Abstract
Aims
The aim of this study was to analyze whether local application of 3% hydrogen peroxide (H2O2) additionally to standard antibiotic prophylaxis following implantation of cardiac implantable electronic devices (CIED) reduces the incidence of pocket infections (PI).
Methods
In this observational case-control study every patient from the group additionally treated with H2O2 was matched with two patients out of the control group for age, male-gender, body-mass-index and operation time. The incidence of PI within 365 days after device implantation was compared.
Results
During the 5-year study period, 429 consecutive patients were additionally treated with H2O2 and matched with 858 patients undergoing standard treatment (mean age 69 ± 12 years, 876 males (67.4%), body-mass-index 28 ± 4.0 kg/m2 and operation time 45 ± 23 min). Except for a more frequent use of dual-platelet-inhibition in the H2O2-group, clinical characteristics were otherwise similar. A total of 23 (1.78%) PIs occurred, most of them (14/23; 61%) during the first 45 days after implantation procedure. The use of H2O2 was associated with a significant reduction (3/429 = 0.69% versus 20/858 = 2.33%; p = 0.04), although patients of the H2O2 treated group received more complex procedures increasing the risk of PI.
Conclusion
Intraoperative local application of 3% H2O2 seems to be associated with a significant reduced incidence of PI following implantation of CIED. Because of its non-randomized character this trial should be considered as a hypothesis generating study.
Keywords: Pocket infection, Hydrogen peroxide, Prevention, Pacemaker, Defibrillator, Implantation
1. Introduction
Since the routine use of antibiotic prophylaxis has been introduced, a significant decline in the number of infections complicating implantations of a cardiovascular implantable electronic devices (CIED) has been observed [1]. However, this complication is still present and pocket infection (PI) is the most common one [2]. A worldwide exponential growth of CIEDs implantation and replacements requires further consideration, as the incidence of infections associated with such procedures ranges between 1.6 and 3.5% [3]. Therefore, an increase of the total numbers of infectious complications is expected.
PI is defined by the inflammation of the subcutaneous tissue containing the device and/or the subcutaneous segment of the leads. In contrast, endovascular infections are spreading to the intravenous parts of the leads with or without involvement of the device pocket [4]. PI is most likely related to contamination during the implantation procedure whereas lead infections are often due to other causes and different pathogenic sources [4].
The aim of this study was to compare retrospectively the infection rate of a standard antibiotic prophylaxis strategy versus the use of intra-pocket 3% hydrogen peroxide (H2O2) in addition to the standard of care in a large cohort of consecutive patients.
2. Methods
2.1. Definition of pocket infection
To confirm the diagnosis PI, a purulent discharge at the pocket site, either spontaneous or expressed upon palpation must be present, regardless of whether an organism is cultured by smear test from the site or not [5]. The following cases are also regarded as an infection of the device: erythema, tenderness, induration at the pocket site with or without a serous or serosanguinous discharge [5]. In the present study, PI related to the implantation was diagnosed after the occurrence of any of the above within 365 days after the implantation procedure [6].
2.2. Data collection
In this case-control study, all consecutive patients receiving a CIED (cardiac pacemakers, implantable cardioverter defibrillator [ICD], cardiac resynchronization therapy [CRT], cardiac contraction modulation [CCM], or implantable loop-recorder), an upgrade (≥1 lead has been placed additionally), or an exchange of the CIED between January 2010 and December 2014 at our institution were included and retrospectively analyzed. The device implantation was performed by eight different physicians (see Table 3). While six physicians treated patients commonly with standard of care, two others irrigate the device pocket with 20 ml of 3% H2O2 before wound closure. In the present paper, we exclusively examined the effect of 3% H2O2 on the reduction of PI. The cases of isolated lead endocarditis without an infection of the device pocket were excluded because they show a different pathogenesis.
Table 3.
