Abstract
Purpose
Surgical resection for gastric adenocarcinoma is associated with significant post-operative morbidity and mortality. The aim of this study was to assess the prognostic significance of sarcopenia in patients undergoing resection for gastric adenocarcinoma with respect to post-operative morbidity and survival.
Materials and Methods
A retrospective analysis was conducted on a cohort of consecutive patients who underwent surgical resection for gastric adenocarcinoma between 2008 and 2014. Patient demographics, radiological parameters, and pathological data were collected. OsiriX software (Pixmeo) was used to measure skeletal muscle area, which was normalized for height to calculate skeletal muscle index.
Results
A total of 56 patients (41 male, 15 female; mean age, 68.4 ± 11.9 years) met the inclusion criteria. Of these, 36% (20 of 56) of the patients were sarcopenic pre-operatively. Both sarcopenic and non-sarcopenic patient groups were equally matched with the exception of weight and body mass index (P=0.036 and 0.001, respectively). Sarcopenia was associated with a decreased overall survival (log-rank P=0.003) and was an adverse prognostic predictor of overall survival in multivariate analysis (hazard ratio, 10.915; P=0.001). Sarcopenia was a predictor of serious in-hospital complications in multivariate analysis (odds ratio, 3.508; P=0.042).
Conclusions
In patients undergoing curative resection for gastric cancer, there was a statistically significant association between sarcopenia and both decreased overall survival and serious post-operative complications. The measurement and reporting of skeletal muscle index on pre-operative computed tomography should be considered.
Keywords: Sarcopenia; Stomach neoplasms; Prognosis; Morbidity; Tomography, X-ray computed
INTRODUCTION
Gastric cancer is the fifth most common malignancy worldwide and comprises 6.8% of all cancers diagnosed. It is the third leading cause of cancer-related deaths worldwide [1]. There is a wide geographical variation in the incidence of cases, with countries in Eastern Asia, such as South Korea and Japan, having age-standardized incidence rates of 41.8 and 29.9 per 100,000, respectively [1], while in the United States, an approximate incidence of 7.3 per 100,000 is observed [2].
Gastric cancer has a poor 5-year overall survival (OS) rate of 30.6% [2], with surgical resection being the only curative intervention [3]. Post-operative complications have been reported to be as high as 39% in patients undergoing surgery with curative intent [4]. A recent study on patients undergoing potentially curative total gastrectomy for gastric adenocarcinoma indicated that 28% of patients required invasive post-operative intervention, based on the grading of the interventions performed (Clavien-Dindo classification) [5].
A number of recent studies have demonstrated an adverse association between sarcopenia and immediate and long-term patient outcomes following surgery, including post-operative complications, length of hospital stay, recurrence-free survival (RFS), and OS [6,7,8]. Sarcopenia is defined by the European Working Group on Sarcopenia in Older People as a low muscle mass and either decreased muscle strength or low physical performance [9]. The deleterious effect of sarcopenia on patient outcomes has been shown to be significant in a number of different cancers such as colorectal and liver cancers.
The skeletal muscle index is used to diagnose sarcopenia and this index may be calculated using computed tomography (CT). CT constitutes an important aspect of patient assessment prior to surgical triage and all such patients will undergo pre-operative staging CT. The calculation of skeletal muscle index on CT prior to surgery may provide addition useful information pertaining to the patient's condition and could help guide patient preparation and counselling. The aim of this study was to investigate the association between sarcopenia in patients undergoing surgery for gastric cancer and their post-operative outcomes in terms of morbidity, RFS, and OS.
MATERIALS AND METHODS
The local Institutional Review Board granted ethical approval (approval number ECM 4 (cc) 04/04/16). All consecutive patients who underwent surgery with curative intent for gastric adenocarcinoma between January 1, 2008 and December 31, 2014 in a single tertiary referral center by one surgeon were included in this study. Patients were identified through surgical logbooks and cross-verified using the histopathology database confirming the diagnosis of gastric adenocarcinoma according to the American Joint Committee on Cancer (AJCC) staging system [10]. Pathological data including tumor location and stage were collected from this database. Patient medical records were analyzed to obtain pre-operative clinical demographics, including age, sex, date of surgery, use of neoadjuvant therapy, and the identification of patients' comorbidities. The Charlson comorbidity index (CCI) was calculated from patient pre-operative information [11].
