Abstract
Background
Atherosclerosis (AS) is defined as chronic inflammation of the vessel wall. The major objective of the this study was to explore the mechanism of Treg/Th17 imbalance and the role of high mobility group box-1 protein (HMGB1) on the balance in AS.
Methods
We detected the apoptotic ratios of Treg and Th17 cells in peripheral blood mononuclear cells (PBMCs) from subjects with AS and normal coronary arteries (NCA) by flow cytometry. The effects of recombinant HMGB1 (rHMGB1) on the proportion, apoptosis and differentiation of Treg and Th17 cells were analyzed using flow cytometry, qRT-PCR and ELISA.
Results
The frequencies of apoptotic Treg cells in the PBMCs from the subjects with AS were significantly higher than in those with NCA (p < 0.01). Stimulation of rHMGB1 obviously increased the level of Th17 cells and acid- related orphan receptor C (RORC) mRNA, and markedly decreased Treg cell frequency and the mRNA expression of factor forkhead family protein 3 (Foxp3) in the PBMCs. rHMGB1 played an obvious role in elevating Treg cell apoptosis ratio (p < 0.01). rHMGB1 treatment significantly decreased Treg cell ratio and IL-10 level, and increased Th17 cell ratio and IL-17A level induced from naïve CD4+ T cells.
Conclusions
HMGB1 may modulate Treg/Th17 balance in patients with AS through inducing Treg cell apoptosis and promoting cell differentiation of Th17.
Keywords: Atherosclerosis, HMGB1, Regulatory T cell, T helper cell 17
INTRODUCTION
Atherosclerosis (AS) is defined as chronic inflammation of the vessel wall, involving the activation of various leukocytes and participation of several inflammatory cytokines along with lipid deposition.1,2 Under different stimulant conditions, naive CD4+ T cells can differentiate into distinct subtypes such as Th1, Th2, Th17 and regulatory T (Treg) cells.3 Treg cells express transcriptional factor forkhead family protein 3 (Foxp3) and secrete anti-inflammatory cytokines such as TGF-β and IL-10, and are critical in restraining inflammation and maintaining immune tolerance. Th17 cells, the special transcriptional factors of which are acid-related orphan receptor C (RORC) in humans, are pro-inflammatory and secrete IL-17A/F, IL-21 and TNF-α. The balance of Treg/ Th17 cells has been shown to be important in AS and associated cardiovascular diseases.4
High mobility group box-1 protein (HMGB1), a lasted inflammatory molecule, can be secreted from activated immunocytes and released from necrotic or injured cells.5 Our previous studies and others have demonstrated that HMGB1 levels in the serum of AS patients are significantly higher than in control subjects.6,7 HMGB1 can stimulate the expression or/and secretion of cytokines, adhesion molecules, chemokines, lipid mediators and plasminogen activator in smooth muscle cells or endothelial cells, and partake in vascular smooth muscle cells (VSMCs) proliferation and migration, endothelium activation, activation of macrophages, and disorder of lipid metabolism, all of which are linked to AS pathology.5
HMGB1 has been reported to induce the apoptosis of macrophages, T lymphocytes and myocardial cells.8-10 Nevertheless, the effects of HMGB1 on Treg and Th17 cells apoptosis still remain unclear. HMGB1 has been shown to facilitate the differentiation of myeloid-derived suppressor cells from bone marrow,11 and also to induce human macrophage polarization from monocytes via collaborating with C1q.12 However, whether HMGB1 affects the differentiation of Treg and Th17 cells is still ambiguous.
In this study, we determined the apoptosis levels of Treg and Th17 cells in peripheral blood mononuclear cells (PBMCs) from AS patients and control subjects. We also assessed the effects of recombinant HMGB1 (rHMGB1) on the apoptosis and differentiation of Treg and Th17 cells in vitro. The major aim of this study was to investigate the potential regulatory mechanisms of HMGB1 on peripheral Treg/Th17 balance in patients with AS.
MATERIALS AND METHODS
Patients
We examined patients at Yichang Central People’s Hospital who underwent diagnostic catheterization (males and females) between March 2016 and January 2017. All patients gave written informed consent previous to enrollment into this study. This study was approved by the Research Ethics Committee of the Central People’s Hospital in Yichang, Hubei Province, China. The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Patients were divided into two groups according to coronary angiography: group 1: AS (23 males and 13 females, mean age = 61.3 ± 7.4 years), patients displaying at least one coronary artery exceeding 50% stenosis; group 2: normal coronary arteries (NCA) (35 males and 28 females, mean age = 58.3 ± 8.1 years), patients showing normal coronary arteries.
The exclusion criteria were as follows:13,14 cardiovascular events < 1 year (such as myocardial infarction or stroke); metabolic diseases (such as diabetes mellitus and hyperlipemia); tumors; autoimmune disorders; connective tissue diseases; various acute and chronic infections; surgery; liver diseases; renal failure; and treatment with immunosuppressive drugs and/or anti-inflammatory agents.
Blood samples and cell isolation
Peripheral venous blood (10-20 mL) was collected in heparin anticoagulant tubes from all of the patients after fasting overnight. All blood samples were used to prepare PBMCs by Ficoll-Hypaque density gradient centrifugation according to the manufacturer’s instructions with Lymphocyte Separation Medium (Lot LTS1077, TBD, China) within 4 hours. The isolated PBMCs were examined by flow cytometry (FCM) or cultured in 1640 complete culture medium at a density of 2 × 106 cells/mL.
