Abstract
Objective
Nesfatin-1 is a novel anorectic neuropeptide with potent metabolic regulatory effects. It regulates blood pressure, heart rate, cardiomyocyte metabolism and permeability. SYNTAX score, which is an angiographic scoring system, defines the grade and complexity of coronary artery disease (CAD). We aimed to evaluate the relationship between nesfatin-1 level and severity of CAD according to the SYNTAX score in patients with non-ST segment elevation myocardial infarction (NSTEMI).
Methods
A total of 109 subjects were enrolled into the study, of whom 80 underwent coronary angiography (CA) with the diagnosis of NSTEMI and 29 had normal coronary arteries detected in CA. NSTEMI patients were divided into 2 groups: low SYNTAX score (< 32) (45 patients) and high SYNTAX score (≥ 32) (35 patients).
Results
The NSTEMI patients with a high SYNTAX score (score ≥ 32) had a lower serum nesfatin-1 level (62 pg/ml; 39-98) compared to the NSTEMI patients with a low SYNTAX score (score < 32) (138 pg/ml; 65-286) and the control group (392 pg/ml; 178-1320). There was also a negative correlation between serum nesfatin-1 level and SYNTAX score (r = -0.594, p < 0.001). A lower serum level of nesfatin-1 (odds ratio = 0.116; 95% confidence interval: 0.138- 0.094; p < 0.001) was an independent predictor for high SYNTAX score in the NSTEMI patients after multiple linear regression analysis.
Conclusions
Serum nesfatin-1 level was lower in the high SYNTAX group than in the low SYNTAX group in patients with NSTEMI. Nesfatin-1could have a role in the pathogenesis of atherosclerotic burden in patients with NSTEMI.
Keywords: Coronary artery disease, Nesfatin-1, SYNTAX score
INTRODUCTION
Nesfatin-1 was identified by Oh et al. as a peptide consisting of 82 amino acids by proteolysis of nucleobindin 2 (NUCB-2). This protein was first discovered in the hypothalamus in 2006.1 At first, it was thought to be a satiety molecule that plays an important role in regulating appetite and metabolism through the melanocortin system due to its acute and chronic anorexigenic effects.2 Recently, it has been found that nesfatin-1 influences adipocyte growth and differentiation in adipose tissue, inflammation, thermoregulation, pancreatic insulin secretion, glucose homeostasis in the liver, nutrient intake in the brain, sleep, fear, anxiety, stress, glucose homeostasis; regulation of gastric emptying, gastric acid secretion, gastric motility and reproductive functions.3,4
The cardiovascular effects of nesfatin-1 on cardiac pathophysiology include the regulation of blood pressure and heart rate, cardiomyocyte metabolism and permeability, increasing cardiac atrial levels in women, reducing cardiac atrial levels in cases of coronary damage, protecting against ischemia/reperfusion injury, reducing its circulatory levels in patients with acute myocardial infarction (AMI) and peripheral artery disease, and increasing its levels in incident paroxysmal subventricular tachycardia.5
Dai et al. found that serum nesfatine levels were lower in individuals with AMI compared to those with angina pectoris and controls in a study performed on 156 individuals. Additionally, plasma nesfatin-1 level was negatively associated with high sensitivity C-reactive protein (CRP), neutrophil percentage and Gensini score in the AMI group. They concluded that low nesfatin-1 concentration may play an important role in the development of AMI.6
SYNTAX score, which is an angiographic scoring system, defines the grade and complexity of coronary artery disease (CAD). Numerous publications have shown that patients with a relatively high SYNTAX score have poor outcomes, and that the score is an independent predictor of major advanced cardiovascular events (MACE) for percutaneous coronary intervention (PCI).7
This study aimed to evaluate the relationship between nesfatin-1 level and the severity of CAD according to the SYNTAX score in patients with non-ST segment elevation myocardial infarction (NSTEMI).