Peri-operative reports were used to obtain patients' height, weight, and the American Society of Anesthesiologists physical status (ASA-PS) [12]. Post-operative information was obtained from patient medical records to include the occurrence of complications, which were graded according to the Clavien-Dindo classification system [13]. The total number of intensive care unit (ICU) and hospital bed days were obtained from the hospital inpatient medical system. Follow-up information regarding tumor recurrence and mortality were obtained from medical and radiological records. OS was defined as the length of time from the date of first therapy to the date of death or loss to follow-up. RFS was defined as the length of time from the date of first therapy to the date of detection of tumor recurrence, death, or loss to follow-up.
Diagnostic work-up and imaging
All patients were discussed at a multi-disciplinary team meeting. As part of the routine pre-operative diagnostic procedure, all patients underwent an esophagogastroduodenoscopy and a staging CT scan of the thorax, abdomen, and pelvis [14]. In all, 80.4% (45 of 56) of the patients had an endoscopic ultrasound for loco-regional staging and 73.2% (41 of 56) of the patients had a staging laparoscopy with peritoneal washings. Survival analysis was performed on data from the pre-operative CT or from before commencement of chemotherapy where applicable. Morbidity analysis was performed on data from the staging CT performed before surgery irrespective of whether chemotherapy was administered as this reflected the patient's skeletal muscle index entering surgery, which could be affected by the administration of chemotherapy. CT scans were electronically stored on the hospital imaging system. Pre-operative CT scans for the identified study patients were anonymized by a third-party imaging technician.
Image analysis
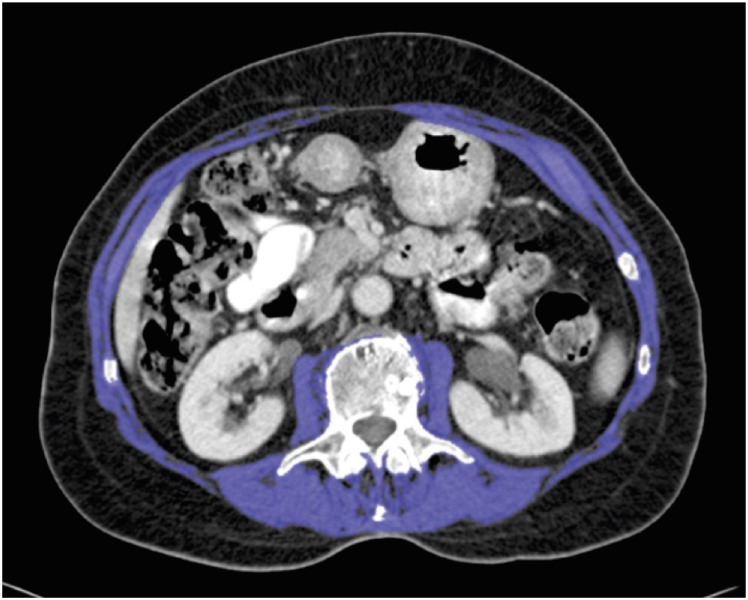
CT scans were analyzed using the OsiriX version 5.6.1 open source software (32-bit, Pixmeo, Geneva, Switzerland; http://www.osirix-viewer.com). The cross-sectional skeletal muscle area (cm2) was measured using a standardized approach [8,15]. Two sequential scans at the level of the third lumbar vertebra, in which both transverse processes were visible, were used. The grow/regrow tool was used to measure the skeletal muscle area on these axial slices by a reviewer blinded to the patients' demographics. The threshold range for skeletal muscle was −30 to +150 Hounsfield units [16,17]. The skeletal muscles included were the psoas, paraspinal, and abdominal wall muscles. The average muscle area of these 2 slices was used. The skeletal muscle area was normalized for height to calculate the skeletal muscle index [18]. The specific cut-off values used for sarcopenia were 52.4 cm2/m2 for men and 38.5 cm2/m2 for women, as published by Prado et al. [17]. An example of an analyzed CT scan is shown in Fig. 1.
Fig. 1. Axial CT scan of a 77-year-old sarcopenic male with a skeletal muscle index of 41.58 cm2/m2. The total skeletal muscle area (indicated in purple) was measured on a CT slice at the level of L3.