Apoptosis analysis of Treg and Th17 cells
We examined apoptosis levels of Treg and Th17 cells in PBMCs from two subjects by staining the cells for Annexin V. For Treg cell apoptosis analysis, aliquots (100 μL) of PBMCs were incubated with anti-human CD4-PE-Cy7 (Lot 25-0049-42, eBioscience, America), anti-human CD25-PE (Lot 12-0259-42, eBioscience, America) and anti-human CD127-APC (Lot 17-1278-42, eBioscience, America) at 4 °C for 30 min. After washing with phosphate-buffered saline (PBS), the cells were suspended in 100 μL binding buffer (Lot KGA108, KeyGENBioTECH, China). Then, the cells were incubated with Annexin V-FITC (Lot KGA108, KeyGENBioTECH, China) at 4 °C for 15 min. After being washed and resuspended in 400 μL binding buffer, the cells were analyzed by FCM.
For Th17 cell apoptosis analysis, 2 × 106 PBMCs were stimulated with BFA/Monensin mixture (4 uL/mL, Lot LK-CS1001, Liankebio, China) and PMA/Ionomycin mixture (4 uL/mL, Lot LK-CS1001, Liankebio, China) for 6 hours in 24-well plates with 5% CO2 at 37 °C. The cell suspension was collected and washed with PBS. Aliquots (100 μL) of PBMCs were incubated with anti-human CD3-PE-Cy7 (Lot 25-0038-42, eBioscience, America) and anti-human CD8-APC (Lot 17-0088-42, eBioscience, America)at 4 °C for 30 min. After being washed with PBS, the cells were suspended in 100 μL binding buffer and incubated with Annexin V-FITC at 4 °C for 15 min. The cells were fixed and permeabilized using an Intracellular Fixation & Permeabilization Buffer Set Kit (Lot 85-88-8824-00, eBioscience, America) according to the manufacturer’s instructions and stained for anti-human IL-17A-PE (Lot 12-7178-42, eBioscience, America). After being washed and resuspended in 400 μL binding buffer, the cells were analyzed by FCM.
The effects of stimulation (BFA/Monensin and PMA/Ionomycin), fixation and permeabilization on the apoptosis of cells were evaluated by examining the apoptosis of CD4+ T cells from the PBMCs under post-fixation/permeabilization, post-stimulation and pre-stimulation before analysis of Th17 cell apoptosis.
Measurement of blood biochemistry
The levels of fasting blood glucose, serum creatinine, triglycerides, total cholesterol, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol were measured by enzymatic methods at the clinical chemistry laboratory of our hospital.
rHMGB1 induction expressions
rHMGB1 was purchased from Sigma, America (Lot H4652, Purity: ≥ 90% verified by SDS-PAGE). rHMGB1 was cleaned with EndoTrap (Hyglos GmbH, Germany) to remove endotoxin. PBMCs from the NCA subjects (n = 8) were incubated with 1640 complete culture medium, including various concentrations of rHMGB1 (0, 10, 100, 1000 ng/mL), at a density of 2 × 106 cells/ mL for 24 h in vitro. The PBMCs from the NCA subjects (n = 5) were incubated with 1640 complete culture medium, including 1000 ng/mL rHMGB1, at a density of 2 × 106 cells/mL for 0, 12, 24 and 48 h in vitro. The frequencies of Treg and Th17 cells were then measured by FCM.
To compare the effects of rHMGB1 on Treg and Th17 cells from the AS and NCA subjects, PBMCs (each group: n = 5) were incubated with 1640 complete culture medium, including 100 ng/mL rHMGB1, at a density of 2 × 106 cells/mL for 24 h in vitro. The frequencies of Treg and Th17 cells were measured by FCM. The expressions of Foxp3 and RORC were determined by qRT-PCR.
For Treg cell frequency analysis, aliquots (100 μL) of PBMCs were incubated with anti-human CD4-FITC (Lot 85-11-0047-42, eBioscience, America), anti-human CD25-PE and anti-human CD127-PE-Cy7 (Lot 85-25-1278-42, eBioscience, America) at 4 °C for 30 min. After being washed and resuspended in 300 μL PBS, the cells were analyzed by FCM.
For Th17 cell frequency analysis, 2 × 106 PBMCs were stimulated with BFA/Monensin mixture (4 uL/mL, Lot LK-CS1001, Liankebio, China) and PMA/Ionomycin mixture (4 uL/mL, Lot LK-CS1001, Liankebio, China) for 6 hours in 24-well plates with 5% CO2 at 37 °C. The cell suspension was then collected and washed with PBS. Aliquots (100 μL) of PBMCs were incubated with anti-human CD3-FITC (Lot 11-0039-42, eBioscience, America) and anti-human CD8-APC (Lot 17-0088-42, eBioscience, America) at 4 ° C for 30 min. After being washed with PBS, the cells were fixed and permeabilized using an Intracellular Fixation & Permeabilization Buffer Set Kit (Lot 85-88-8824-00, eBioscience, America) according to the manufacturer’s instructions and stained for anti-human IL-17A-PE (Lot 12-7178-42, eBioscience, America). After being washed and resuspended in 300 μL PBS, the cells were analyzed by FCM.