METHODS
Study population
The study was planned prospectively. Eighty consecutive patients who underwent coronary angiography (CA) with the diagnosis of NSTEMI and 29 randomly chosen patients with normal coronary artery detected in CA at the Turkiye Yuksek Ihtisas Training and Research Hospital were included in the study between November 2015 and December 2015. Patients with previous coronary artery bypass grafting (CABG) were excluded from the study, since SYNTAX score is suitable only for patients with native coronary artery lesions. Patients with active infection, chronic inflammatory diseases, severe hepatic or renal dysfunction and malignancy were excluded from the study. If the patients did not have ST elevation, those with acute chest pain or overwhelming shortness of breath and with a classic rise and fall in at least one cardiac enzyme (troponin or creatine kinase-myocardial band) were accepted as having NSTEMI. Patients with normal CAD were selected from those who underwent CA as a result of a positive stress test (exercise stress test or myocardial perfusion scintigraphy test) or clinically high suspicion of CAD (e.g., patients with strong family history of CAD or early death with or without associated risk factors, and patients with unexplained chest pain after careful clinical and laboratory evaluation if there was strong suspicion of ischemic heart disease). Normal coronary arteries were defined as those with no visible disease or luminal irregularity (less than 50%) as judged visually on CA. The study was approved by the local Ethical Committee, and all patients provided written informed consent.
Coronary angiography
We performed CA using the Judkins technique (Siemens Axiom Artis Zee 2011; Siemens Healthcare, Erlangen, Germany) through the femoral or radial artery. Each coronary artery was visualized in at least 2 different plane images. PCI procedures were performed using standard techniques. According to baseline CA, the SYNTAX score was calculated for all patients by two experienced interventional cardiologists unaware of the patients’ clinical or laboratory results. SYNTAX score was determined for all coronary lesions with > 50% diameter stenosis in a vessel > 1.5 mm based on SYNTAX score calculator 2.1 (www.syntaxscore.com). The NSTEMI patients were divided into 2 groups: low SYNTAX score (< 32) (45 patients), and high SYNTAX score (≥ 32) (35 patients).
Reproducibility
To define intra-observer variability, 15 patients were randomly selected from the study group. Measurements were repeated under the same basal conditions, and reproducibility of the SYNTAX score by CA was assessed according to the coefficient of variation between measurements. Intra-observer variability was 5.5% for SYNTAX score.
Laboratory measurements
Samples were taken from the antecubital vein when the patients were admitted to the hospital. Basal creatinine level, white blood cell (WBC) count, platelet count and hemoglobin concentration were measured in the first 6 hours of hospitalization. The following morning after admission to the hospital, lipid profile and other biochemical parameters were measured using standard techniques. Peak and basal levels of troponin and creatinine kinase myocardial band levels were measured.
The blood samples used for nesfatin-1 measurement were centrifuged immediately, and serum samples were stored at -80 °C until the day of analysis. Serum nesfatin-1 levels were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (sensitivity: < 10 pg/ml; assay range: 31.2 pg/ml-2000 pg/ml; Boster Immunoleader/USA) as recommended by the manufacturer’s protocol. Serum adropin was measured using a commercial human adropin ELISA kit (catalog no. 201-12-3107, limit of determination 5-10000 pg/mL; Sunred Biological Technology Co., Shanghai, PRC) as recommended by the manufacturer’s protocol.
Statistical analysis
Data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, Il, USA). Continuous variables were reported as mean ± SD, and categorical variables were reported as percentages and counts. The Student’s t-test was used for comparisons of normally distributed variables, and the Mann-Whitney U test was used for non-normally distributed variables if 2 groups existed. One-way analysis of variance was used to compare normally distributed variables between 3 groups. Tukey’s test was used for post-hoc analysis. Categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate. Pearson’s correlation coefficients were used to assess the strength of relationships between continuous variables, and Spearman correlation analysis was performed for non-continuous and categorical variables. Univariate and multiple models consisted of SYNTAX ≥ 32 score and variables [male gender, nesfatin-1, peak creatine kinase-myocardial band (CK-MB), WBC, high-density lipoprotein (HDL) cholesterol]. In all analyses, a p value of < 0.05 was considered to be statistically significant.
RESULTS
The baseline clinical and angiographic characteristics of the study population are shown in Table 1. There was no statistically significant difference between groups in terms of age, body mass index, blood pressure (systolic and diastolic), diabetes mellitus (DM), and smoking status. The high SYNTAX group (score ≥ 32) had significantly higher rates of previous myocardial infarction (MI), multi-vessel coronary involvement, chronic total occlusion, CABG procedures, collateral vessels, and fewer stent implantations compared with the low SYNTAX group (score < 32) (p = 0.015, p < 0.001, p < 0.001, p < 0.001, p = 0.018, and p < 0.001, respectively). There was no statistically significant difference between the patient groups in terms of the use of antihypertensive, anticoagulant, antidepressant, antidiabetic, antidepressant and cholesterol lowering drugs.