CT = computed tomography.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) v20.0 software (SPSS Inc., Chicago, IL, USA). The independent t-test and Mann-Whitney U test were used to compare continuous variables including age, number of positive lymph nodes (LNs), number of LNs resected, patient height, weight, and body mass index (BMI). The χ2 test and Fisher's exact test were used to compare categorical variables such as sex, tumor site, TNM stage, use of neoadjuvant therapy, ASA-PS grade, and CCI score. The impact of sarcopenia on morbidity was analyzed by univariate and multivariate regression analyses. Morbidity was assessed according to serious in-hospital complications (Clavien-Dindo ≥3a), the number of ICU bed days, and the total length of hospital stay. OS and RFS were evaluated by Kaplan-Meier survival analysis and the log-rank test. The association of sarcopenia with OS was analyzed using univariate and multivariate Cox regression analyses. Graphs were constructed on GraphPad Prism v6.07 (GraphPad Software Inc., La Jolla, CA, USA). A P-value of <0.05 was used as the level of significance for the study.
RESULTS
A total of 56 patients (41 male, 15 female; mean age, 68.4 ± 11.9 years) were included in the study. Follow-up was completed for all included patients. There was a mean of 28 days (range, 0–74 days) from the date of CT scan to the date of surgery. The majority of the patients (87.5%, 49 of 56) had the CT scan within 6 weeks of the surgery. In all, 20 patients (35.7%) were sarcopenic pre-operatively, which included three of 29 patients who received neoadjuvant chemotherapy and subsequently became sarcopenic. Patient demographics, clinical indices, and pathological data are summarized in Table 1.
Table 1. Clinical indices and pathological data of patients stratified by the presence of pre-operative sarcopenia.
| Characteristics | All Patients (n=56) | Sarcopenic (n=20) | Non-sarcopenic (n=36) | P-value | |
|---|---|---|---|---|---|
| Age (yr) | 68.4±11.9 | 67.4±12.7 | 70.3±10.2 | 0.385* | |
| Sex | 0.393† | ||||
| Male | 41 | 16 | 25 | ||
| Female | 15 | 4 | 11 | ||
| Height (cm) | 168.4±8.8 | 169±7.5 | 167±9.5 | 0.356* | |
| Weight (kg) | 73.8±14.8 | 68.3±11.6 | 76.9±15.7 | 0.036* | |
| BMI (kg/m2) | 25.9±4.3 | 23±2.9 | 27.2±4.4 | 0.001* | |
| ASA-PS | 0.803† | ||||
| Grade 1 | 5 | 2 | 3 | ||
| Grade 2 | 21 | 8 | 13 | ||
| Grade 3 | 29 | 10 | 19 | ||
| Grade 4 | 1 | 0 | 1 | ||
| CCI | 0.144† | ||||
| 0 | 5 | 0 | 5 | ||
| 1 | 2 | 1 | 1 | ||
| 2 | 17 | 7 | 10 | ||
| 3 | 11 | 7 | 4 | ||
| 4 | 11 | 2 | 9 | ||
| 5 | 6 | 2 | 4 | ||
| 6 | 3 | 1 | 2 | ||
| 7 | 1 | 0 | 1 | ||
| Neoadjuvant therapy | 0.610† | ||||
| Yes | 28 | 10 | 18 | ||
| No | 28 | 10 | 18 | ||
| Type of surgery | 0.087† | ||||
| Total gastrectomy | 34 | 16 | 18 | ||
| Distal gastrectomy | 12 | 2 | 10 | ||
| Proximal gastrectomy | 10 | 2 | 8 | ||
| Tumor site | 0.416† | ||||
| Cardia | 16 | 5 | 11 | ||
| Fundus | 4 | 2 | 2 | ||
| Body | 15 | 8 | 7 | ||
| Antrum | 14 | 3 | 11 | ||
| Pylorus | 7 | 2 | 5 | ||
| Stage | 0.282† | ||||
| 0 | 7 | 3 | 4 | ||
| 1A | 11 | 3 | 8 | ||
| 1B | 7 | 2 | 5 | ||
| 2A | 6 | 1 | 5 | ||
| 2B | 7 | 2 | 5 | ||
| 3A | 3 | 0 | 3 | ||
| 3B | 9 | 6 | 3 | ||
| 3C | 6 | 3 | 3 | ||
| Number of LNs resected (range) | 28.7 (4–75) | 28.3 (13–79) | 29 (4–58) | 0.308‡ | |
| Number of positive LNs (range) | 2.2 (0–23) | 2.05 (0–10) | 2.33 (0–23) | 0.827‡ | |
Continuous data presented as mean±1 standard deviation unless otherwise stated. Statistically significant values in bold.