Total RNA isolation and qRT-PCR
Total RNA was extracted from PBMCs using an RNA simple Total RNA Kit (Lot DP419, TIANGEN BIOTECH, China), and cDNA was synthesized using an Revert Aid First Strand cDNA Synthesis Kit (Lot K1621, Thermo Scientific, Australia) according to the manufacturer’s instructions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize mRNA expression level as a housekeeping gene. The primer pairs were as follows: Foxp3 forward: AACAGCACATTCCCAGAGTTCC; Foxp3 reverse: CATTGAGTGTCCGCTGCTTC (NM_014009.3); RORC forward: CCGAGGATGAGATTGCCCTCT; RORC reverse: GGTGGCAGCTTTGCCAGGAT (NM_005060.3); GAPDH (Lot PHS04, Sangon Biotech, China). qRT-PCR was performed using SYBR® Premix Ex TaqTM II (Lot RR82LR, TAKARA, Japan) on an Agilent StrataGene Mx3000P system. The PCR conditions were 95 °C for 2 min, 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. We calculated the relative gene expressions using the comparative CT method. Samples were examined in triplicate.
The effect of rHMGB1 on apoptosis of Treg and Th17 cells
PBMCs (n = 8) from the NCA subjects were incubated with 1640 complete culture medium, including 100 ng/mL rHMGB1, at a density of 2 × 106 cells/mL for 24 h in vitro. The apoptosis frequencies of Treg and Th17 cells were measured by FCM.
The role of rHMGB1 in differentiation assay
Naive CD4+ T cells were isolated from PBMCs (n = 6) of the NCA subjects using a Human Naive CD4+ T Cell Isolation Kit (Lot 130-094-131, MiltenyiBiotec, Germany) according to the manufacturer’s instruction. We determined the purity of the sorted cells by FCM (> 95% for CD4+CD45RA+ cells).
Naive CD4+ T cells were cultured in 1640 complete culture medium containing 2 ug/mL soluble anti-CD28 Ab (Lot 16-0289-85, eBioscience, America) in 96-well plates coated with 5 ug/mL anti-CD3 Ab (Lot 16-0039-85, eBioscience, America) for 7 days. The culture medium included 5 ng/mL TGF-β1 (Lot 100-21, PeproTech, America) and 500 IU/mL IL-2 (Lot 200-02, PeproTech, America) for Treg cell differentiation, and 10 ng/mL IL-1β (Lot 200-01b, PeproTech, America), 20 ng/mL IL-6 (Lot 200-06, PeproTech, America) and 10 ng/mL IL-23 (Lot 200-23, PeproTech, America) for Th17 cell differentiation.
To analyze the role of rHMGB1 in Treg cell differentiation, naive CD4+ T cells were divided into two groups: the control group (incubated in conditions for Treg cell differentiation) and rHMGB1 group (incubated in conditions for Treg cell differentiation and 100 ng/mL rHMGB1).
To analyze the role of rHMGB1 in Th17cell differentiation, naive CD4+ T cells were divided into two groups: the control group (incubated in conditions for Th17 cell differentiation) and rHMGB1 group (incubated in conditions for Th17 cell differentiation and 100 ng/mL rHMGB1).
Cells were collected for Treg and Th17 cell frequency analysis by FCM. The supernatants were stored at -80 °C for measurements of IL-10 and IL-17A level by enzyme-linked immunosorbent assay (ELISA).
Detection of IL-10 and IL-17A by ELISA
IL-10 and IL-17A secretions in the supernatants from each culture group were measured using a Human IL-10 ELISA kit (Lot EHC009.96, NeoBioscience, China) and a Human IL-17A ELISA kit (Lot EHC170.96, NeoBioscience, China) according to the manufacturer’s protocol.
Statistical analysis
SPSS statistical software (version 18) was used for all data analyses. Data were expressed as the mean ± standard deviation (SD). Comparisons between groups were performed using one-way ANOVA. Statistical significance for the differences between two groups was tested using the Student’s t-test. p < 0.05 was considered to be statistically significant.
RESULTS
Basic characteristics of the patients
Table 1 shows the basic characteristics of the patients. There were no statistically significant differences in gender, age, hypertension, fasting plasma glucose, serum creatinine, total cholesterol, triglycerides, high-density lipoprotein-cholesterol and low-density lipoprotein-cholesterol among the patients in the AS and NCA groups.
Table 1. Clinical characteristics of the groups.
| Item | NCA group (n = 63) | AS group (n = 36) |
| Gender (male/female) | 35/28 | 23/13 |
| Age (years) | 58.3 ± 8.1 | 61.3 ± 7.4 |
| Hypertension, n (%) | 21 (33%) | 18 (50%) |
| FPG (mmol/L) | 4.7 ± 0.6 | 4.7 ± 0.5 |
| Serum Cr (umol/L) | 71.1 ± 16.5 | 75.3 ± 18.0 |
| TC (mmol/L) | 4.0 ± 0.9 | 4.0 ± 1.0 |
| TG (mmol/L) | 1.5 ± 1.0 | 1.6 ± 0.8 |
| HDL-C (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.2 |
| LDL-C (mmol/L) | 2.4 ± 0.8 | 2.3 ± 0.9 |
Values are expressed as mean ± SD.
AS, atherosclerosis; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; NCA, normal coronary arteries; Serum Cr, serum creatinine; TC, total cholesterol; TG, triglycerides.