Data given as n (%). The table represents all cases of each implanting physician. regardless whether H2O2 was used or not. There was no significant difference in the incidence of PI between each physician.
| Incidence of pocket infection related to the implanting physician | ||
|---|---|---|
| Cases with Pocket Infection (n = 23) | Cases without Pocket Infection (n = 1.287) | |
| Physician 1 | 5 (1.5%) | 324 |
| Physician 2 | 0 (0.0%) | 91 |
| Physician 3 | 7 (2.3%) | 305 |
| Physician 4 | 6 (2.0%) | 297 |
| Physician 5 | 0 (0.0%) | 34 |
| Physician 6 | 0 (0.0%) | 31 |
| Physician 7 | 5 (2.9%) | 175 |
| Physician 8 | 0 (0.0%) | 30 |
2.3. Subjects
For each patient treated with 20 ml of 3% H2O2 additionally to the standard of care, two control subjects matching in age, sex, body-mass-index and operation time were used for comparison. To characterize the two groups, the following characteristics were assessed: (1) Patient related characteristics like concomitant diseases (e.g. coronary heart disease, arterial hypertension, renal insufficiency, and diabetes mellitus). Prior coronary interventions and coronary artery bypass grafts were documented as well.
Furthermore, the systolic function was evaluated during the hospital stay by echocardiography.
(2) Procedure related characteristics were recorded: e.g. type of implanted CIED, operation duration, need for prior temporary pacing, type of venous access, device placement (sub-fascial vs. sub-muscular), and the type of the wound closure (intracutaneous suture vs. dermal glue). We also determined whether the procedure was the patient's first implantation of a CIED, an aggregate exchange, a device upgrade or a revision caused by lead dysfunction or pocket hematoma. (3) We also recorded current medication influencing the patients' hemostasis because antiplatelet-therapy or oral anticoagulation in cardiac patients is often needed and bleeding complications are known to be associated with infectious complications [7]. Finally, laboratory values [international-normalized-ratio (INR), number of platelets, leucocytes and C-reactive protein (CRP)] were assessed as well.
2.4. Operation techniques and institutional standard of care
Each implanting physician had an experience of at least 200 procedures. Intravenous application of prophylactic antibiotics (2-g cefazolin) was given half an hour before the procedure. Patients being allergic to cefazolin were excluded from the study to avoid different effects of the antibiotic treatment on the result. After local anesthesia (50 ml lidocaine 1%), a pectoral incision under the clavicle was made, and a sub-fascial or sub-muscular pocket was created. Venous access was achieved by cephalic preparation or puncture of the subclavian vein (one puncture per lead) and the leads were implanted under fluoroscopic guidance. An active fixation mechanism was chosen for all atrial leads and for ventricular leads in case of severe tricuspid regurgitation, pulmonary hypertension, or cardiac defibrillator implantation. All left ventricular leads were inserted via the coronary sinus.
Hemostasis was appropriately obtained by electro-cautery, non-treated cotton pledges and insertion of drainage or hemostatic agents at the discretion of the implanting physician. In patients treated with additional H2O2, the pocket was irrigated with 20 ml of a 3% solution.
The patients being part of the control group did not receive any kind of irrigation. Finally, the device pocket was closed by subcutaneous stitches. Then, either an intracutaneous suture or the application of dermal glue (cyanoacrylate-based medical adhesive) was performed. After the procedure, bed rest was suggested while a pressure dressing was applied for 24 h. Whenever the insertion of wound drainage had been performed, the system was removed 24 h later.
2.5. Follow–up
After 24 h of bed rest and pressure dressing, pneumo-thorax was excluded by chest-x-ray if new leads had been added, and the programming of the implanted CIED was checked for the first time after implantation. The patients underwent a daily examination of the device pocket until hospital discharge and thereafter check-ups in larger intervals (every three to six months) in the outpatient clinic of the division. In addition, patients were encouraged to return to our clinic in case they felt uncomfortable in any way. The duration of the follow up was 365 days after the implantation.