Table 1. Baseline clinical and angiographic characteristics of the study population.
| Variables | NSTEMI SYNTAX score ≥ 32 (n = 35) | NSTEMI SYNTAX score < 32 (n = 45) | NCA (n = 29) | p value* | p value# | p value† | p value‡ |
| Age,years | 62.94 ± 9.49 | 62.29 ± 8.25 | 58.90 ± 10.64 | 0.187 | |||
| BMI, kg/m2 | 30.03 ± 5.67 | 30.09 ± 4.99 | 29.90 ± 6.13 | 0.989 | |||
| Waist circumference, cm | 90.28 ± 7.21 | 88.02 ± 6.67 | 87.45 ± 5.42 | 0.168 | |||
| Systolic blood pressure, mmHg | 136.48 ± 7.35 | 135.66 ± 10.87 | 136.79 ± 5.75 | 0.843 | |||
| Diastolic blood pressure, mmHg | 84.65 ± 6.36 | 84.60 ± 9.53 | 85.00 ± 4.51 | 0.973 | |||
| Female, n (%) | 12 (34.3) | 11 (22.4) | 8 (27.6) | 0.622 | |||
| Hyperlipidemia, n (%) | 17 (48.6) | 21 (46.7) | 6 (20.7) | 0.041 | |||
| Diabetes mellitus, n (%) | 13 (37.1) | 10 (22.2) | 7 (24.1) | 0.298 | |||
| Smoking, n (%) | 17 (48.6) | 28 (62.2) | 8 (27.6) | 0.014 | < 0.001 | < 0.001 | < 0.001 |
| Previous MI, n (%) | 5 (14.3) | 11 (24.4) | 0 (0.0) | 0.015 | < 0.001 | ||
| Multi-vessel disease, n (%) | 32 (91.4) | 30 (66.7) | 0 (0.0) | < 0.001 | < 0.001 | ||
| Chronic total occlusion, n (%) | 13 (37.1) | 7 (15.6) | 0 (0.0) | < 0.001 | < 0.001 | ||
| Stent implantation, n (%) | 15 (42.8) | 29 (64.4) | 0 (0.0) | < 0.001 | < 0.001 | ||
| Decision for CABG, n (%) | 15 (42.8) | 5 (11.1) | 0 (0.0) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Collateral vessel, n (%) | 10 (28.6) | 4 (8.9) | 0 (0.0) | 0.018 | |||
| SYNTAX score | 37.77 ± 3.66 | 17.88 ± 7.13 | 0 ± 0 | < 0.001 | |||
| LVEF, % | 53.92 ± 10.86 | 51.82 ± 8.89 | 58.04 ± 6.85 | 0.035 | 0.619 | 0.24 | 0.026 |
| Drug usage, n (%) | |||||||
| β blockers | 8 (22.9) | 7 (15.6) | 2 (6.9) | 0.193 | |||
| ACEi/ARB | 7 (20.0) | 14 (31.1) | 5 (17.2) | 0.318 | |||
| Calcium channel blocker | 6 (17.1) | 7 (15.6) | 5 (17.2) | 0.975 | |||
| Spironolactone/diuretics | 7 (20.0) | 7 (15.6) | 4 (13.8) | 0.784 | |||
| Statins | 13 (37.1) | 10 (22.2) | 7 (24.1) | 0.298 | |||
| Antidepressant | 5 (14.3) | 7 (15.6) | 5 (17.2) | 0.949 | |||
| ASA | 7 (20.0) | 7 (15.6) | 1 (3.4) | 0.145 | |||
| Klopidogrel/ticagrelor/prasugrel | 2 (5.7) | 2 (4.4) | 0 (0.0) | 0.273 | |||
| Warfarin/NOAC | 2 (5.7) | 2 (4.4) | 0 (0.0) | 0.273 | |||
| Insulin | 3 (8.6) | 2 (4.4) | 0 (0.0) | 0.154 | |||
| Oral antidiabetic | 13 (37.1) | 10 (22.2) | 7 (24.1) | 0.298 |
Data are given as mean ± SD, n (%) or median (lower-upper limit).
ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ASA, acetylsalicylic acid; BMI, body mass index; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NCA, normal coronary artery; NOAC, novel oral anticoagulants; NSTEMI, non ST-segment elevation myocardial infarction.