Apoptosis levels of Treg and Th17 cells in the AS and NCA groups
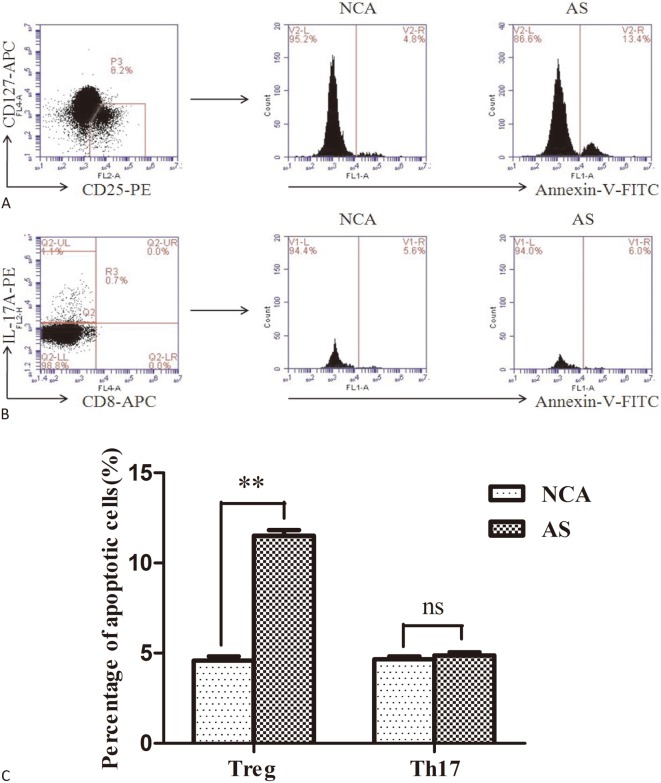
Figure 1A and Figure 1B show the representative flow cytometric dot plots for apoptotic Treg cells (gated by CD4+CD25+CD127- Annexin V+ cells) and apoptotic Th17 cells (gated by CD3+CD8-IL-17+ Annexin V+ cells) in the AS and NCA groups. As shown in Figure 1C, the frequencies of apoptotic Treg cells were significantly higher in the AS group than in the NCA group (p < 0.01), whereas there was no significant difference in Th17 cell apoptosis ratio between the two groups (p > 0.05).
Figure 1.
Apoptosis level of Treg and Th17 cells in atherosclerosis (AS) and normal coronary arteries (NCA) group. (A) and (B) Representative flow cytometric dot plots for apoptotic Treg cells (gated by CD4+CD25+CD127- Annexin V+ cells) and apoptotic Th17 cells (gated by CD3+CD8-IL-17+ Annexin V+ cells) in each group; (C) A summary of the percentage of apoptotic Treg and Th17 cells in AS (n = 31) and NCA (n = 31) group. (** p < 0.01; ns, not significant)
Effects of rHMGB1 on Treg and Th17 cells in vitro
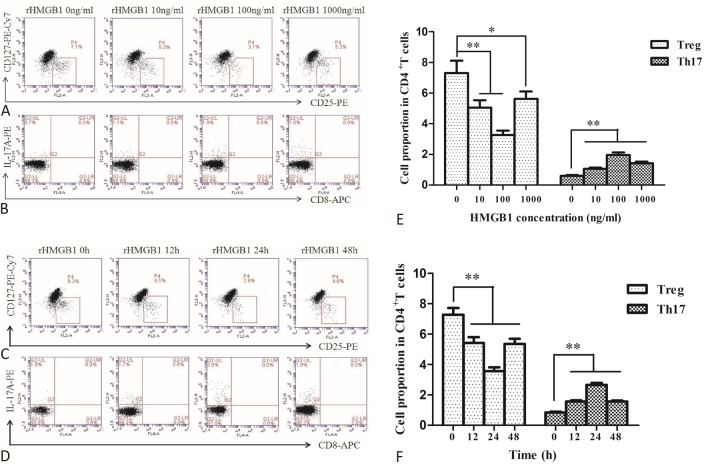
Figure 2A and Figure 2B show the representative flow cytometric dot plots for Treg cells (gated by CD4+CD25+ CD127- cells) and Th17 cells (gated by CD3+CD8-IL-17+ cells) after rHMGB1 stimulation at various concentrations (0, 10, 100, 1000 ng/mL)for 24 h. Figure 2C and Figure 2D show the representative flow cytometric dot plots for Treg cells and Th17 cells after rHMGB1 stimulation (at 100 ng/ml) for different incubation times (0, 12, 24 and 48 h). As shown in Figure 2E and Figure 2F, stimulation of rHMGB1 obviously increased the level of Th17 cells, and markedly decreased the frequency of Treg cells. The effect of rHMGB1 was more obvious at 100 ng/ml concentration (p < 0.01, Figure 2E) and after 24 h of incubation (p < 0.01, Figure 2F).
Figure 2.
Change of Treg and Th17 cell frequencies after rHMGB1 stimulation at various concentrations for 24 h (NCA, n = 8) and after 100 ng/mL rHMGB1 stimulation for different incubation times (NCA, n = 5). (A) and (B) Representative flow cytometric dot plots for Treg cells (gated by CD4+CD25+CD127- cells) and Th17 cells (gated by CD3+CD8-IL-17+ cells) after rHMGB1 stimulation at various concentrations; (C) and (D) Representative flow cytometric dot plots for Treg cells (gated byCD4+CD25+CD127- cells) and Th17 cells (gated by CD3+CD8-IL-17+ cells) after rHMGB1 stimulation for different incubation times; (E) Comparison of Treg and Th17 cell frequencies after rHMGB1 stimulation at various concentrations for 24 h; (F) Comparison of Treg and Th17 cell frequencies after 100 ng/mL rHMGB1 stimulation for different incubation times. (* p < 0.05, ** p < 0.01)
The Treg cell frequencies were decreased (p < 0.01, Figure 3A) and the Th17 cell frequencies were increased (p < 0.01, Figure 3B) in PBMCs from both AS and NCA groups after rHMGB1 stimulation. The rHMGB1-mediated decrease of Treg cells in the AS group was more significant than in the NCA group (p < 0.01, Figure 3C). The rHMGB1-mediated increase of Th17 cells in the AS group was more significant than in the NCA group (p < 0.01, Figure 3D).