2.6. Statistical analysis
Continuous variables are reported as mean value ± standard deviation or median and interquartile ranges (25th–75th percentiles). Categorical variables are presented as absolute (n) and relative (%) frequencies. Normal distribution of variables was assessed using the D'Agostino-Pearson omnibus normality test. The T-test, Mann-Whitney-test, Fisher's exact test were used, as appropriate. All tests were two-tailed, and a probability value of p ≤ 0.05 was considered statistically significant. Clinical outcome (pocket infection) is presented using the Kaplan-Meier graph. Statistical analysis was performed using the GraphPad Prism version 6.02 or Windows (GraphPad Software, La Jolla, California, USA).
3. Results
Throughout the study period, a total of 1287 consecutive patients received a CIED. Following the matching characteristics, patients were mean 69 ± 12 years old, 876 (67.4%) of them were males and the body-mass-index was mean 28 ± 4.0 kg/m2.
Longer implantation duration has not been identified as a predictive factor for a device infection yet [6]. Because it is generally accepted that longer procedure duration is associated with an increased risk for infection, we also matched patients depending on duration of the procedure (average 45 ± 23 min).
429 patients underwent treatment with 20 ml of 3% H2O2 in addition to standard antibiotic treatment. In the H2O2-group a dual-platelet-inhibition was found more frequent in current medication and more complex procedures [implantation of CRT-pacemakers (12.1% vs 6.6%, p < 0.01), CRT-defibrillators (32.2% vs 20.2%, p < 0.01) and device-upgrades (19.6% vs 10.5%; p < 0.01)] were recorded as well (Table 1, Table 2).
Table 1.
Data given as mean ± SD or n (%).* Significant value. BMI (body mass index). CABG (coronary artery bypass grafting). CRP (C-reactive protein). INR (international normalized ratio). PCI (percutaneous coronary intervention).
| Clinical Baseline Characteristics | |||
|---|---|---|---|
| H2O2-group (n = 429) | Control-group (n = 858) | p-Value | |
| Age [years] | 69 ± 12 | 69 ± 12 | case-control matching characteristics |
| Sex [male] | 289 (67.4%) | 578 (67.4%) | |
| BMI [kg/m2] | 28 ± 4.0 | 28 ± 4.0 | |
| Procedure Time [min] | 45 ± 23 | 45 ± 23 | |
| Medical History | |||
| Dilative Cardiomyopathy | 100 (23.3%) | 182 (21.2%) | 0.39 |
| Coronary Heart Disease | 233 (54.3%) | 448 (52.2%) | 0.51 |
| Prior CABG | 96 (22.4%) | 180 (21.0%) | 0.72 |
| Prior PCI | 176 (41.0%) | 339 (33.5%) | 0.63 |
| Hypertension | 357 (83.2%) | 736 (85.7%) | 0.25 |
| Diabetes | 172 (40.1%) | 324 (37.8%) | 0.43 |
| Chronic Kidney Disease | 126 (29.4%) | 271 (31.6%) | 0.44 |
| Echocardiographic Findings | |||
| Ejection Fraction [%] | 40 ± 15 | 43 ± 16 | 0.90 |
| Current Medication | |||
| Single-Platelet-Inhibition | 117 (27.3%) | 274 (31.9%) | 0.09 |
| Dual-Platelet-Inhibition | 108 (25.2%) | 170 (19.8%) | 0.03* |
| Anticoagulation | 166 (38.7%) | 300 (34.9%) | 0.20 |
| Laboratory Values | |||
| CRP [mg/l] | 12.9 ± 24.1 | 12.4 ± 22.4 | 0.98 |
| Leucocytes [×10³/μl] | 8.0 ± 2.5 | 7.9 ± 2.9 | 0.98 |
| Platelets [×10³/μl] | 216 ± 79 | 216 ± 100 | 1.00 |
| INR | 1.3 ± 0.49 | 1.3 ± 0.44 | 1.00 |
Table 2.