BMI = body mass index; ASA-PS = American Society of Anesthesiologists physical status; CCI = Charlson comorbidity index; Stage = overall TNM staging as per American Joint Committee on Cancer 7th edition; LN = lymph node.
*Independent t-test, †χ2 test ‡Mann-Whitney U test.
Stratification of patients based on the presence or absence of sarcopenia demonstrated that only patient weight and BMI were statistically different between the groups (P=0.036 and 0.001, respectively). There was no statistical difference in the patients' comorbidities, assessed by the ASA-PS and CCI scores (P=0.803 and 0.144, respectively). No statistically significant difference was observed in the tumor profile between the patient groups. This was assessed by tumor site, stage, and the number of LNs resected. In all, 50% (28 of 56) of patients received neoadjuvant chemotherapy and 64.3% (18 of 28) of these patients went on to have adjuvant chemotherapy. The seven patients with stage 0 gastric cancer were treated surgically and did not receive neoadjuvant chemotherapy. A mean of 28.7 LNs were resected in the whole patient cohort. In total, 52 patients (92.9%) of the whole patient cohort had a resection of ≥15 LNs. Of the remainder, three patients had stage 1A disease.
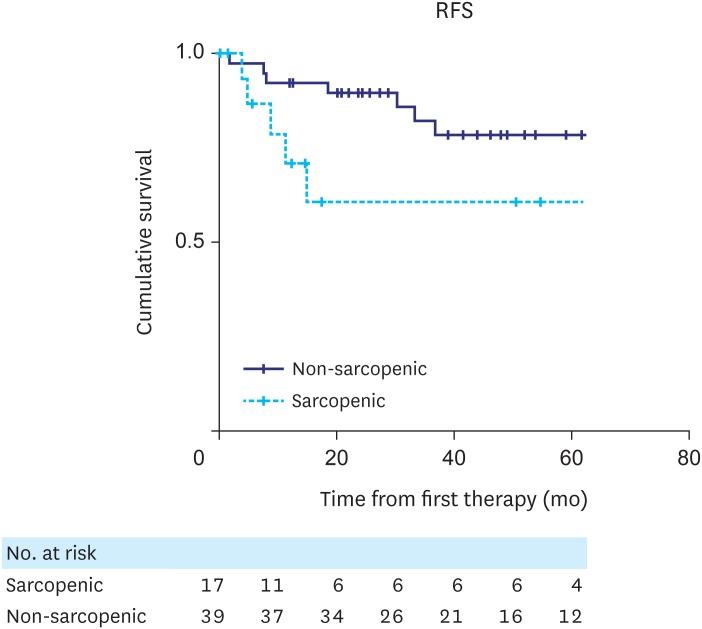
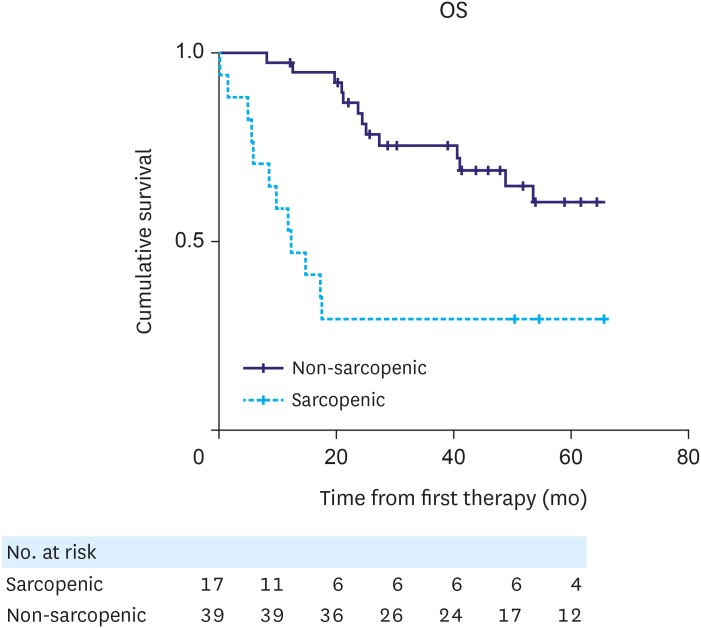
The median follow-up time for the whole patient cohort was 39.88 months (interquartile range, 17.43–61.2 months). RFS and OS curves for patients with and without sarcopenia are shown in Figs. 2 and 3, respectively. No statistically significant difference was observed in RFS between patient groups (P=0.084, log-rank test). However, there was a statistically significant difference in OS between the 2 groups (P=0.003, log-rank test), with sarcopenic patients having reduced survival.