Figure 3.
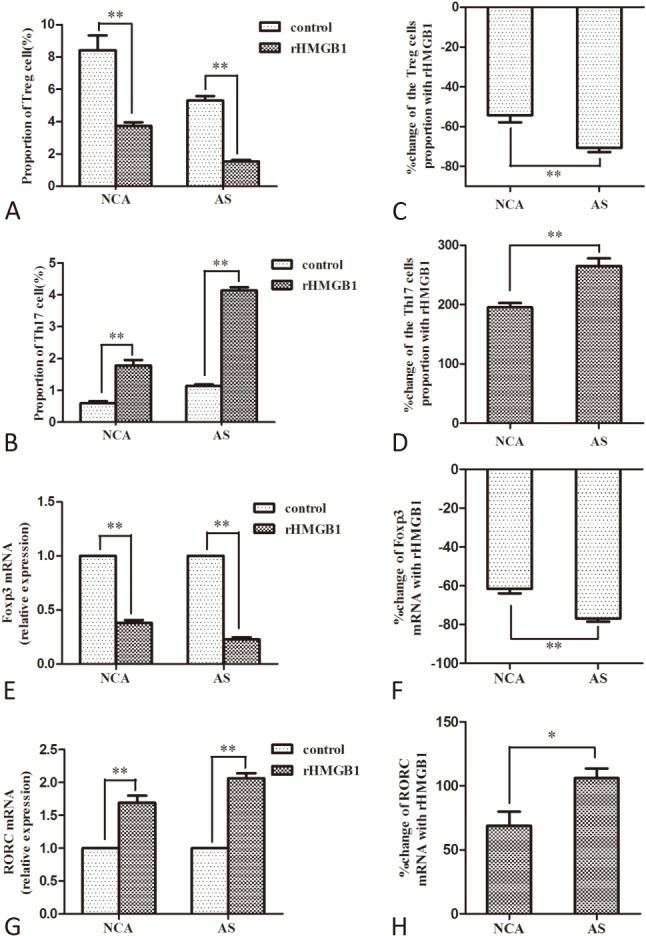
Comparison of frequencies changes of Treg and Th17 cell and expression changes of Foxp3 and RORC mRNA induced by rHMGB1 in atherosclerosis (AS) and normal coronary arteries (NCA) group (AS, n = 5; NCA, n = 5). (A) and (B) Comparison of Treg and Th17 cell frequencies with or without 100 ng/mL rHMGB1 stimulation for 24 h in each group; (C) and (D) Average change of Treg and Th17 cell numbers in two groups after rHMGB1 stimulation; (E) and (F) Comparison of Foxp3 and RORC mRNA levels with or without 100 ng/mL rHMGB1 stimulation for 24 h in each group; (G) and (H) Average change of Foxp3 and RORC mRNA levels in two groups after rHMGB1 stimulation. (* p < 0.05, ** p < 0.01)
The Foxp3 mRNA levels were decreased (p < 0.01, Figure 3E) and RORC mRNA levels were increased (p < 0.01, Figure 3F) in PBMCs from both AS and NCA groups after rHMGB1 stimulation. The rHMGB1-mediated decrease of Foxp3 mRNA levels in the AS group was more significant than in the NCA group (p < 0.01, Figure 3G). The rHMGB1-mediated increase of RORC mRNA levels in the AS group was more significant than in the NCA group (p < 0.05, Figure 3H).
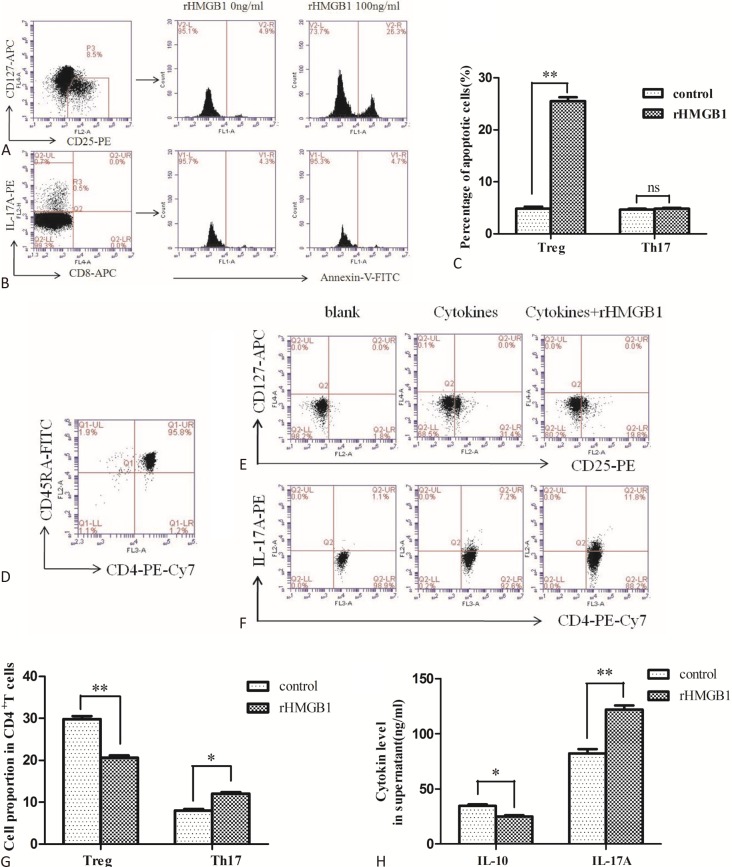
Effects of rHMGB1 on apoptosis of Treg and Th17 cells in vitro
Figure 4A and Figure 4B show the representative flow cytometric dot plots for apoptotic Treg cells (gated by CD4+CD25+CD127- Annexin V+ cells) and apoptotic Th17 cells (gated by CD3+CD8-IL-17+ Annexin V+ cells) with or without 100 ng/mL rHMGB1 stimulation for 24 h in the NCA group. Stimulation of rHMGB1 obviously elevated the Treg cell apoptosis ratio (p < 0.01, Figure 4C), but it had no significant effect on the ratio of Th17 cell apoptosis (p > 0.05, Figure 4C).
