Abstract
Purpose
This study aimed to examine the outcomes of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis (PM) of advanced gastric cancer (AGC).
Materials and Methods
Between May 2015 and June 2017, 38 CRS and HIPEC procedures were performed in patients with PM of AGC at the Dankook University Hospital. We prospectively collected and analyzed data regarding PM grade, morbidity and mortality rates, and short-term follow-up results (median, 13.5 months).
Results
The mean peritoneal cancer index was 15 (range, 0–39). Complete cytoreduction was achieved in 21 patients (55.2%), whereas complications occurred in 16 (42.1%) and 2 (5.7%) patients died. The overall median patient survival time was 19 months. The patients who underwent complete cytoreduction had a median survival time of 26 months, which was significantly longer than the median survival time of 16 months in the patients who did not undergo complete cytoreduction (P=0.006).
Conclusions
CRS with HIPEC may have a beneficial effect in patients with PM of AGC. However, the rates of complications and mortality associated with this combined therapeutic approach are high. Therefore, this treatment should be performed only in selected patients by surgeons experienced in the field of gastric cancer with PM.
Keywords: Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy, Gastric cancer
INTRODUCTION
Peritoneal metastasis (PM) arising from advanced gastric cancer (AGC) is the most common pattern of recurrence and is generally associated with poor prognosis [1]. Several studies have assessed treatment methods (systemic chemotherapy, surgery, and intraperitoneal chemotherapy) that are effective for treating this fatal disease. However, there is no consensus regarding direct treatment for patients with PM. To date, palliative systemic chemotherapy is the only treatment method that increases the survival rate of patients with PM, but the median survival time is still shorter than 10 months [2,3]. Therefore, a new therapeutic modality is required for patients with PM of AGC.
Complete cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is widely performed for treating isolated PM of colorectal cancer and for rare primary peritoneal malignancies, malignant peritoneal mesotheliomas, and peritoneal pseudomyxoma peritonei [4,5,6]. The survival benefits of the combined treatment for these diseases led surgeons worldwide to propose this therapeutic strategy for PM of AGC [7,8]. However, only few institutions continue to perform these procedures because of technical difficulties and safety issues.
At our institution, CRS and intraperitoneal chemotherapy have been performed since the early 2000 in patients with colorectal cancer, and we reported the short-term outcomes of these treatments in 2016 [9]. We continued to improve the techniques in CRS and HIPEC. Thereafter, we expanded the indication and adopted the techniques in other malignancies, such as gastric cancer. The present study aimed to assess the outcomes of patients who underwent surgery for PM of AGC at a single institution in Korea. Here, we describe our experience and the short-term follow-up results of CRS with HIPEC.
MATERIALS AND METHODS
Between May 2015 and June 2017, CRS with HIPEC was performed 38 times in patients with PM of AGC at the Dankook University Hospital. All the patients had PM confirmed on computed tomography (CT) or laparoscopic examination. Patients who had distant metastasis, aged >70 years, and who had poor general health conditions (Eastern Cooperative Oncology Group performance status >2) were excluded from the study.
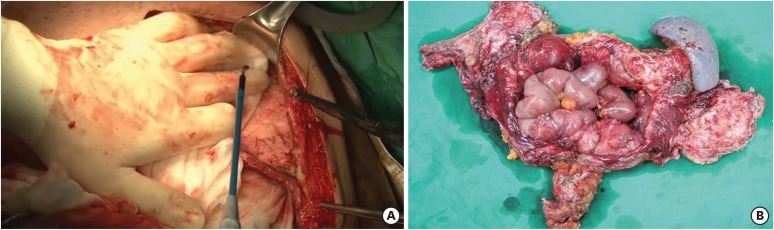
CRS was performed in accordance with the techniques described by Sugarbaker [10] and Yonemura et al. [11]. All grossly visible seeding nodules were removed via peritonectomy using ball-type electrocautery (Fig. 1A). The visceral organs were also resected in accordance with the extent of invasion (Fig. 1B). The extent of PM was routinely measured using the peritoneal cancer index (PCI), which ranges from 0 to 39. Three PCI grades (0–10, 11–20, and 21–39) were used for the analysis. After cytoreduction, the completeness of the cytoreduction score (from CCR-0 to CCR-3) was recorded in accordance with the Sugarbaker classification [12]. CCR-0 and CCR-1 were defined as complete cytoreduction. Total or distal gastrectomy with D2 lymph node dissection was conducted in accordance with the Japanese gastric cancer treatment guidelines [13].