Fig. 2. Kaplan-Meier analysis for RFS indicated that there was no statistically significant difference in RFS between sarcopenic and non-sarcopenic patients (P=0.084, log-rank test).
RFS = recurrence-free survival.
Fig. 3. Kaplan-Meier analysis for OS indicated a statistically significant difference in OS between non-sarcopenic and sarcopenic patients (P=0.003, log-rank test).
OS = overall survival.
Overall, 20 patients experienced severe post-operative complications (Clavien-Dindo >3A). The sarcopenic group had a higher rate of severe complications than the non-sarcopenic group (11 patients vs. 9 patients, respectively). Radiologically guided pleural drainage or chest tube insertion was required by 8 patients: 3 from the sarcopenic group and 5 from the non-sarcopenic group. Surgical intervention under general anesthetic was required by 5 patients for wound debridement (n=1), perforation (n=1), tracheostomy insertion (n=1), and small bowel obstruction (n=2): 3 from the sarcopenic group and 2 from the non-sarcopenic group. ICU admission was required by 7 patients: 5 from the sarcopenic group and 2 from the non-sarcopenic group.
A summary of univariate and multivariate cox regression analyses for OS is presented in Table 2. In the univariate regression analysis, sarcopenia, BMI, and AJCC tumor stages 2A–2B and 3A–3C were statistically significant prognosticators of OS. Due to the small study group, only the variables that were statistically significant in the univariate analysis were included in the multivariate regression analysis. Only sarcopenia and AJCC tumor stages 2A–2B and 3A–3C were statistically significant predictors of poor OS in the multivariate analysis (hazard ratio [HR], 10.915; P=0.001; HR, 50.177, P≤0.001; and HR, 56.377; P≤0.001, respectively).
Table 2. Univariate and multivariate Cox regression analysis of clinicopathological factors and OS.
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Age | 1.030 | 0.991–1.070 | 0.135 | ||||
| Sex | 0.605 | 0.227–1.613 | 0.315 | ||||
| Height | 1.013 | 0.972–1.056 | 0.542 | ||||
| Weight | 0.981 | 0.955–1.007 | 0.144 | ||||
| BMI | 0.896 | 0.809–0.993 | 0.037 | 0.894 | 0.791–1.011 | 0.074 | |
| Sarcopenia | 3.885 | 1.754–8.607 | 0.001 | 10.915 | 3.195–37.288 | 0.001 | |
| ASA-PS group ≥2 | 0.680 | 0.310–1.494 | 0.337 | ||||
| CCI ≥3 | 1.416 | 0.625–3.209 | 0.404 | ||||
| Neoadjuvant therapy | 1.255 | 0.569–2.768 | 0.573 | ||||
| Type of surgery | |||||||
| Total gastrectomy | 1 | ||||||
| Distal gastrectomy | 1.330 | 0.467–3.791 | 0.594 | ||||
| Proximal gastrectomy | 1.596 | 0.609–4.182 | 0.341 | ||||
| Tumor site | |||||||
| Cardia | 1 | ||||||
| Fundus | 1.225 | 0.259–5.786 | 0.798 | ||||
| Body | 1.370 | 0.514–3.687 | 0.525 | ||||
| Antrum | 0.683 | 0.223–2.093 | 0.505 | ||||
| Pylorus | 0.737 | 0.156–3.481 | 0.700 | ||||
| Stage | |||||||
| 0–1B | 1 | 1 | |||||
| 2A–2B | 10.749 | 2.320–49.804 | 0.002 | 50.177 | 7.682–327.752 | <0.001 | |
| 3A–3C | 19.610 | 4.366–88.079 | <0.001 | 56.377 | 9.175–346.407 | <0.001 | |
| Number of LNs resected | 1.012 | 0.979–1.047 | 0.485 | ||||
Statistically significant values in bold.
OS = overall survival; HR = hazard ratio; CI = confidence interval; BMI = body mass index; ASA-PS = American Society of Anesthesiologists physical status; CCI = Charlson comorbidity index; Stage = overall TNM staging as per American Joint Committee on Cancer 7th edition.