Figure 4.
Effects of rHMGB1 on apoptosis and differentiation of Treg and Th17 cells from normal coronary arteries (NCA) group. (A) and (B) Representative flow cytometric dot plots for apoptotic Treg cells (gated by CD4+CD25+CD127- Annexin V+ cells) and apoptotic Th17 cells (gated by CD3+CD8-IL-17+ Annexin V+ cells) with or without 100 ng/mL rHMGB1 stimulation for 24 h in NCA group. (C) A summary of the percentage of apoptotic Treg and Th17 cells with or without 100 ng/mL rHMGB1 stimulation for 24 h in NCA group (n = 8); (D) Representative flow cytometric dot plots for purified naive CD4+ T cells (gated by CD4+CD45RA+ cells) from NCA group; (E) and (F) Representative flow cytometric dot plots for Treg cells (gated by CD4+CD25+CD127- cells) and Th17 cells (gated by CD4+IL-17+ cells) in blank (without induction), control (induction without rHMGB1 stimulation) and rHMGB1 (induction with 100 ng/mL rHMGB1 sitmulation) group; (G) Sum up Treg and Th17 cells frequencies after induction from naive CD4+ T cells with or without rHMGB1 stimulation (n = 6); (H) A summary of IL-10 and IL-17A levels in supernatants from each culture group (n = 6). (* p < 0.05; ** p < 0.01; ns, not significant)
Effects of rHMGB1 on differentiation of Treg and Th17 cells from naive CD4+ T cells in vitro
The purity of the sorted naive CD4+ T cells from the NCA subjects was determined by FCM (> 95% for CD4+ CD45RA+ cells, Figure 4D). Figure 4E and Figure 4F show the representative flow cytometric dot plots for Treg cells (gated by CD4+CD25+CD127- cells) and Th17 cells (gated by CD4+IL-17+ cells) in blank (without induction), control (induction without rHMGB1 stimulation) and rHMGB1 (induction with 100 ng/mL rHMGB1 stimulation) groups. The frequencies of Treg cells and Th17 cells were (1.5 ± 0.45)% and (0.9 ± 0.38)% before treating naive CD4+ T cells with induction conditions. The CD4+ CD25+CD127- Treg cell ratio was elevated to (29.8 ± 1.75)% after stimulating naive CD4+ T cells with TGF-β 1 and IL-2 for 7 days. The CD4+IL-17+Th17 cell ratio was elevated to (8.0 ± 0.84)% after stimulating naive CD4+ T cells with IL-1β, IL-6 and IL-23 for 7 days. The Treg cell ratio was decreased to (20.6 ± 1.41)% in the rHMGB1 treatment group, which was significantly lower than in the control group (p < 0.01, Figure 4G). The IL-10 level in the rHMGB1 treatment group was lower than in the control group (p < 0.05, Figure 4H). The Th17 cell ratio was elevated to (12.1 ± 0.81)% in the rHMGB1 treatment group, which was significantly higher than in the control group (p < 0.05, Figure 4G). The IL-17A level in the rHMGB1 treatment group was higher than in the control group (p < 0.01, Figure 4H).
DISCUSSION
HMGB1 levels have been shown to be significantly higher in the serum of AS patients than in those with normal arteries6,7 and to be independently associated with the burden of non-calcified plaque in patients with stable coronary artery diseases.7 In the present study, we showed that rHMGB1 influenced Treg/Th17 balance in the peripheral blood of AS patients by promoting the balance to the pro-inflammatory Th17 cell side. HMGB1 has been shown to partake in VSMC proliferation and migration, endothelium activation, activation of macrophages and platelets, as well as disorder of lipid metabolism, all of which can accelerate plaque development and thrombus formation in the pathology of AS.15-17 The role of HMGB1 on lymphocyte immunity and local inflammation in AS is still inconclusive. Our previous study on AS6 and previous papers on acute coronary syndrome18,19 indicated that the frequencies of transcriptional factor Foxp3 and cytokines (IL-10 and TGF-β1) secretion of Treg cells were significantly reduced, while the frequencies of transcriptional factor RORC and cytokines (IL-17, IL-6, IL-23 and TNF-α) secretion of Th17 cells were obviously elevated in these patients. Treg cells and their secreted IL-10 can reduce plaque sizeand the risk of AS,20-22 and Th17 cells associated with IL-17 can promote plaque formation.23
The mechanism of breaking the Treg/Th17 balance and promoting the balance to the pro-inflammatory Th17 cell side in the progression of AS is still unclear. HMGB1 has been demonstrated to promote Th17 differentiation through TLR2 or TLR4-IL-6 pathways in studies of rheumatoid arthritis24 and chronic hepatitis B,25 which may be through promoting the secretion of Th17 differentiation-associated cytokines (IL-23 and IL-1β) from antigen presenting cells.26-28 In the present study, we showed that simulation of PBMCs with rHMGB1 significantly decreased Treg cell frequencies and increased Th17 cell ratios.
