To the Editors:
Atypical hemolytic uremic syndrome (aHUS) is a form of complement-mediated thrombotic microangiopathy (TMA), mainly characterized by chronic, uncontrolled, and excessive activation of the alternative pathway of the complement system, resulting in microvascular thrombosis, thereby causing multiple organ dysfunction. The complement system activation may be due to mutations in the complement regulatory proteins, or is caused by acquired neutralizing autoantibody inhibitors of these complement system components. aHUS episodes can be triggered by infection, cancer, organ transplantation, pregnancy, and the use of certain antiplatelet agents (e.g., ticlopidine and clopidogrel), anticancer drugs and immunosuppressant medications (e.g., cyclosporine and tacrolimus).1
Before the development of eculizumab, aHUS was frequently associated with refractoy hypertention and acute kidney injury even under various therapy, including plasmapheresis.2 We report a 56-year-old woman with aHUS who presented with hypertensive crisis and cardiogenic pulmonary edema, and acute renal failure. Her extremely elevated blood pressure was refractory to multiple antihypertensive medications, hemodialysis and plasmapheresis, until the administration of eculizumab.
The patient, a 56-year-old woman, was well until she caught a cold about 1 month before the incident during her travel to the U.S.A. Fatigue and dizziness developed in the next few weeks. She was sent to the emergency department of a medical center in the U.S.A. due to productive cough with pink-colored sputum and progressive orthopnea for 2 days. On examination, she was in respiratory distress. She had a body temperature of 36.7 °C, a blood pressure of 266/162 mmHg, a pulse rate of 148 beats per minute, a respiratory rate of 28 breaths per minute, and an oxygen saturation of 87% on room air. Pale conjunctiva with jugular vein distention were noted. Auscultation of the chest revealed bilateral basal rales and expiratory wheezing. There was also grade 2+ edema on both legs. Acute decompensated heart failure with pulmonary edema was impressed. Oxygen supplementation with continuous positive airway pressure (CPAP) was then administered. The patient’s serial blood examination results are shown in Table 1. Blood count upon admission revealed hemoglobin 7.5 g/dL, platelet count 68,000/μL and poor renal function (creatinine 8.9 mg/dL). Electrocardiogram revealed sinus rhythm, nonspecific ST-T wave abnormalities in precordial leads, and evidence of left ventricular enlargement. Chest radiograph and chest computed tomography findings demonstrated cardiomegaly, bilateral central opacities, and massive right-sided pleural effusion with passive atelectasis. Furosemide and nitroglycerin were intravenously administered. However, 1 hour later, respiratory failure developed; thus, endotracheal intubation was performed with subsequent mechanical ventilatory support. Intermittent hemodialysis (for 7 times) was also arranged due to the presence of acute pulmonary edema. Subsequent blood examination showed negative Coombs test result, low haptoglobin level (< 10 mg/dL), and reticulocytosis (0.141 × 1012/L, 4.38%). Red blood cell (RBC) morphology revealed at least 2 to 3 schistocytes per high-power field. Since TMA was suspected, plasmapheresis was performed twice as thrombotic thrombocytopenic purpura (TTP) could not be ruled out. Due to improvement in clinical conditions, the patient was extubated on Day 4. She was later transferred back to Taiwan and admitted to our hospital for further evaluation and management on Day 13.
Table 1. Serial laboratory results at admission and after discharge.
| Variable | Reference range | Day 0 | Day 32 | Day 35 | Day 59* | Day202# |
| White-cell count (/mm3) | 4000-10000 | 9400 | 11200 | 7300 | 5400 | 5200 |
| Hemoglobin (g/dl) | Men: 13-17 | 7.5 | 8.9 | 10.8 | 11.5 | 13.2 |
| Woman: 12-15 | ||||||
| Platelet (/mm3) | 150000-400000 | 68000 | 172000 | 189000 | 241000 | 207000 |
| INR | 0.9-1.2 | 1 | 0.94 | - | - | - |
| aPTT (sec) | 20-40 | 30 | 19.6 | - | - | - |
| Reticulocytes (%) | 0.6-2.1 | 4.38 | - | - | - | - |
| Hepatoglobin (mg/dL) | 30-200 | < 10 | 10.3 | 81.3 | 112.9 | 81.2 |
| LDH (U/L) | 50-150 | 922 | 200 | 249 | 241 | 216 |
| GOT (U/L) | 43250 | 49 | - | - | - | - |
| GPT (U/L) | 5-35 | 25 | - | - | - | - |
| Total bilirubin (mg/dL) | 0.3-2.0 | 2.4 | - | - | - | - |
| Direct bilirubin (mg/dL) | 0-0.3 | 0.3 | - | - | - | - |
| Alk-P (U/L) | 50-100 | 84 | - | - | - | - |
| Total protein (mg/dL) | 6.0-8.0 | - | - | - | - | |
| Albumin (g/dL) | 3.5-5.5 | 3.5 | - | - | 4.3 | 4.3 |
| Urea nitrogen (mg/dl) | 43333 | 85 | 33 | 63 | 52 | 56 |
| Creatinine (mg/dl) | 0.8-1.3 | 8.9 | 5.73 | 6.98 | 4.63 | 2.69 |
| eGFR (mL/min/1.73 m2) | > 90 | 4.86 | 8.0 | 6.0 | 10 | 18 |
| Sodium (mmol/L) | 136-145 | 126 | 132 | 136 | 138 | 136 |
| Potassium (mmol/L) | 3.5-5.0 | 3.5 | 3.6 | 3.8 | 3.5 | 4 |
| Calcium (mg/dl) | 9.0-10.5 | - | 9.8 | 9.5 | 9.8 | 9.7 |
| Phosphorus (mg/dl) | 3.0-4.5 | - | 3.2 | 3.4 | 3.6 | 4.2 |
| bicarbonate (mmol/L) | 24-26 | - | 23 | 19 | 26 | 23 |
| NT-proBNP (pg/ml) | < 125 | 14500 | - | - | - | - |
| C-reactive protein (mg/dL) | < 0.5 | 0.3 | - | - | - | - |
| Troponin I (ng/ml) | < 0.3 | 0.35 | - | - | - | - |
* After 4 doses of Eculizumab (post induction phase) injection. # After 15 doses of Eculizumab injection.