Data given as number or mean ± SD (%).* Significant value. CRT (cardiac resynchronization therapy).
| Procedural Characteristics | |||
|---|---|---|---|
| H2O2-group (n = 429) | Control-group (n = 858) | P-value | |
| Heart Rhythm Device | |||
| Pacemaker [PM] | 197 (45.9%) | 409 (47.7%) | 0.59 |
| Single Chamber PM | 23 (5.4%) | 60 (7.0%) | 0.28 |
| Dual Chamber PM | 122 (28.4%) | 292 (34.0%) | 0.04* |
| CRT-PM | 52 (12.1%) | 57 (6.6%) | <0.01* |
| Implantable-Cardioverter-Defibrillator [ICD] | 230 (53.6%) | 397 (46.3%) | 0.01* |
| Single Chamber ICD | 61 (14.2%) | 167 (19.5%) | 0.02* |
| Dual Chamber ICD | 31 (7.2%) | 57 (6.6%) | 0.72 |
| CRT-D | 138 (32.2%) | 173 (20.2%) | <0.01* |
| Implantable-Loop-Recorder | 2 (0.5%) | 43 (5%) | <0.01* |
| Cardiac Contractility Modulation | 0% | 9 (1.0%) | – |
| Temporary Pacing | |||
| Prior temporary pacing | 53 (12.7%) | 83 (9.7%) | 0.15 |
| Duration [days] | 2.9 ± 1.8 | 3.3 ± 3.0 | 0.92 |
| Implantation Procedure | |||
| 1st Procedure | 252 (58.7%) | 538 (62.7%) | 0.18 |
| Follow-Up Intervention | 177 (41.3%) | 320 (37.3%) | 0.18 |
| Generator exchange | 29 (6.8%) | 104 (12.1%) | <0.01* |
| Device upgrade | 84 (19.6%) | 90 (10.5%) | <0.01* |
| Device explantation | 17 (4.0%) | 35 (4.1%) | 1.0 |
| Complications needing revision | 47 (11.0%) | 91 (10.6) | 0.84 |
| Lead dysfunction | 35 (8.2%) | 78 (9.1%) | 0.60 |
| Pocket Hematoma | 6 (1.4%) | 16 (1.9%) | 0.65 |
| Device Placement | |||
| Site of operation [left] | 367 (85.5%) | 740 (86.2%) | 0.73 |
| Sub-cutaneous/sub-fascial | 392 (91.4%) | 757 (88.2%) | 0.13 |
| Sub-muscular | 36 (8.4%) | 97 (11.3%) | 0.12 |
| Venous Access | |||
| Puncture (subclavian/axillary vein) | 378 (88.1%) | 667 (77.7%) | <0.01* |
| Cephalic cut-down | 5 (1.2%) | 8 (0.9%) | 0.77 |
| Wound Closure | |||
| Intracutaneous suture | 21 (4.9%) | 189 (22.0%) | <0.01* |
| Dermal Glue | 408 (95.1%) | 669 (77.9%) | <0.01* |
| Bleeding Management | |||
| Drainage | 50 (11.7%) | 117 (13.6%) | 0.33 |
| D-stat Flowable Hemostat™ | 39 (9.1%) | 30 (3.5%) | <0.01* |
3.1. Primary endpoint
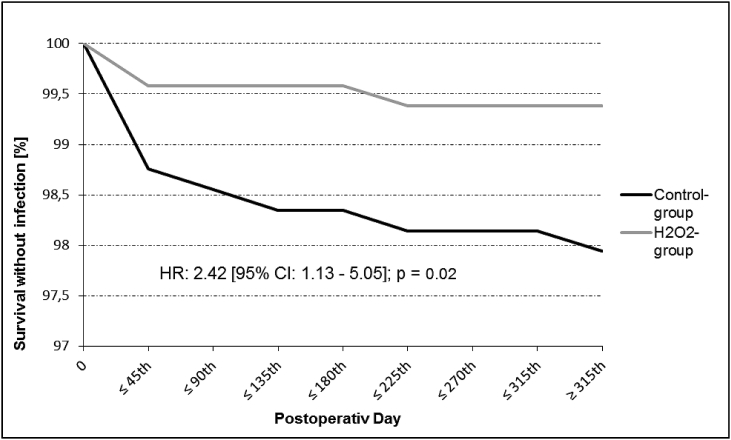
Patients undergoing treatment with 3% H2O2 developed significantly less pocket infections than the standard group (3 [0.69%] vs 20 patients [2.33%]; p = 0.04), resulting in a relative risk reduction of 70%. Pocket infection-free survival by Kaplan-Meier estimate is shown in Fig. 2.