Fig. 1. Operative findings in cytoreductive surgery. (A) Parietal peritonectomy and (B) specimen of the resected organ.
HIPEC and early postoperative intraperitoneal chemotherapy (EPIC) were performed in accordance with the aforementioned standard procedure followed at our institution [9]. HIPEC was performed using the closed method with a Belmont hyperthermic pump (Belmont Instrument Corp., Billerica, MA, USA). Mitomycin (30 mg) and cisplatin (90 mg) were used as chemotherapeutic agents. The perfusion duration was 90 minutes, and the inflow temperature was 42°C. EPIC was delivered from days 1 to 5 after surgery. The chemotherapeutic agent used for EPIC was mitomycin on day 1 and 5-fluorouracil for the following 4 days.
All procedures were performed by two experienced surgeons, a colorectal surgeon who had performed >350 procedures of CRS with HIPEC since 2012 and a gastric surgeon who had handled >1,000 gastrectomy cases. The institutional review board of the Dankook University Hospital approved the protocol (Institutional Review Board approval No. 201807027), and written informed consent was obtained from all the patients. This study was performed in accordance with the Declaration of Helsinki.
Categorical data were assessed using the χ2 test or Fisher exact test. In addition, the t-test or Mann-Whitney U test was used to assess continuous variables. Survival outcomes were analyzed using the Kaplan-Meier method and log-rank test. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using the SPSS version 22.0 software package for Windows (IBM Corp., Chicago, IL, USA).
RESULTS
In this study, 38 patients (12 male and 26 female; mean age, 45.8±15.5 years; mean body mass index, 22.3±3.1 kg/m2) were included. The baseline characteristics of the participants are summarized in Table 1. Among the 38 patients, 5 were initially diagnosed at our hospital and 33 were transferred from other hospitals for a second opinion. PM was synchronously and metachronously identified in 24 (63.2%) and 14 patients (36.8%), respectively. Most patients (81.6%) received palliative chemotherapy during the preoperative period.
Table 1. Demographic data and baseline characteristics of the patients (n=38).
| Characteristics | Values | |
|---|---|---|
| Age (yr) | 45.8±15.5 | |
| Sex | ||
| Male | 12 (31.6%) | |
| Female | 26 (68.4%) | |
| BMI (kg/m2) | 22.3±3.1 | |
| ASA score | ||
| 1 | 19 (50.0%) | |
| 2 | 15 (39.5%) | |
| ≥3 | 4 (10.5%) | |
| Number of comorbidities | ||
| 0 | 20 (52.6%) | |
| 1 | 11 (28.9%) | |
| 2 | 5 (13.1%) | |
| ≥3 | 2 (5.4%) | |
| Location of the primary tumor | ||
| Cardia | 3 (7.8%) | |
| Upper | 8 (21.0%) | |
| Middle | 11 (28.9%) | |
| Lower | 16 (42.3%) | |
| Disease presentation | ||
| Synchronous | 24 (63.2%) | |
| Metachronous | 14 (36.8%) | |
| Tumor size (mm) | 52.4±31.3 | |
| Preoperative chemotherapy | ||
| Yes | 31 (81.6%) | |
| No | 7 (18.4%) | |
BMI = body mass index; ASA = American Society of Anesthesiologists.
Table 2 presents the surgical results of CRS with HIPEC. The mean operation time was 530.7±98 minutes. Other organs (colon, gallbladder, and other such organs) were simultaneously resected. The mean PCI was 15 (grade I, 42%; grade II, 29%; and grade III, 29%), and complete cytoreduction was performed in 21 patients (55.2%). Two, 23, and 13 patients underwent EPIC, HIPEC, and both procedures, respectively.