The association between sarcopenia and post-operative morbidity is shown in Table 3. There was a statistically significant difference between the 2 groups with respect to serious post-operative complications (Clavien-Dindo ≥3a) (P=0.025), in-hospital mortality (P=0.041), and number of ICU bed days (P=0.007). There was no statistically significant difference in the total length of hospital stay between the 2 groups (P=0.373). Univariate and multivariate regression analyses were used to identify factors associated with hospital complications (Table 4). In the univariate analysis, being male and having sarcopenia were found to be statistically significant factors associated with in-hospital complications (odds ratio [OR], 5.087; P=0.048 and OR, 3.667; P=0.028). However, in the multivariate analysis, only sarcopenia was found to be a significant prognosticator of serious in-hospital complications (OR, 3.508; P=0.042).
Table 3. Analysis of hospital outcomes by pre-operative sarcopenia.
| Outcomes | All patients (n=56) | Sarcopenic (n=20) | Non-sarcopenic (n=36) | P-value |
|---|---|---|---|---|
| Serious complications (%) | 20 (35.7) | 11 (55.0) | 9 (25.0) | 0.025* |
| Length of hospital stay | 21.46 (7–71) | 25.1 (7–57) | 19.44 (8–71) | 0.373† |
| Total number of ICU/HDU bed days | 6.68 (0–62) | 9.45 (0–43) | 5.08 (0–62) | 0.007† |
| In-hospital mortality (%) | 3 (5.4) | 3 (15.0) | 0 (0) | 0.041‡ |
Results of length of hospital stay and number of ICU bed days are presented as mean number of days (range). Serious complications (Clavien-Dindo ≥3a) and in-hospital mortality are recorded as number (%).
ICU = intensive care unit; HDU = high-dependency unit.
*χ2 test, †Mann-Whitney U test, ‡Fisher's exact test.
Table 4. Univariate and multivariate regression analyses for risk factors of serious complications (Clavien-Dindo ≥3A).
| Characteristics | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | ||
| Age | 1.008 | 0.962–1.057 | 0.743 | ||||
| Sex (male) | 5.087 | 1.015–25.485 | 0.048 | 4.836 | 0.922–25.361 | 0.062 | |
| Height | 1.053 | 0.985–1.125 | 0.129 | ||||
| Weight | 1.010 | 0.973–1.048 | 0.591 | ||||
| BMI | 0.979 | 0.859–1.116 | 0.752 | ||||
| Sarcopenic | 3.667 | 1.150–11.694 | 0.028 | 3.508 | 1.048–11.739 | 0.042 | |
| ASA-PS ≥3 | 0.584 | 0.194–1.760 | 0.340 | ||||
| CCI ≥3 | 0.636 | 0.211–1.918 | 0.422 | ||||
| Neoadjuvant therapy | 0.431 | 0.139–1.333 | 0.144 | ||||
| Type of surgery | |||||||
| Total gastrectomy | 1 | ||||||
| Distal gastrectomy | 0.000 | 0.00–Infinity | 0.999 | ||||
| Proximal gastrectomy | 2.143 | 0.509–9.024 | 0.299 | ||||
| Location of tumor | |||||||
| Cardia | 1 | ||||||
| Fundus | 3.857 | 0.326–45.570 | 0.284 | ||||
| Body | 1.125 | 0.273–4.635 | 0.870 | ||||
| Antrum | 0.214 | 0.036–1.288 | 0.092 | ||||
| Pylorus | 0.214 | 0.021–2.216 | 0.196 | ||||
| Stage | |||||||
| 0–1B | 1 | ||||||
| 2A–2B | 2.204 | 0.545–8.910 | 0.267 | ||||
| 3A–3C | 1.636 | 0.451–5.936 | 0.454 | ||||
| Number of LNs resected | 0.967 | 0.918–1.019 | 0.211 | ||||
Statistically significant values in bold.
OR = odds ratio; CI = confidence interval; BMI = body mass index; ASA-PS = American Society of Anesthesiologists physical status; CCI = Charlson comorbidity index; Stage = overall TNM staging as per American Joint Committee on Cancer 7th edition; LN = lymph node.