§ Parameters without normally distributed (One-Way ANOVA test was used to show the differences between the three groups in continuous numeric parameters with normal distribution. Tukey's test was used for the post-hoc analysis. Kruskal-Wallis test was used for the differences between the three groups in parameters without normally distributed, if there was statistical significance the Mann-Whitney U test was used for nonpaired groups. Differences between three groups for the categorical variables were analyzed using the chi-square test.)
* p value between all groups. # p value between NSTEMI SYNTAX score ≥ 32 and NSTEMI SYNTAX score < 32 groups. † p value between NSTEMI SYNTAX score ≥ 32 and NCA groups. ‡ p value between NSTEMI SYNTAX score < 32 groups and NCA groups groups.
Biochemical, hematological and serum nesfatin-1 measurements of the study population are also provided in Table 2. There were no statistically significant differences not between the groups except in WBC (p = 0.003), adropin (< 0.001), hs-CRP (< 0.001), and HDL cholesterol (p = 0.036). The NSTEMI patients with a high SYNTAX score (score ≥ 32) had lower serum nesfatin-1 levels (392 pg/ml ± 320.6) compared to the NSTEMI patients with a low SYNTAX score (score < 32) (138 pg/ml ± 51.2) and the control group (62 pg/ml ± 19.0). Nesfatin-1 levels were statistically significantly different between all groups (p < 0.001). Additionally, there were statistically significant differences between the high SYNTAX score group and controls (p < 0.001), low SYNTAX score group and controls (p < 0.001), and high SYNTAX score group and low SYNTAX score group (p < 0.001). As demonstrated in Figure 1, there was a negative correlation between serum nesfatin-1 level and high SYNTAX score (r = -0.594, p < 0.001).
Table 2. Biochemical and hematological measurements of the study patients.
| Variables | NSTEMI SYNTAX score ≥ 32 (n = 35) | NSTEMI SYNTAX score < 32 (n = 45) | NCA (n = 29) | p value* | p value# | p value† | p value‡ |
| Glucose, mg/dL | 151.92 ± 72.33 | 131.08 ± 47.76 | 133.75 ± 45.02 | 0.234 | |||
| Creatinine, mg/dL | 0.99 ± 0.26 | 1.12 ± 0.78 | 0.91 ± 0.113 | 0.234 | |||
| Total cholesterol, mg/dL | 199.71 ± 49.03 | 188.44 ± 55.27 | 176.20 ± 45.28 | 0.237 | |||
| LDL cholesterol, mg/dL | 118.96 ± 42.78 | 112.18 ± 45.09 | 98.75 ± 37.77 | 0.215 | |||
| HDL cholesterol, mg/dL | 47.65 ± 12.56 | 46.18 ± 10.11 | 58.54 ± 16.91 | 0.001 | 0.876 | 0.006 | 0.001 |
| Triglyceride, mg/dL | 174.84 ± 113.24 | 150.76 ± 91.30 | 147.13 ± 79.68 | 0.479 | |||
| Hemoglobin, g/dL | 13.79 ± 1.63 | 13.82 ± 1.76 | 13.37 ± 1.28 | 0.494 | |||
| WBC, 103/mm3 | 9.20 ± 2.15 | 8.81 ± 2.12 | 7.52 ± 1.59 | 0.003 | 0.668 | 0.003 | 0.024 |
| Platelet, 103/mm3 | 238.91 ± 70.66 | 248.83 ± 74.45 | 249.46 ± 66.39 | 0.787 | |||
| HbA1c (%) | 6.58 ± 1.04 | 6.69 ± 1.24 | 7.05 ± 1.62 | 0.365 | |||
| hs-CRP, mg/L | 4.02 ± 0.81 | 3.32 ± 1.23 | 1.58 ± 0.59 | < 0.001 | 0.005 | < 0.001 | < 0.001 |
| Adropin, pg/mL | 2357.30 ± 821.58 | 3077.00 ± 912.86 | 3688.00 ± 956.65 | < 0.001 | 0.003 | < 0.001 | 0.016 |
| Peak CK-MB, U/L | 46 (15-229) | 38 (0-131) | 0.488 | ||||
| Peak troponin-T, ng/mL | 4.51 (0-34.7) | 5.27 (0.09-31.00) | 0.884 | ||||
| Nesfatin-1, pg/ml | 62 (39-98) | 138 (65-286) | 392 (178-1320) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Data are given as mean ± SD, n (%) or median (lower-upper limit).