Alk-P, alkaline phosphatase; aPTT, activated partial thromboplastin time; eGFR, estimated glomerular filtration rate; GOT, glutamate oxaloacetate transaminase; GPT, alanine aminotransferase; INR, international normalized ratio; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Her blood pressure remained high despite multiple antihypertensive medications (Carvedilol 25 mg 1# BID, Nifedipine 30 mg 1# Q12H, Azilsartan 40 mg 1# HS, Doxazosin 4 mg 1# Q12H). Hemodialysis (Day 15) and plasmapheresis (Day 16) were continued. There was no evidence of hypertensive retinopathy upon ophthalmological examination. Initial laboratory tests showed that urea was 44 mg/dL, creatinine 5.16 mg/dL, sodium 140 mmol/L, potassium 4.4 mmol/L, free calcium 1.15 mmol/L (reference range: 1.13-1.31 md/dL), LDH 393 U/L, total bilirubin 0.56 mg/dL, and direct bilirubin 0.2 mg/dL. Urinalysis showed 1+ protein, 3-5 erythrocyte per high-power field, and daily urine albumin excretion of 0.3916 g/day. Blood count was as following: hemoglobin 9 g/dL, platelet count 204,000/μL, reticulocyte count 2.78% (reference range: 0.6-2.1%). Haptoglobin was 9.5 mg/dL (reference range: 30-200 md/dL) and both direct and indirect Coombs were negative. Peripheral blood smear examination showed 1-3 schistocytes per high-power field. C3/C4 and immunoglobulins G, A, and M were in normal range. Anti-nuclear antibody, anti-nuclear cytoplasmic antibody, and anti-glomerular basement membrane were negative. Cardiac echocardiography revealed eccentric left ventricular hypertrophy and hypokinesis of ventricular septum and inferior wall of the left ventricle with an ejection fraction of 44%. Thallium-201 myocardial perfusion scan showed no definite evidence of stress-induced ischemia in the vascular territories. The results of further studies for secondary hypertension were unremarkable, including serum adrenocorticotropic hormone, cortisol, renin, aldosterone, urine vanillylmandelic acid, and catecholamine. Abdominal ultrasonography showed normal renal size (right: 10.7 cm; left: 11.6 cm) and patent bilateral main renal arteries without visible adrenal lesion. In view of these findings, the patient was diagnosed with microangiopathic hemolytic anemia (MAHA). Since her ADAMTS13 (von Willebrand factor-cleaving metalloprotease) activity was normal (64.3%) and there was no diarrhea throughout the clinical course, a diagnosis of aHUS was then made. Renal biopsy was later performed on Day 31. The pathological findings revealed endothelial swelling and narrowing of capillary lumen filled with numerous red cells, compatible with the diagnosis of microangiopathic nephropathy associated with acute tubular injury and chronic tubulointerstitial changes. Immunoflorescent stainings were negative for IgA, IgG, IgM, lambda, kappa, C3 and C4. After discontinuation of hemodialysis (Day 31) and plasmapheresis (Day 33), creatinine level still increased from 5.73 mg/dL (Day 32) to 6.98 mg/dL (Day 35). Treatment with eculizumab at a dose of 900 mg weekly for 4 weeks, then 1200 mg every 2 weeks, was initiated on Day 37. Following the initiation of eculizumab, continued improvement of her blood pressure (Figure 1), renal function, and hemoglobin levels were observed. The number of anti-hypertensive medications was reduced gradually (Carvedilol 25 mg 1# BID). Haptoglobin level also gradually rose. The serum lactate dehydrogenase decreased to the normal range. Laboratory data during eculizumab treatment are shown in Table 1.