Fig. 2.
Pocket infection free survival in “H2O2-group” (grey lined) and “Control-group” (black lined). Hazard Ratio (HR). Confidence interval (CI).
The most causative bacteria identified for PI was staphylococcus epidermidis (n = 10; 47.6%) followed by staphylococcus aureus (n = 8; 38.1%). Other detected bacteria were enterobacter cloacae, actinomyces viscosus and gemella morbillorum. They accounted for one case each (4.8%). In two cases (9.5%) no predominant bacteria could be found at all, but PI was confirmed by a purulent discharge from the pocket site (Table 4). There were no differences regarding the spectrum of bacteria between the H2O2-group (two cases of staphylococcus epidermidis and one case of staphylococcus aureus) and the control group.
Table 4.
All cases of pocket infections are shown. In two cases no bacteria could be identified but pocket infection seems to be very likely in the presence of purulent discharge. CRP (C-reactive protein. Staph. (Staphylococcus).
| Pocket Infection Related Characteristics | ||||||
|---|---|---|---|---|---|---|
| N | Bacteria | Fever [≥38 °C] | CRP [mg/l] | Leucocytes [x103/μL] | Lead affected | Signs of infection |
| 1 | Staph. epidermidis | – | 24.0 | 6.4 | – | purulent discharge. tenderness |
| 2 | Staph. aureus | Yes | 129.0 | 7.9 | – | erythema |
| 3 | Enterobacter cloacae | – | 12.7 | 9.2 | Yes | – |
| 4 | Staph. epidermidis | – | 37.7 | 4.5 | – | tenderness |
| 5 | Staph. aureus | – | 44.0 | 7.6 | – | erythema |
| 6 | Staph. epidermidis | – | 32.5 | 21.9 | – | purulent discharge |
| 7 | Staph. epidermidis | Yes | 17.8 | 14.5 | Yes | erythema |
| 8 | Staph. epidermidis | 1.4 | 3.9 | – | erythema. | |
| 9 | Staph. epidermidis | Yes | 237.0 | 18.3 | – | tenderness |
| 10 | – | – | 99.3 | 9.4 | – | purulent discharge. tenderness. erythema |
| 11 | Actinomyces viscosus | – | 4.3 | 8.7 | – | tenderness |
| 12 | Staph. aureus | Yes | 316.0 | 3.3 | – | purulent discharge. erythema. tenderness |
| 13 | Staph. aureus | – | 13.3 | 7.9 | – | purulent discharge. erythema. tenderness |
| 14 | Staph. epidermidis | – | 15.9 | 8.2 | – | purulent discharge. erythema. tenderness |
| 15 | Staph. aureus | Yes | 129.0 | 7.9 | – | serous discharge. erythema |
| 16 | Staph. aureus | Yes | 158.5 | 11.5 | Yes | purulent discharge. erythema |
| 17 | Staph. aureus | – | 3.8 | 7.4 | – | purulent discharge. erythema |
| 18 | – | – | 1.6 | 6.4 | – | purulent discharge |
| 19 | Staph. epidermidis | Yes | 22.7 | 10.6 | – | purulent discharge. erythema |
| 20 | Gemella morbillorum | – | 119.3 | 7.0 | – | tenderness |
| 21 | Staph. epidermidis | – | 22.9 | 8.8 | – | purulent discharge |
| 22 | Staph. epidermidis | – | 5.2 | 8.1 | – | purulent discharge |
| 23 | Staph. aureus | Yes | 366.0 | 5.7 | – | purulent discharge |
4. Discussion
Our large single-center case-control-study demonstrates a significantly reduced incidence of PI (2.33% vs. 0.69%) after using 3% H2O2 locally in addition to the standard treatment in patients undergoing a permanent CIED implantation.