Table 2. Surgical results (n=38).
| Variables | Values | |
|---|---|---|
| Operation time (min) | 530.7±98.0 | |
| Hospital stay (day) | 25.8±9.4 | |
| Estimated blood loss (mL) | 623.2±333.3 | |
| Combined resection | ||
| Colon | 10 | |
| Gallbladder | 18 | |
| Small bowel | 12 | |
| Spleen | 13 | |
| Pancreas | 8 | |
| Uterus or ovary | 14 | |
| Appendix | 22 | |
| PCI | ||
| Grade I (1–10) | 16 (42.0%) | |
| Grade II (11–20) | 11 (29.0%) | |
| Grade III (21–39) | 11 (29.0%) | |
| CCR score | ||
| 0 | 17 (44.7%) | |
| 1 | 4 (10.5%) | |
| 2 | 5 (13.2%) | |
| 3 | 12 (31.6%) | |
| Type of intraperitoneal chemotherapy | ||
| EPIC | 2 (5.7%) | |
| HIPEC | 23 (60.5%) | |
| EPIC+HIPEC | 13 (33.8%) | |
| Cell type | ||
| Well or moderately differentiated | 6 (15.8%) | |
| Poorly differentiated | 12 (31.6%) | |
| Signet ring cell | 14 (36.8%) | |
| Unknown | 6 (15.8%) | |
PCI = peritoneal cancer index; CCR = completeness of cytoreduction; EPIC = early postoperative intraperitoneal chemotherapy; HIPEC = hyperthermic intraperitoneal chemotherapy.
The surgical morbidity rate was 42.1%. Intra-abdominal and wound complications occurred in 36.8% and 21.1% of the patients, respectively. Most medical complications were respiratory problems. Two patients (5.7%) postoperatively died from multiorgan failure and hypovolemic shock (Table 3). Four patients who were diagnosed with PM on CT scan had no evidence of PM in laparotomy; therefore, these patients were excluded from the statistical analysis (Table 4).
Table 3. Post-operative morbidity and mortality (n=38).
| Variables | Values | |
|---|---|---|
| Number of patients with postoperative morbidity* | 16 (42.1%) | |
| Intra-abdominal complications | 14 (36.8%) | |
| Fluid collection/abscess | 5 | |
| Intraluminal bleeding | 1 | |
| Intra-abdominal bleeding | 2 | |
| Anastomosis site leakage | 4 | |
| Intestinal obstruction/ileus | 5 | |
| Wound complication | 8 (21.1%) | |
| Dehiscence | 6 | |
| Evisceration | 2 | |
| Medical complication | 11 (28.9%) | |
| Respiratory | 10 | |
| Cardiovascular | 3 | |
| Renal | 7 | |
| Urinary | 2 | |
| Hepatic | 2 | |
*Number of case with postoperative mortality was 2 (5.7%); multiorgan failure (anastomosis site leakage) and hypovolemic shock (intra-abdominal bleeding).
Table 4. Patients not diagnosed as having peritoneal metastasis using laparotomy (initially diagnosed as having synchronous peritoneal metastasis on CT).
| Patient No. | Preoperative chemotherapy | Laparoscopic examination | Final pathology |
|---|---|---|---|
| 1 | Capecitabine/oxaliplatin 5 | None | T3 N2 M0 |
| 2 | None | None | T3 N0 M0 |
| 3 | Capecitabine/oxaliplatin 6 | None | T2 N3a M0 |
| 4 | None | None | T1a N0 M0 |
CT = computed tomography.