CK-MB, creatine kinase-myocardial band; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein; WBC, white blood cell.
§ Parameters without normally distributed (One-Way ANOVA test was used to show the differences between the three groups in continuous numeric parameters with normal distribution. Tukey's test was used for the post-hoc analysis. Kruskal-Wallis test was used for the differences between the three groups in parameters without normally distributed, if there was statistical significance the Mann-Whitney U test was used for nonpaired groups. Differences between three groups for the categorical variables were analyzed using the chi-square test.)
* p value between all groups. # p value between NSTEMI SYNTAX score ≥ 32 and NSTEMI SYNTAX score < 32 groups. † p value between NSTEMI SYNTAX score ≥ 32 and NCA groups. ‡ p value between NSTEMI SYNTAX score < 32 groups and NCA groups groups.
Figure 1.
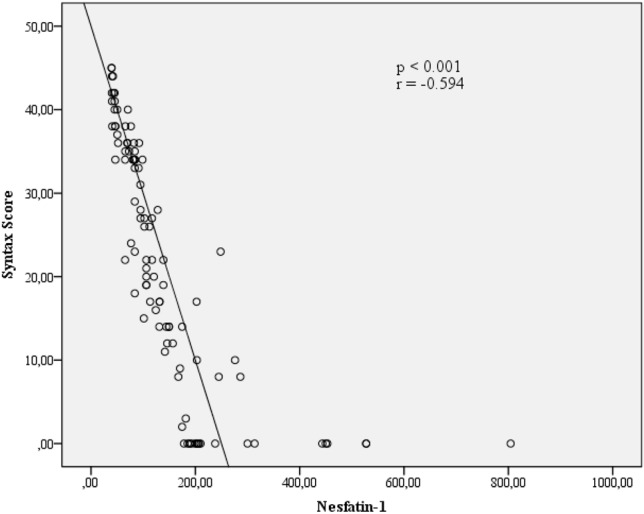
The correlation between nesfatin-1 level and SYNTAX score.
As shown in Table 3 and Figure 1, the serum nesfatin-1 level and SYNTAX score showed a statistically significant negative correlation (r = -0.594, p < 0.001). The peak CK-MB level, which is indicative of myocardial damage during MI, had a statistically significant negative correlation with serum nesfatin-1 level (r = -0.284, p = 0.013). A positive correlation between nesfatin-1 levels and HDL cholesterol (r = 0.175, p = 0.096) and a negative correlation between nesfatin-1 levels and troponin T (r = 0.192, p = 0.094) were nearly statistically significant.
Table 3. Pearson analysis of Nesfatin-1 with other parameters.
| Variables | r value | p value |
| Age | 0.005 | 0.959 |
| BMI | -0.054 | 0.591 |
| SYNTAX score | -0.594 | < 0.001 |
| LDL cholesterol | -0.154 | 0.143 |
| HDL cholesterol | 0.175 | 0.096 |
| Triglyceride | -0.013 | 0.904 |
| WBC | -0.167 | 0.101 |
| Peak CK-MB | -0.284 | 0.013 |
| Peak troponin-T | -0.192 | 0.094 |
| hs-CRP | -0.147 | 0.394 |
| Adropin | 0.102 | 0.245 |
BMI, body mass index; CK-MB, creatine kinase-myocardial band; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein; WBC, white blood cell.
We performed univariate and multiple linear regression analyses for the predictors of SYNTAX ≥ 32 score as depicted in Table 1 and Table 2 (Table 4). In univariate regression analysis, nesfatin-1 [odds ratio (OR) = 0.047; 95% confidence interval (CI): 0.059-0.034; p < 0.001], peak CK-MB level (OR = 0.111; 95% CI: 0.019-0.203; p = 0.018), hs-CRP (OR = 1.874; 95% CI: 1.179-2.979; p = 0.012), (OR = 0.999; 95% CI: 0.998-1.001; p < 0.001), and WBC (OR = 1.800; 95% CI: 0.410-3.190; p = 0.012) were associated with a high SYNTAX score. Lower serum level of nesfatin-1 (OR = 0.116; 95% CI: 0.138-0.094; p < 0.001) and adropin (OR = 1.003; 95% CI: 1.002-1.004; p = 0.046) were independent predictor for a high SYNTAX score in the NSTEMI patients after multiple linear regression analysis.