This result seems to be relevant, as several variables known to increase the likelihood of PI were more frequent in the H2O2-group. Such variables included dual-platelet-inhibition [3], implantation of CRT-D/PM devices [3] and application of local hemostats [8]. The implantation of devices for cardiac-contraction modulation (CCM) has to be considered as a complex procedure as well. There were nine cases of a CCM-implantation in the control group, but none in the H2O2-group. There were no cases of PI in patients receiving a CCM-device.
One procedure related characteristic needs to be further discussed: In the H2O2-group were only a few cases where the device pocket was closed by intracutaneous sutures (23/429; 4.9%). This type of wound closure was more frequent in control-cases (189/858; 22.0%) resulting in a significant difference (p < 0.001). There are many advantages in cyanoacrylate-based medical adhesive use e.g. shorter duration of the procedure, immediate wound sealing and elimination of needle-stick injuries resulting also in a lower infection rate compared to intra-cutaneous suturing [9]. In the present study intra-cutaneous suturing was performed in 210 cases, nevertheless H2O2 was use or not. In 6 of 210 cases PI (2.9%) occurred. The incidence of PI in cases when dermal glue was applied is 1.6% (17/1060). There was no significant difference regarding the type of wound closure related to the occurrence of PI (p = 0.25). Although there is no statistical impact on an elevated incidence of PI depending on type of wound closure in the present study, an effect cannot be excluded.
4.1. Mechanisms of action of H2O2
H2O2 is a weak acid built of hydrogen and oxygen. In contact with surfaces of metal, organic tissues and even spontaneously this fluid releases water and hydrogen [10]. H2O2 works as antimicrobial agent by producing hydroxyl free radicals (*OH) being able to destroy cell components like deoxyribonucleic acid, lipids and proteins [11], and although its antiseptic effect is commonly accepted, some controversies exist regarding the effect of H2O2 in vivo [12].
Its use is attractive, because H2O2 is more effective against gram-positive than gram-negative bacteria [11]. More than 90% of the PIs are caused by gram positive agents like coagulase-negative Staphylococcus aureus, Staphylococcus aureus and Streptococci [12]. The presence of peroxidases and catalases in body tissues and in some bacteria are responsible for a reduced efficacy especially at dilutions below 3% [11]. This might explain why we found three cases of PI caused by staphylococcus aureus and staphylococcus epidermidis in H2O2 treated patients.
Regarding the safety of hydrogen peroxide, two points merit further discussion: wound healing and gas embolism. First, one might consider that H2O2 inhibits wound healing by its hydroxyl free radicals, but there are animal's as well as human's studies concluding that there are no negative effects of hydrogen peroxide on wound healing if concentrations of three percent or less are used [12]. Even positive effects on wound healing after H2O2 application (improved blood flow and re-epithelization) are described [11].
Several cases of gas embolism following H2O2 applications have been published [[13], [14], [15], [16]]. These case reports critically discuss the benefit of H2O2 balanced against this rare but severe complication. In all cited cases, volumes of more than 250 ml were used and flushed with pressure in large wounds or highly vascularized regions (e.g. large thigh trauma, cavity of a spinal cord abscess, and fracture of the femoral shaft with introduction of osteosynthesis material). One milliliter of H2O2 releases 10 ml of oxygen which is a large amount of gas that can potentially diffuse into the vascular system [15]. A relationship between the occurrence of gas embolism and the volume of used H2O2 is likely, explaining the absence of this potentially dangerous complication in our study and suggesting that irrigation with small volumes (20 ml) of 3% H2O2 is safe.