Figure 1.
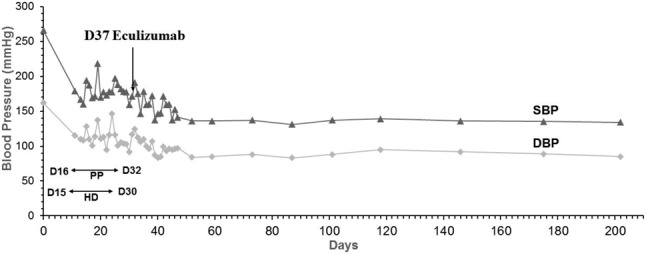
Serial blood pressure measurements with reference to the administration of eculizumab. DBP, diastolic blood pressure; HD, hemodialysis; PP, plasmapheresis; SBP, systolic blood pressure.
Hypertension is a common complication of aHUS, which is characterized by the triad of renal failure, MAHA and thrombocytopenia.3 aHUS is usually associated with a poor prognosis and may progress to end-stage renal disease in half of the patients.4 Although the patient presented with hypertensive crisis, it was overshadowed by other presentations such as fatigue, dizziness, respiratory distress, hemolytic anemia and uremia, leading to the first impression highly suspected to be hematologic origin. Both clinical manifestations and renal biopsy findings supported the diagnosis of aHUS in our case. Eculizumab is the first-line treatment for patients with aHUS.5 However, plasma exchange should be considered and act as a bridging therapy while awaiting ADAMTS-13 activity level since numerous causes of thrombotic microangiopathies may present similar laboratory and clinical findings.6
This case highlights the control of blood pressure merely via medication, dialysis and plasmapheresis seemed unlikely in patient with aHUS, given that the complement hyperactivation causing microvascular thrombosis further activates renin-angiotensin-aldosterone system, leading to vicious cycles of intractable hypertension and progressive renal injury.7 The rapid clinical response to eculizumab in our case supports that therapeutic complement blockade is essential for managing blood pressure in patient with aHUS.8 Eculizumab, a humanized monoclonal anti-C5 inhibitor that targets selective complement activating pathways, markedly decreases the microvascular thrombosis, leading to striking remissions of refractory hypertension.9 Occasionally, hypertension may be the only manifestation of aHUS for years before its characteristic triad (renal failure, MAHA and/or thrombocytopenia) appears, depending on the sites and range of the endothelial injury. When TMA mainly affects pre-glomerular hemodynamics, abnormal renin release and hypertension may develop without renal insufficiency, MAHA or thrombocytopenia.10
In patients with hypertensive crisis and acute renal failure, aHUS should be considered when high blood pressure cannot be controlled by maximum dosages of multiple antihypertensive medications and dialysis. Timely recognition of the disease and treatment with eculizumab may dramatically improve the patients’ prognosis. Moreover, as demonstrated in the present case, critical care with a multi-disciplinary approach including respiratory support by mechanical ventilator and renal replacement therapy by hemodialysis is also essential for a favorable clinical outcome in patients presenting with a multi-system disorder. The hypertensive crisis may still relegate to empirical treatment with available anti-hypertensive agents while awaiting a more likely mechanism that may explain the clinical picture and offer an opportunity for novel treatments.
REFERENCES
- 1.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 2.Ohta T, Urayama K, Tada Y, et al. Eculizumab in the treatment of atypical hemolytic uremic syndrome in an infant leads to cessation of peritoneal dialysis and improvement of severe hypertension. Pediatr Nephrol. 2015;30:603–608. doi: 10.1007/s00467-014-2975-4. [DOI] [PubMed] [Google Scholar]
- 3.Loirat C, Frémeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011;6:60. doi: 10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jokiranta TS. HUS and atypical HUS. Blood. 2017;129:2847–2856. doi: 10.1182/blood-2016-11-709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Licht C, Greenbaum LA, Muus P, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:1847–1848. doi: 10.1056/NEJMc1410951. [DOI] [PubMed] [Google Scholar]
- 7.Raghunathan V, Sethi SK, Dragon-Durey MA, et al. Targeting renin-angiotensin system in malignant hypertension in atypical hemolytic uremic syndrome. Indian J Nephrol. 2017;27:136–140. doi: 10.4103/0971-4065.181462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 9.Asif A, Nayer A, Haas CS. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J Nephrol. 2017;30:347–362. doi: 10.1007/s40620-016-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai HM. Atypical hemolytic uremic syndrome may present as severe hypertension without hemolysis or thrombocytopenia. Austin J Nephrol Hypertens Obstet Gynecol. 2016;127:907–910. doi: 10.1097/AOG.0000000000001340. [DOI] [PubMed] [Google Scholar]