DISCUSSION
The role of sarcopenia in the management of patients with cancer is an evolving area of research. Numerous studies encompassing different tumor biologies have demonstrated that a low skeletal muscle index has an adverse effect on the outcomes of oncology patients. Sarcopenia has been associated with increased length of hospital stay, serious in-hospital complications, increased inpatient mortality, and lower disease-free survival and OS [6,8,19,20].
The present study investigated the effect of sarcopenia on patients undergoing potentially curative surgery for gastric adenocarcinoma with respect to post-operative morbidity, RFS, and OS. The study groups were very well-matched and the surgical approach was standardized, given that only 1 surgeon was involved. Sarcopenia was adversely associated with serious post-operative hospital complications (Clavien-Dindo ≥3a) and decreased OS. Interestingly, there was no statistically significant difference observed in the length of hospital stay between the patient groups, which was expected due to the association of sarcopenia with major in-hospital complications and increased length of stay in the ICU. This may be due in part to the small study group. However, the differences observed in the incidence of major complications and the number of ICU bed days indicate that sarcopenic patients are more resource intensive than non-sarcopenic patients.
Sarcopenic thresholds reported by Prado et al. [17] are frequently referenced and used for patient stratification; these are derived from an obese cohort of cancer patients with a number of different tumor etiologies. Despite the potential for bias in these cut-off values, significant associations with many disease outcomes have been found. This may be due to the general population becoming increasingly obese and the study's restriction to obese patients may, in fact, be representative of modern society [21]. Hence, it was deemed appropriate to use these threshold values for the purposes of the present study. Almost 59% (33 of 56) of patients in the present study were overweight or obese and although there was a statistical difference in BMI between the 2 patient groups, BMI was not associated with a worse outcome in terms of morbidity or survival in the multivariate analysis. A similar finding was reported in a study of patients with hepatocellular carcinoma [19]. Cachexia associated with cancer is characterized by loss of a muscle mass, which may not be accompanied by a loss of fat mass and may be poorly reflected by changes in BMI [22]. With the rising prevalence of obesity in the community, sarcopenia may be a more suitable prognostic factor than BMI. The use of CT to identify patients who have sarcopenia despite having a normal BMI has not been widely exploited. The reporting of skeletal muscle index represents a potential added value that radiologists could provide to guide referring physicians.
Some studies have failed to demonstrate these relationships; a recent study that investigated the effect of sarcopenia on patients with gastric cancer undergoing surgery did not show an association between sarcopenia and short-term post-operative morbidity and mortality [23]. There are fundamental differences in the profile of patients' in that study: 57.7% of patients in that study were sarcopenic compared with only 35.7% in the present study, and 30.6% of patients in that study underwent palliative surgery, with 37.1% having stage 4 disease, whereas the present study group was restricted to those undergoing curative surgery. These differences may explain the different study results. Another recent paper reported that sarcopenia is associated with decreased OS in esophago-gastric cancer patients [24]. The incidence of sarcopenia in that study was 49.4% and a larger variety of cancers were studied (esophageal, esophago-gastric junction, and gastric cancers) in comparison to the present, more focused study.
The incorporation of skeletal muscle index calculation into pre-operative risk stratification and prognostic models may require tumor-specific cut-off values for a low skeletal muscle index. Tumor pathophysiology and prognosis can vary widely, as can the physiologic challenge of surgery. The concept of tumor-specific values has been investigated in several studies. An adverse association has been demonstrated between sarcopenia and RFS and OS in patients with colorectal cancer [25]. Similarly, an association between sarcopenia and OS in patients with pancreatic cancer has also been shown [26]. Both of these studies used the lowest sex-specific quartile to define a low skeletal muscle index.
The identification of patients who are sarcopenic at the time of diagnosis may aid the selection of patients for early nutritional and physical intervention. This might be particularly suitable for patients with gastric cancer who require neoadjuvant chemotherapy. In a study which analyzed the change in body composition in patients after neoadjuvant chemotherapy for esophago-gastric cancer, there was a statistically significant increase in the number of patients with sarcopenia post-chemotherapy [27]. This was reflected in the present study with previously non-sarcopenic patients becoming sarcopenic post-chemotherapy.
The present study included consecutive patients who underwent curative surgery for gastric cancer at a single tertiary referral center. The study was retrospective and although comparable with similar papers studying other cancers, the patient group was relatively small. A multicenter prospective study with a larger patient population may be necessary to better elucidate the association between sarcopenia and post-operative outcomes in specific subgroups of patients undergoing curative resection for gastric cancer.