4.2. Incidence and time course of PI
The implanted CIED influence cellular adhesion and integration as well as inflammatory and immunological processes, depending on the physicochemical characteristics of the material they are built of [17]. There is also a relationship between time of infection onset and causative micro-organism. Staphylococcus aureus is often found in infected device pockets which become symptomatic a few days after implantation whereas staphylococcus epidermidis is more frequently found in PI cases with a delayed onset or chronical inflammation processes [17,18]. Furthermore, certain bacteria like pseudomonas aeruginosa, escherichia coli and klebsiella pneumoniae building up a bio-film layer surrounding the implanted device [5,7,19]. There is evidence for an altered immune response and markedly reduced efficacy of systemically applied antibiotics against bacterial colonization facilitated by these bacteria protecting layer around the device [5,17]. On the other hand, biofilms themselves may inhibit bacterial growth so that there can be a kind of steady state when the device is contaminated, but the infection does not become clinically apparent [20]. It remains unclear which circumstances make a contaminated device pocket symptomatic.
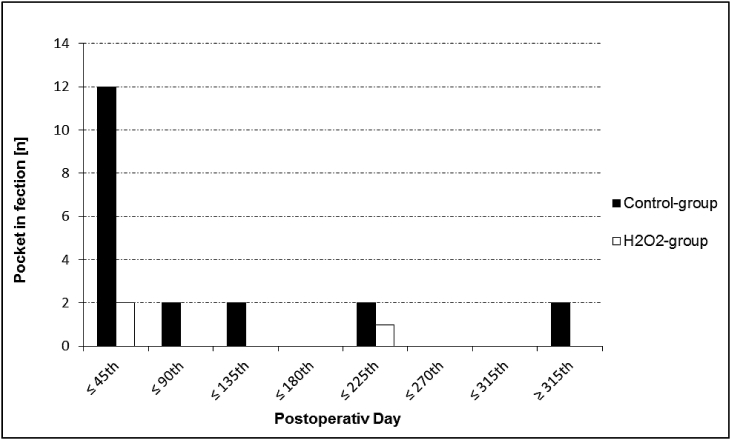
These facts may explain the significantly reduced incidence of PI especially within the first 45 days after implantation in the present study (see Fig 1). Interestingly, H2O2 may even inhibit bio-film formation on CIED which is, beside to its proven bactericidal characteristics, another possible mechanism to reduce bacterial infection [19].
Fig. 1.
The number of pocket infections is presented in a time dependent manner. Black columns indicate infections of the control group. White columns indicate infections of the cases group.
4.3. Study limitations
This study is subject to limitations inherent to all non-randomized studies. In addition, no clear evidence exists that the type of wound closure (intracutaneous suture vs dermal glue) affects the incidence of PI, such variables were more prevalent in the “standard” group and might have affected the results.
5. Conclusion
The local application of 20 ml 3% H2O2 before wound closure in patients undergoing implantation of CIED was associated with significant reduction in the incidence of postoperative PI. Furthermore, instillation of 3% H2O2 on top of standard antibiotic prophylaxis is safe, easy to realize and adds little costs, making it a possible additional therapeutic option to reduce the incidence of pocket infection following CIED implantation. However, because of the observational character of this study the results should be considered as a hypothesis generating and randomized trials should prospectively evaluate the role of H2O2 in patients undergoing CIED implantation.