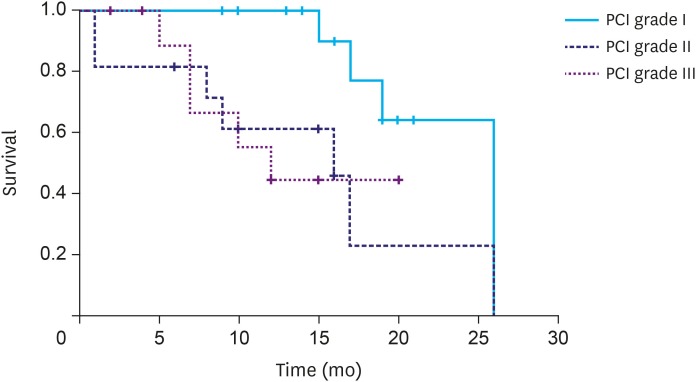
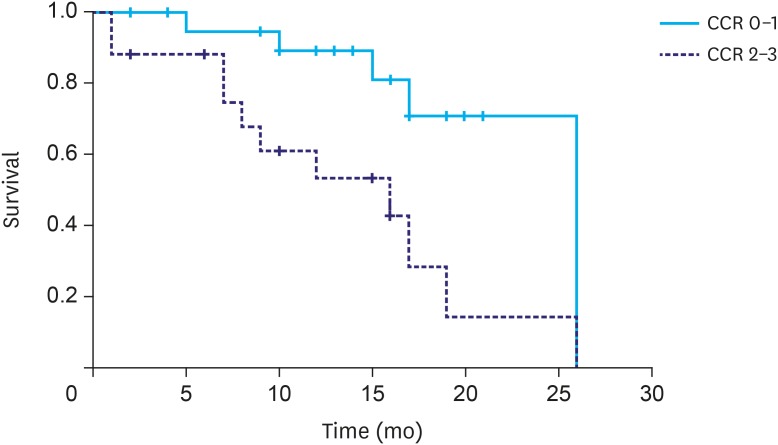
The overall median survival time was 19 months. There was no statistical difference in the survival rate according to disease presentation (synchronous, 19 months; metachronous, 17 months; P=0.897). However, a significant difference was observed in the survival rate according to the PCI score (grade I, 26 months; grade II, 16 months; and grade III, 12 months; P=0.041; Fig. 2). The patients who underwent a complete cytoreduction had a median survival time of 26 months, which was significantly longer than the median survival time of 16 months in the patients who did not undergo complete cytoreduction (P=0.006; Fig. 3).
Fig. 2. Median survival time according to the peritoneal cancer index (PCI; grade I, 26 months; grade II, 16 months; and grade III, 12 months; P=0.041).
PCI = peritoneal cancer index.
Fig. 3. Median survival time according to the CCR (CCR 0/1, 26 months and CCR 2/3, 16 months; P=0.006).
CCR = completeness of cytoreduction.
DISCUSSION
Gastric cancer is the second most common cancer in Korea, with approximately 30,000 newly diagnosed cases every year. Recently, owing to the increased use of mass screening programs and advanced diagnostic instruments, the incidence of early gastric cancer has been increasing as compared with that of AGC [14,15]. Despite this trend, the number of patients with stage IV gastric cancer still remains the same. In patients with distant metastasis, palliative systemic chemotherapy is the gold standard treatment [2]. However, there is no consensus regarding the treatment for patients with PM. Recently, the treatment of patients with PM has become an important issue in the field of gastric cancer.
Theoretically, CRS with HIPEC can be a good treatment option for patients with PM of AGC. PM has been considered a locoregional disease in recent years [7], and radical resection of visible tumors might postpone death or possibly cure some patients. Furthermore, compared with systemic chemotherapy, HIPEC is beneficial owing to its antitumor effects with minimal systemic toxicity [16]. Many institutions, however, do not perform this procedure, and our center remains to be one of the few institutions to conduct this procedure in Korea. This may be due to several reasons. First, the procedure itself is extremely complicated. Thus, the rates of associated complications and mortality can be high. In this study, the surgical morbidity and mortality rates were 42.1% and 2.7%, respectively. To obtain satisfactory results, all visible seeding nodules should be removed, and concomitant resection of other organs is inevitable. For example, the pelvic cavity is often a primary site of PM. However, pelvic peritonectomy is challenging owing to the complicated anatomical structure of the pelvis. The rectum, uterus, and sometimes the bladder should be resected if necessary. Therefore, the surgeons should have a considerable level of surgical spectrum and must be experienced enough to perform this operation. Numerous reports have discussed the importance of surgical experience in decreasing morbidity and mortality rates [17,18,19,20]. Second, a longer surgical duration is required. In addition to the long surgical duration of CRS itself, HIPEC (90 minutes), hemostasis, and anastomosis also take time (average time taken, 530 minutes). It is not feasible for most hospitals to afford the time-consuming and low-cost nature of this procedure. Third, the evidence of its effectiveness is still unclear. Moreover, the number of well-designed phase III studies on PM from gastric cancer is limited.
