hsu T. The ADAS-cog in Alzheimer's disease clinical trials: psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatry. 2010 doi: 10.1136/jnnp.2009.204008. jnnp. 2009.204008. [DOI] [PubMed] [Google Scholar]
- 33.Farlow M, Anand R, Messina J, Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer's disease. Eur Neurol. 2000;44:236–241. doi: 10.1159/000008243. [DOI] [PubMed] [Google Scholar]
- 34.Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dement Geriatr Cogn Dis. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 35.Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 36.Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klingberg T. Development of a superior frontal–intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 38.van Ewijk H, Weeda WD, Heslenfeld DJ, Luman M, Hartman CA, Hoekstra PJ. Neural correlates of visuospatial working memory in attention-deficit/hyperactivity disorder and healthy controls. Psychiatry Res. 2015;233:233–242. doi: 10.1016/j.pscychresns.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]