Treg cells have been shown to drive a shift from a Th1 to a Th2 response,29 and that this plays a key role in AS. In a study of atherogenesis in apo E(-/-) mice, dendritic cell overactivation was shown to inhibit Treg cell function probably through secreting IL-6.30 Inflammation leads to lipid deposition in the walls of arteries, causing AS of varying severities.31 Th17 cells, as inflammatory cells, can be detected within plaques. More importantly, treatment with neutralizing anti-IL-17A Ab has been shown to significantly inhibit the development of plaque, whereas rIL-17 treatment has been shown to markedly promote plaque formation in AS in apoE-deficient mice.23 Therefore, HMGB1 probably influences the progression of AS by affecting the inflammatory environment and lipid deposition by modulating the balance of Treg/Th17.
The present study showed that the frequencies of apoptotic Treg cells in the AS subjects were significantly higher than in the NCA subjects. A large body of evidence has suggested that HMGB1 can induce the apoptosis of macrophages, T lymphocytes, and myocardial cells.8-10,32 Our findings suggest that rHMGB1 can promote Treg cell apoptosis. The Fas/FasL/caspase-3 signaling pathway has been shown to becritical in T cell apoptosis, including Treg cells.14 Our results suggest that rHMGB1 probably influences the Treg/Th17 balance partially through inducing Treg cell apoptosis. However, the concrete me-chanism needs further investigation.
The immunologic functions of Treg and Th17 cells are mutually antagonistic, and their differentiation progress are interrelated. Naive CD4+ T cells can differentiate into Treg cells under stimulation of TGF-β1 and IL-2, and polarize to Th17 cells after stimulation of IL-1β, IL-6 and IL-23.3 In the present study, we showed that interference of rHMGB1 significantly promoted Th17 cell differentiation from naive CD4+ T cells. Although the Treg cell frequency was obviously decreased in the differentiation assay with rHMGB1, we could not confirm the effect of rHMGB1 on Treg differentiation due to the induction of apoptosis on Treg cells by rHMGB1. Signal transducer and activator of transcription 3 (STAT3) has been found to bind to the IL17A promoter directly through chromatin immunoprecipitation, accompanied by increasing the number of IL-17-producing cells.33 Treg cell differentiation has been shown to be mediated by the combination of signal transducer and activator of transcription 5 (STAT5) with Foxp3 promoter.34 We will investigate the possible signal pathway involved in the differentiation of Treg and Th17 cells in future studies.
CONCLUSIONS
Our findings suggest that HMGB1 may influence Treg/Th17 balance in AS patients by inducing Treg cell apoptosis, promoting Th17 cell differentiation and probably inhibiting Treg cell differentiation. This study may provide new targets for a better diagnosis and treatment of AS. Nevertheless, the specific mechanisms of how HMGB1 modulates Treg cell apoptosis and the differentiation of Treg and Th17 cells need to be investigated in further studies.
Acknowledgments
This work was supported by the Natural Science Foundation of Hubei Province, China (2014CFC1035), and the National Natural Science Foundation of China (81400794).
CONFLICT OF INTEREST
The authors have no financial conflicts of interest.