Despite its low feasibility, we have been conducting CRS with HIPEC in patients with PM of AGC since 2015. At the Dankook University Hospital, CRS has been performed in patients with colorectal cancer since the early 2000s. Thus, we have >10 years of experience and have organized a team approach system that is comprised of >5 specialists and professional nurses. Consequently, we can conclude that the patients in this study who underwent complete cytoreduction had increased survival rates (Fig. 3). Although this is not an immediate outcome, it is the only information available in Korea, and this experience has helped us consider the role of CRS with HIPEC in the treatment of gastric cancer.
The most important issue is patient selection. The patients who underwent complete cytoreduction showed a considerable increase in survival rate. Conversely, the survival rate of those with CCR-2 or CCR-3 did not considerably differ. CRS with HIPEC is a procedure associated with high morbidity, as mentioned earlier. Thus, surgery for all indicated patients poses more risks than achievable benefits. We conducted CRS in all patients in the early stage of our hospital operation. However, recently, the strategy was revised. Laparoscopic examination was routinely performed, and PCI score was assessed. We performed CRS only when complete cytoreduction was achievable with minimal organ resection. Otherwise, palliative intraperitoneal chemotherapy was performed using laparoscopic HIPEC (marked involvement of the seeding nodule in the small bowel mesentery, disseminated seeding in bowel serosa, etc.). After more cases are recorded, we will analyze other factors, such as PCI, age, and general condition to make a surgical indication that will provide the best results.
The diagnosis of PM in patients with gastric cancer should be reconsidered. CT is one of the most effective diagnostic tools for PM. However, CT alone has the potential of overdiagnosis. Some institutions do not perform diagnostic laparoscopy in patients with typical findings of PM on CT. Table 4 presents the four patients who were diagnosed as having PM at other institutions. However, these patients were not diagnosed with PM via laparotomy. Patients 1 and 3 could be considered to be in complete remission after chemotherapy. However, in the other 2 patients, no seeding nodule was observed in the abdominal cavity. Both patients were first encouraged to undergo palliative chemotherapy without diagnostic laparoscopy, and even stage I resulted in the final pathological findings in patient 4. Unless patients show signs of massive malignant ascites or frozen abdomen, diagnostic laparoscopy should be performed.
Obtaining informed consent after providing sufficient explanation and coordinating with other departments is also important in CRS with HIPEC. Minimally invasive surgery is the current trend in gastric cancer surgery with regard to patient quality of life after operation. Reduced port or single-incision gastrectomy is popular for its cosmetic effect and pain control ability. However, performing CRS is another consideration. A long midline abdominal incision is essential, and sometimes, diversions, such as ileostomy or colostomy, should be conducted during the surgery. Surgeons should provide patients with accurate information regarding the risks and benefits of this procedure and other available treatments. Moreover, various departments should collaborate with each other. A multidisciplinary approach is required for diagnosing PM and managing postoperative complications.
Finally, the importance of systemic chemotherapy must be considered. In addition, the role of the surgical oncologist must be reassessed. CRS with HIPEC is a procedure that can play a crucial role in the treatment of patients with PM. Theoretically, reducing tumor loading and directly applying anticancer drugs can treat PM in patients with AGC [21,22]. However, this procedure alone is not sufficient for increasing the survival rate of patients. Conventional systemic chemotherapy should be consecutively performed before and after surgery. The role of the surgical oncologist in chemotherapy has been assessed and may be even more important in chemotherapy after major surgeries such as CRS with HIPEC. Understanding the complex anatomy, the ability to cope with complications, and treatment continuity help surgeons to easily manage patients.