Acknowledgement
None of the authors has to declare any conflict of interest.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Da Costa A., Kirkorian G., Cucherat M. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998;97:1796–1801. doi: 10.1161/01.cir.97.18.1796. [DOI] [PubMed] [Google Scholar]
- 2.Sohail M.R., Uslan D.Z., Khan A.H. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007;45:166–173. doi: 10.1086/518889. [DOI] [PubMed] [Google Scholar]
- 3.Mittal S., Shaw R.E., Michel K. Cardiac implantable electronic device infections: incidence, risk factors, and the effect of AigisRx antibacterial envelope. Heart Rhythm. 2014;4:595–601. doi: 10.1016/j.hrthm.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Karchmer A.W., Longworth D.L. Infections of intracardiac devices. Infect Dis Clin. 2002;16:477–505. doi: 10.1016/s0891-5520(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 5.Baddour L.M., Bettmann M.A., Bolger A.F. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 6.Klug D., Balde M., Pavin D., PEOPLE Study Group Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 7.Zilberman M., Elsner J.J. Antibiotic-eluting medical devices for various applications. J Contr Release. 2008;130:202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 8.MA Ohlow, Lauer B., Buchter B. Pocket related complications in 163 patients receiving anticoagulation or dual antiplatelet therapy: D-Stat Hemostat™ versus standard of care. Int J Cardiol. 2012;159:177–180. doi: 10.1016/j.ijcard.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mubarak L., Al-Haddab M. Cutaneous wound closure materials: an overview and update. J Cutan Aesthetic Surg. 2013;6:178–188. doi: 10.4103/0974-2077.123395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouerghi S., Abdelhafidh K., Merghli A. Iatrogenic gas embolism after use of hydrogen peroxide in the treatment of lung hydatid cyst: a report of 2 cases. Tunis Med. 2010;88:851–854. [PubMed] [Google Scholar]
- 11.McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drosou A., Falabella A., Kirsner R.S. Antiseptics on wound: an area of controversy. Wounds. 2003;15:149–166. [Google Scholar]
- 13.Chua J.D., Wilkof B.L., Lee I. Diagnosis and management of infections involving implantable electrophyciologic cardiac devices. Ann Intern Med. 2000;133:604–648. doi: 10.7326/0003-4819-133-8-200010170-00011. [DOI] [PubMed] [Google Scholar]
- 14.Patankar P.S., Joshi S.S., Choudhari K.A. Air-embolism and cerebral ischaemia following epidural hydrogen peroxide irrigation in a closed lumbar cavity. Br J Neurosurg. 2014;28:556–558. doi: 10.3109/02688697.2013.865706. [DOI] [PubMed] [Google Scholar]
- 15.Benali Zel A., Abdedaim H., Omari D. Massive gas embolism secondary in the use of intraoperative hydrogen peroxide: still use to lavage with this liquid? Pan Afr Med J. 2013;16:124. doi: 10.11604/pamj.2013.16.124.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beattie C., Harry L.E., Hamilton S.A. Cardiac arrest following hydrogen peroxide irrigation of a breast wound. J Plast Reconstr Aesthetic Surg. 2010;63:253–254. doi: 10.1016/j.bjps.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Schierholz J.M., Beuth J. Implant infections: a haven for opportunistic bacteria. J Hosp Infect. 2001;49:87–93. doi: 10.1053/jhin.2001.1052. [DOI] [PubMed] [Google Scholar]
- 18.Pichlmaier M., Marwitz V., Kühn C. High prevalence of asymptomatic bacterial colonization of rhythm management devices. Europace. 2008;10:1067–1072. doi: 10.1093/europace/eun191. [DOI] [PubMed] [Google Scholar]
- 19.Olmedo G.M., Grillo-Puertas M., Cerioni L. Removal of pathogenic bacterial biofilms by combinations of oxidizing compounds. Can J Microbiol. 2015;12:1–6. doi: 10.1139/cjm-2014-0747. [DOI] [PubMed] [Google Scholar]
- 20.Cooper M., Batchelor S.M., Prosser J.I. Is cell density signalling applicable to biofilms? In: Wimpenny J., Handley P., Gilbert P., Lappin-Scott H., editors. Cardiff. Bioline Press; 1995. pp. 93–97. [Google Scholar]