REFERENCES
- 1.Ateş AH, Aytemir K, Koçyiğit D, et al. Association of neutrophil-to-lymphocyte ratio with the severity and morphology of coronary atherosclerotic plaques detected by multidetector computerized tomography. Acta Cardiol Sin. 2016;32:676–683. doi: 10.6515/ACS20160225A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilhan F, Kalkanli ST. Atherosclerosis and the role of immune cells. World J Clin Cases. 2015;3:345–352. doi: 10.12998/wjcc.v3.i4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iborra S, Gonzalez-Granado JM. In vitro differentiation of naive CD4(+) T cells: a tool for understanding the development of atherosclerosis. Methods Mol Biol. 2015;1339:177–189. doi: 10.1007/978-1-4939-2929-0_12. [DOI] [PubMed] [Google Scholar]
- 4.Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding JW, Zheng XX, Zhou T, et al. HMGB1 modulates the Treg/ Th17 ratio in atherosclerotic patients. J Atheroscler Thromb. 2016;23:737–745. doi: 10.5551/jat.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrassy M, Volz HC, Maack B, et al. HMGB1 is associated with atherosclerotic plaque composition and burden in patients with stable coronary artery disease. PLoS One. 2012;7:e52081. doi: 10.1371/journal.pone.0052081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu XM, Yao YM, Liang HP, et al. Effect of high mobility group box-1 protein on apoptosis of peritoneal macrophages. Arch Biochem Biophys. 2009;492:54–61. doi: 10.1016/j.abb.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Wu ZS, Yao YM, Hong GL, et al. Role of mitofusin-2 in high mobility group box-1 protein-mediated apoptosis of T cells in vitro. Cell Physiol Biochem. 2014;33:769–783. doi: 10.1159/000358651. [DOI] [PubMed] [Google Scholar]
- 10.Ding HS, Yang J, Chen P, et al. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene. 2013;527:389–393. doi: 10.1016/j.gene.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Su Z, Ni P, She P, et al. Bio-HMGB1 from breast cancer contributes to M-MDSC differentiation from bone marrow progenitor cells and facilitates conversion of monocytes into MDSC-like cells. Cancer Immunol Immunother. 2016;66:391–401. doi: 10.1007/s00262-016-1942-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Son M, Porat A, He M, et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood. 2016;128:2218–2228. doi: 10.1182/blood-2016-05-719757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Wang Y, Yu F, et al. Peripheral Th17/Treg imbalance in patients with atherosclerotic cerebral infarction. Int J Clin Exp Pathol. 2013;6:1015–1027. [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Wang Y, Zhou Q, et al. Distinct different sensitivity of Treg and Th17 cells to Fas-mediated apoptosis signaling in patients with acute coronary syndrome. Int J Clin Exp Pathol. 2013;6:297–307. [PMC free article] [PubMed] [Google Scholar]
- 15.Hu N, Kong L, Qian A, et al. HMGB1 silencing potentiates the anti-inflammatory effects of sodium ferulate in ox-LDL-stimulated vascular smooth muscle cells. Cell Biochem Biophys. 2015;72:297–304. doi: 10.1007/s12013-014-0455-x. [DOI] [PubMed] [Google Scholar]
- 16.Kanellakis P, Agrotis A, Kyaw TS, et al. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:313–319. doi: 10.1161/ATVBAHA.110.218669. [DOI] [PubMed] [Google Scholar]
- 17.Montes VN, Subramanian S, Goodspeed L, et al. Anti-HMGB1 antibody reduces weight gain in mice fed a high-fat diet. Nutr Diabetes. 2015;5:e161. doi: 10.1038/nutd.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Xu LJ, Wu JX. Changes of circulating CD4(+)CD25(+) CD127(low) regulatory T cells in patients with acute coronary syndrome and its significance. Genet Mol Res. 2015;14:15930–15936. doi: 10.4238/2015.December.7.4. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Wang Y, Chen K, et al. Treg/Th17 ratio acts as a novel indicator for acute coronary syndrome. Cell Biochem Biophys. 2014;70:1489–1498. doi: 10.1007/s12013-014-9993-5. [DOI] [PubMed] [Google Scholar]
- 20.Feng J, Zhang Z, Kong W, et al. Regulatory T cells ameliorate hyperhomocysteinaemia-accelerated atherosclerosis in apoE-/- mice. Cardiovasc Res. 2009;84:155–163. doi: 10.1093/cvr/cvp182. [DOI] [PubMed] [Google Scholar]
- 21.Arjuman A, Chandra NC. Effect of IL-10 on LOX-1 expression, signalling and functional activity: an atheroprotective response. Diab Vasc Dis Res. 2013;10:442–451. doi: 10.1177/1479164113489042. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Liu HP, Weng D, Ge BX. IL-10 negatively regulates oxLDL-P38 pathway inhibited macrophage emigration. Exp Mol Pathol. 2014;97:590–599. doi: 10.1016/j.yexmp.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Erbel C, Chen L, Bea F, et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Shotorbani SS, Jiao Z, et al. HMGB1 promotes the differentiation of Th17 via up-regulating TLR2 and IL-23 of CD14+ monocytes from patients with rheumatoid arthritis. Scand J Immunol. 2012;76:483–490. doi: 10.1111/j.1365-3083.2012.02759.x. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Wang FP, She WM, et al. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. J Viral Hepat. 2014;21:129–140. doi: 10.1111/jvh.12152. [DOI] [PubMed] [Google Scholar]
- 26.Xia Q, Duan L, Shi L, et al. High-mobility group box 1 accelerates early acute allograft rejection via enhancing IL-17+ gammadelta T-cell response. Transpl Int. 2014;27:399–407. doi: 10.1111/tri.12264. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Huang G, Hu B, et al. Anti-HMGB1 neutralizing antibody ameliorates neutrophilic airway inflammation by suppressing dendritic cell-mediated Th17 polarization. Mediators Inflamm. 2014;2014:257930. doi: 10.1155/2014/257930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Huang G, Hu B, et al. Recombinant HMGB1 A box protein inhibits Th17 responses in mice with neutrophilic asthma by suppressing dendritic cell-mediated Th17 polarization. Int Immunopharmacol. 2015;24:110–118. doi: 10.1016/j.intimp.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 29.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 30.Chen YL, Jian Y, Liu MJ, et al. Role of the Th17/Treg functional imbalance on the development of atherosclerosis in apo E knockout mice. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41:416–421. [PubMed] [Google Scholar]
- 31.Yao H, Lv J. Statin attenuated myocardial inflammation induced by PM2.5 in rats. Acta Cardiol Sin. 2017;33:637–645. doi: 10.6515/ACS20170518A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang F, Huang H, Zhang M, et al. HMGB1 induces apoptosis and EMT in association with increased autophagy following H/R injury in cardiomyocytes. Int J Mol Med. 2016;37:679–689. doi: 10.3892/ijmm.2016.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 34.Burchill MA, Yang J, Vogtenhuber C, et al. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]