Table 4. Multivariate logistic regression analysis showing the predictors for the SYNTAX ≥ 32 score.
| Variables | Univariable | Multivariable | ||
| Beta (95% CI) | p value | Beta (95% CI) | p value | |
| Smoking | 1.744 (0.712-4.272) | 0.224 | ||
| Nesfatin-1 | 0.047 (0.059-0.034) | < 0.001 | 0.116 (0.138-0.094) | < 0.001 |
| Peak CK-MB | 0.111 (0.019-0.203) | 0.018 | 0.067 (0.052-0.083) | 0.098 |
| Dyslipidemia | 0.926 (0.383-2.244) | 0.866 | ||
| hs-CRP | 1.874 (1.179-2.979) | 0.008 | 1.154 (0.753-1.556) | 0.139 |
| Adropin | 0.999 (0.998-1.000) | 0.002 | 1.003 (1.002-1.004) | 0.046 |
| WBC | 1.091 (0.883-1.349) | 0.012 | 0.485 (0.527-1.497) | 0.245 |
| HDL cholesterol | 1.012 (0.971-1.055) | 0.571 |
CK-MB, creatine kinase-myocardial band; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; WBC, white blood cell.
DISCUSSION
Our study showed that serum nesfatin-1 levels were lower in the NSTEMI patients than in the NCA patients. In addition, the NSTEMI patients with a high SYNTAX score had lower serum nesfatin-1 levels than the NSTEMI patients with a low SYNTAX score. Furthermore, nesfatin-1 levels showed a negative correlation with SYNTAX score, and a low level of nesfatin-1 was found to be an independent risk factor for CAD with a high SYNTAX score.
NSTEMI is a common presentation in patients with acute coronary syndrome. Even though patients with NSTEMI have lower in-hospital mortality than those with ST-segment elevation, the 6-month mortality rate is similar. Furthermore, the 4-year mortality rate in patients with NSTEMI has been reported to be two times higher than in patients with ST-segment elevation MI.8,9 Intensive medical treatment and invasive procedures can reduce the morbidity and mortality of patients with NSTEMI,9 however, the severity of CAD in CA is the leading factor in determining the most useful treatment strategy.
SYNTAX score is an angiographic tool used to grade CAD complexity by taking into account the number of lesions and their functional and anatomic components including location, presence of bifurcations, tortuosity, total occlusions, collaterals, thrombus and calcification. It can help physicians to make decisions about optimal revascularization strategies. Especially among patients with complex CAD. A high SYNTAX score indicates a more complex disease and is a sign of a therapeutic challenge. Patients with a high SYNTAX score have been reported to have more major adverse cardiac or cerebrovascular events.10-12 The patients with a high SYNTAX (≥ 32) score in this study had more chronic total occlusion, a higher likelihood of having undergone CABG and collateral vessels than those with a low to moderate SYNTAX (< 32) score.
Some studies in the last decade have shown that pathological functions of adipose tissue may be associated with an increased risk of cardiovascular disease, not only because of the effect of the hypothalamus nucleus1,13 on the regulation of cardiovascular function, but also by activating the autocrine/paracrine/endocrine pathway of chemical mediators released from adipose tissue such as nesfatin-1.5 Nesfatin-1 is primarily a satiety hormone.1 Intracerebroventricular injections of this peptide in rats or the intraperitoneal application in mice has been shown to reduce food intake in some studies.1,14 Recently, a close relationship has been shown between this peptide and diabetes,15 polycystic ovary syndrome16 psychiatric disorders17,18 and neurogenic diseases.19
Bonnet et al.20 found an association between inflammation of the brainstem and hypothalamus and activation of neuron expressing nesfatin-1. The main mechanism of acute cardiac events is inflammation, and recent studies have shown the anti-inflammatory effect of nesfatin-1 on a damaged brain,21,22 In addition, it is possible that there is a close relationship between SYNTAX score and inflammation.23-25 It has been demonstrated that the intravenous application of nesfatin-1 can induce vasoconstriction via inhibition of nitric oxide production and to cause high blood pressure.26 Ayada et al. demonstrated that chronic peripheral infusion of nesfatin-1 decreased endothelial nitric oxide synthesis especially in chronic restraint stressed rats.27 Thus, it may be possible that the reduced expression of nesfatin-1 plays a role in the pathogenesis of endothelial dysfunction which then leads to acute cardiac events. Osaki et al. demonstrated that 6-hydroxydopamine, which achieves chemical sympathectomy, increased