This study has some limitations. First, this was an observational study that might have been biased by unrecognized factors. Our patients might have received various treatments, including palliative surgery or chemotherapy, which may be relevant to their survival. In addition, most patients were transferred from other hospitals. Thus, checking the date of PM diagnosis was challenging. Second, the diagnostic procedures and surgical treatments for PM have not been standardized. In Korea, until now, when surgeons encounter patients with PM of AGC, the decision regarding the treatment strategy is still based on the surgeon's experience. A standard protocol for the treatment of these patients is not available.
In conclusion, CRS with HIPEC may have beneficial effects in patients with PM of AGC. However, the rates of complications and mortality associated with this combined therapeutic approach is high. Therefore, this treatment should be performed only in selected patients by surgeons experienced in the field of gastric cancer with PM.
Footnotes
- Conceptualization: J.Y.S.
- Data curation: K.D.W., S.S.
- Formal analysis: K.D.W., S.S.
- Supervision: J.Y.S., P.D.G.
- Writing - original draft: K.D.W.
- Writing - review & editing: J.Y.S., P.D.G.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Lee JH, Son SY, Lee CM, Ahn SH, Park DJ, Kim HH. Factors predicting peritoneal recurrence in advanced gastric cancer: implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer. 2014;17:529–536. doi: 10.1007/s10120-013-0306-2. [DOI] [PubMed] [Google Scholar]
- 2.Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–318. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 3.Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–628. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 4.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 5.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 6.Esquivel J, Elias D, Baratti D, Kusamura S, Deraco M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol. 2008;98:263–267. doi: 10.1002/jso.21053. [DOI] [PubMed] [Google Scholar]
- 7.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776–1782. [PubMed] [Google Scholar]
- 9.Jo MH, Suh JW, Yun JS, Namgung H, Park DG. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer: 2-year follow-up results at a single institution in Korea. Ann Surg Treat Res. 2016;91:157–164. doi: 10.4174/astr.2016.91.4.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370–375. doi: 10.1002/bjs.4695. [DOI] [PubMed] [Google Scholar]
- 12.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on gastric cancer in 2014. J Gastric Cancer. 2016;16:131–140. doi: 10.5230/jgc.2016.16.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007) Gastric Cancer. 2018;21:144–154. doi: 10.1007/s10120-017-0716-7. [DOI] [PubMed] [Google Scholar]
- 16.Rudloff U, Langan RC, Mullinax JE, Beane JD, Steinberg SM, Beresnev T, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol. 2014;110:275–284. doi: 10.1002/jso.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan TD, Links M, Fransi S, Jacques T, Black D, Saunders V, et al. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal surface malignancy--a journey to becoming a Nationally Funded Peritonectomy Center. Ann Surg Oncol. 2007;14:2270–2280. doi: 10.1245/s10434-007-9406-8. [DOI] [PubMed] [Google Scholar]
- 18.Polanco PM, Ding Y, Knox JM, Ramalingam L, Jones H, Hogg ME, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol. 2015;22:1673–1679. doi: 10.1245/s10434-014-4111-x. [DOI] [PubMed] [Google Scholar]
- 19.Kusamura S, Moran BJ, Sugarbaker PH, Levine EA, Elias D, Baratti D, et al. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br J Surg. 2014;101:1758–1765. doi: 10.1002/bjs.9674. [DOI] [PubMed] [Google Scholar]
- 20.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94:1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 21.Kim SW. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastric Cancer. 2014;14:266–270. doi: 10.5230/jgc.2014.14.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarter MD, Fong Y. Role for surgical cytoreduction in multimodality treatments for cancer. Ann Surg Oncol. 2001;8:38–43. doi: 10.1007/s10434-001-0038-0. [DOI] [PubMed] [Google Scholar]