The relationship between the systemic inflammatory response and sarcopenia and its influence on this study's findings were not included due to lack of relevant data (C-reactive protein, etc.). Results from the recent C-SCANS study indicated that pre-diagnosis systemic inflammation and at-diagnosis sarcopenia were associated with an increased mortality risk in patients with non-metastatic colorectal cancer [28]. Further study of the influence of the systemic inflammatory response on skeletal muscle in gastric cancer may help guide interventions to prevent sarcopenia and potentially improve survival outcomes.
In conclusion, the present study demonstrated that, in patients undergoing curative resection for gastric cancer, there was a statistically significant association between sarcopenia and both decreased OS and serious post-operative complications. The measurement and reporting of skeletal muscle index from pre-operative CT should be considered for patient preparation purposes.
ACKNOWLEDGMENTS
Fiona O'Callaghan and Patrick Foley from Central Statistics Office, Cork, Ireland provided statistical support.
Footnotes
- Conceptualization: P.D., M.M.M., O'C.O.J., Ó'S.C.
- Data curation: O'B.S., T.M., M.F., K.R.G., C.B.W.
- Formal analysis: M.M.M., O'C.O.J.
- Investigation: P.D., M.M.M.
- Methodology: O'C.O.J., Ó'S.C.
- Project administration: M.M.M., O'C.O.J
- Supervision: P.D., M.M.M., O'C.O.J., Ó'S.C.
- Validation: M.M.M., O'C.O.J.
- Visualization: T.M., M.F., K.R.G.
- Writing - original draft: O'B.S., K.R.G., C.B.W.
- Writing - review & editing: K.R.G., C.B.W.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ferlay JS, Soerjomataram I, Ervik M, Forman D, Bray F, Dikshit R, et al. GLOBOCAN 2012: cancer incidence, and mortality and prevalence worldwide in 2012 [Internet] Lyon: International Agency for Research on Cancer; 2012. [cited 2014 Nov 14]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.National Cancer Institute. Surveillance, epidemiology, and end results program. Cancer stat facts: stomach cancer [Internet] Bethesda (MD): National Cancer Institute; [cited 2017 Nov 10]. Available from: https://seer.cancer.gov/statfacts/html/stomach.html. [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Bösing NM, Goretzki PE, Röher HD. Gastric cancer: which patients benefit from systematic lymphadenectomy? Eur J Surg Oncol. 2000;26:498–505. doi: 10.1053/ejso.1999.0930. [DOI] [PubMed] [Google Scholar]
- 5.Selby LV, Vertosick EA, Sjoberg DD, Schattner MA, Janjigian YY, Brennan MF, et al. Morbidity after total gastrectomy: analysis of 238 patients. J Am Coll Surg. 2015;220:863–871.e2. doi: 10.1016/j.jamcollsurg.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 8.Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217–222. doi: 10.1093/bja/77.2.217. [DOI] [PubMed] [Google Scholar]
- 13.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 14.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi57–vi63. doi: 10.1093/annonc/mdt344. [DOI] [PubMed] [Google Scholar]
- 15.Dello SA, Lodewick TM, van Dam RM, Reisinger KW, van den Broek MA, von Meyenfeldt MF, et al. Sarcopenia negatively affects preoperative total functional liver volume in patients undergoing liver resection. HPB. 2013;15:165–169. doi: 10.1111/j.1477-2574.2012.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 17.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 19.Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short-and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015;261:1173–1183. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 20.Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the Consensus-Definition for Cancer Cachexia and evaluation of a classification model--a study based on data from an international multicentre project (EPCRC-CSA) Ann Oncol. 2014;25:1635–1642. doi: 10.1093/annonc/mdu086. [DOI] [PubMed] [Google Scholar]
- 22.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Tegels JJ, van Vugt JL, Reisinger KW, Hulsewé KW, Hoofwijk AG, Derikx JP, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112:403–407. doi: 10.1002/jso.24015. [DOI] [PubMed] [Google Scholar]
- 24.Tan BH, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41:333–338. doi: 10.1016/j.ejso.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22:2663–2668. doi: 10.1245/s10434-014-4281-6. [DOI] [PubMed] [Google Scholar]
- 26.Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awad S, Tan BH, Cui H, Bhalla A, Fearon KC, Parsons SL, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31:74–77. doi: 10.1016/j.clnu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3:e172319. doi: 10.1001/jamaoncol.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]