nesfatin/NUCB2 expressions in subcutaneous fat tissues.28 Therefore, it may be possible that sympathetic activity can suppress nesfatin-1 expression. Adropin is a recently discovered bioactive peptide hormone that is encoded by the energy homeostasis associated gene and is released by the brain, liver, heart, and coronary arteries.29 Ignarro et al. showed that serum adropin can increase the expression of endothelial nitric oxide synthase in the endothelium.30 In addition, Topuz et al. found that the adropin level is closely associated with endothelial dysfunction.31 Serum adropin levels showed a close correlation with nesfatin-1 levels in our study. One of the most important mechanisms of NSTEMI is sympathetic activation.32,33 Plasma nesfatin-1 levels have been shown to be significantly higher in hypertensive patients, and plasma nesfatin-1 levels have been positively correlated with systolic and diastolic blood pressure, all of which are important risk factors for the development of atherosclerosis.34
ST-segment elevation MI and NSTEMI have a similar pathophysiology.33 Daia et al. found that plasma nesfatin-1 levels were significantly lower in AMI patients than in those with stable angina pectoris and controls. In addition, plasma nesfatin-1 levels were negatively correlated with high-sensitivity CRP, neutrophil percentage and Gensini scores in the AMI patients.6 These findings are consistent with our study.
Nesfatin-1 levels have been closely associated with glucose and lipid metabolism, endothelial function, limbic system and sympathetic system, and drugs acting on these systems have the potential to affect nesfatin-1 levels.17,27,28,32 However, there were no statistically significant differences between the patient groups in terms of the use of antihypertensive, anticoagulant, antidepressant, antidiabetic, antidepressant and cholesterol lowering drugs in the present study.
Study limitations
The present study is a cross-sectional study with a relatively small sample size. We did not measure nesfatin-1 level after discharge and did not have follow-up MACE data. Therefore, our results should be verified in multi-center prospective longitudinal studies with a larger sample size. The limitations of this study should be considered when interpreting the results.
CONCLUSIONS
In conclusion, the results of this study showed that serum nesfatin-1 level was lower in the high SYNTAX group than in the low SYNTAX group in patients with NSTEMI. Nesfatin-1 could have a role in the pathogenesis of atherosclerotic burden in patients with NSTEMI.
REFERENCES
- 1.Oh IS, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 2.Maejima Y, Sedbazar U, Suyama S, et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–365. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Aydin S. Multi-functional peptide hormone NUCB2/nesfatin-1. Endocrine. 2013;44:312–325. doi: 10.1007/s12020-013-9923-0. [DOI] [PubMed] [Google Scholar]
- 4.Ayada C, Toru U, Korkut Y. Nesfatin-1 and its effects on different systems. Hippokratia. 2015;19:4–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Feijoo-Bandin S, Rodriguez-Penas D, Garcia-Rua V, et al. Nesfatin-1: a new energy-regulating peptide with pleiotropic functions. Implications at cardiovascular level. Endocrine. 2016;52:11–29. doi: 10.1007/s12020-015-0819-z. [DOI] [PubMed] [Google Scholar]
- 6.Dai H, Li X, He T, et al. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides. 2013;46:167–171. doi: 10.1016/j.peptides.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5:50–56. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
- 8.Mandelzweig L, Battler A, Boyko V, et al. The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J. 2006;27:2285–2293. doi: 10.1093/eurheartj/ehl196. [DOI] [PubMed] [Google Scholar]
- 9.Terkelsen CJ, Lassen JF, Norgaard BL, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J. 2005;26:18–26. doi: 10.1093/eurheartj/ehi002. [DOI] [PubMed] [Google Scholar]
- 10.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 11.Farooq V, Serruys PW, Bourantas C, et al. Incidence and multivariable correlates of long-term mortality in patients treated with surgical or percutaneous revascularization in the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial. Eur Heart J. 2012;33:3105–3113. doi: 10.1093/eurheartj/ehs367. [DOI] [PubMed] [Google Scholar]
- 12.Garg S, Serruys PW, Silber S, et al. The prognostic utility of the SYNTAX score on 1-year outcomes after revascularization with zotarolimus- and everolimus-eluting stents: a substudy of the RESOLUTE all comers trial. JACC Cardiovasc Interv. 2011;4:432–441. doi: 10.1016/j.jcin.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–R336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu H, Oh IS, Hashimoto K, et al. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 15.Li QC, Wang HY, Chen X, et al. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159:72–77. doi: 10.1016/j.regpep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Deniz R, Gurates B, Aydin S, et al. Nesfatin-1 and other hormone alterations in polycystic ovary syndrome. Endocrine. 2012;42:694–699. doi: 10.1007/s12020-012-9638-7. [DOI] [PubMed] [Google Scholar]
- 17.Ari M, Ozturk OH, Bez Y, et al. High plasma nesfatin-1 level in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:497–500. doi: 10.1016/j.pnpbp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Gunay H, Tutuncu R, Aydin S, et al. Decreased plasma nesfatin-1 levels in patients with generalized anxiety disorder. Psychoneuroendocrinology. 2012;37:1949–1953. doi: 10.1016/j.psyneuen.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Aydin S, Dag E, Ozkan Y, et al. Nesfatin-1 and ghrelin levels in serum and saliva of epileptic patients: hormonal changes can have a major effect on seizure disorders. Mol Cell Biochem. 2009;328:49. doi: 10.1007/s11010-009-0073-x. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet MS, Pecchi E, Trouslard J, et al. Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27. doi: 10.1186/1742-2094-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özsavcí D, Erşahin M, Şener A, et al. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage–induced oxidative brain damage in rats. Neurosurgery. 2011;68:1699–1708. doi: 10.1227/NEU.0b013e318210f258. [DOI] [PubMed] [Google Scholar]
- 22.Tang CH, Fu XJ, Xu XL, et al. The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in the traumatic rat brain. Peptides. 2012;36:39–45. doi: 10.1016/j.peptides.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Kundi H. Syntax score and inflammation. Herz. 2016;41:535–536. doi: 10.1007/s00059-016-4454-0. [DOI] [PubMed] [Google Scholar]
- 24.Cagdas M, Karakoyun S, Yesin M, et al. The association between monocyte HDL-C ratio and SYNTAX score and SYNTAX score II in STEMI patients treated with primary PCI. Acta Cardiol Sin. 2018;34:23–30. doi: 10.6515/ACS.201801_34(1).20170823A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ates AH, Aytemir K, Kocyigit D, et al. Association of neutrophil-to-lymphocyte ratio with the severity and morphology of coronary atherosclerotic plaques detected by multidetector computerized tomography. Acta Cardiol Sin. 2016;32:676–683. doi: 10.6515/ACS20160225A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamawaki H, Takahashi M, Mukohda M, et al. A novel adipocytokine, nesfatin-1 modulates peripheral arterial contractility and blood pressure in rats. Biochem Biophys Res Commun. 2012;418:676–681. doi: 10.1016/j.bbrc.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 27.Ayada C, Turgut G, Turgut S, et al. The effect of chronic peripheral nesfatin-1 application on blood pressure in normal and chronic restraint stressed rats: related with circulating level of blood pressure regulators. Gen Physiol Biophys. 2015;34:81–88. doi: 10.4149/gpb_2014032. [DOI] [PubMed] [Google Scholar]
- 28.Osaki A, Shimizu H, Ishizuka N, et al. Enhanced expression of nesfatin/nucleobindin-2 in white adipose tissue of ventromedial hypothalamus-lesioned rats. Neurosci Lett. 2012;521:46–51. doi: 10.1016/j.neulet.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 29.Kumar KG, Trevaskis JL, Lam DD, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 31.Topuz M, Celik A, Aslantas T, et al. Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. J Investig Med. 2013;61:1161–1164. doi: 10.2310/JIM.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 32.Manfrini O, Pizzi C, Trere D, et al. Parasympathetic failure and risk of subsequent coronary events in unstable angina and non-ST-segment elevation myocardial infarction. Eur Heart J. 2003;24:1560–1566. doi: 10.1016/s0195-668x(03)00345-2. [DOI] [PubMed] [Google Scholar]
- 33.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Ma X, Wang Q, et al. Nesfatin-1 correlates with hypertension in overweight or obese Han Chinese population. Clin Exp Hypertens. 2015;37:51–56. doi: 10.3109/10641963.2014.897722. [DOI] [PubMed] [Google Scholar]


