Abstract
Lymphocytes are an integral component of the immune system. Classically, all lymphocytes were thought to perpetually recirculate between secondary lymphoid organs and only traffic to non-lymphoid tissues upon activation. In recent years, a diverse family of non-circulating lymphocytes have been identified. These include innate lymphocytes, innate-like T cells and a subset of conventional T cells. Spanning the innate-adaptive spectrum, these tissue-resident lymphocytes carry out specialized functions and cross-talk with other immune cell types to maintain tissue integrity and homeostasis both at the steady state and during pathological conditions. In this review, we provide an overview of the heterogeneous tissue-resident lymphocyte populations, discuss their development, and highlight their functions both in the context of microbial infection and cancer.
Keywords: tissue resident, innate lymphocyte, innate-like T cells, conventional T cells, cancer, infection
Introduction
A fundamental role of the immune system is to maintain host integrity. For metazoan species, an effective immune response must address invading threats in a rapid and specific manner such that the afflicted tissues remain uncompromised and continue to carry out their vital functions for the host. The innate immune system provides the first line of defense through the recognition of stereotypic motifs associated with a broad spectrum of pathogens (1–3). In contrast, the adaptive immune system, equipped with antigen receptors of near-limitless diversity, exerts its effector functions in an antigen specific manner (3). This expanded population of antigen-specific adaptive lymphocytes in turn forms the basis of immunological memory, bestowing the hosts with long-lasting immunity against previously encountered pathogens (3).
For mammalian species, the adaptive immune response is initiated in secondary lymphoid structures by antigen presenting cells (APCs). Upon activation by danger-associated signals, APCs migrate from the site of insult to draining lymph nodes, carrying with them components of the menacing agents. There they present these captured antigens to naïve T lymphocytes, which in turn triggers the successive rounds of cell division by T lymphocytes and initiates their differentiation into effector and memory subsets. Whereas, effector T cells home back to the primary sites of insult, mediate clearance of pathogen and undergo population contraction, memory T cells persist after the resolution of infection and are poised to mount recall responses. Under this classical view, the secondary lymphoid tissues are the integral component of the adaptive immune system, for the constant migration of adaptive lymphocytes within such a network maximizes their chance of antigen encounter (4). Teleologically, this circulatory behavior of naïve adaptive lymphocytes is a necessary consequence of their anticipatory antigen receptor repertoire (5). The antigen receptor genes of adaptive lymphocytes are assembled through random somatic recombination without prior knowledge of their cognate antigen. This anticipatory nature of the adaptive antigen receptor repertoire underlies its tremendous diversity, but greatly limits the frequency of lymphocytes with a given specificity. As such, a given naïve T cell clone cannot be present in all tissues at once. By necessity, they patrol strategically placed lymph nodes, which collect information on the statuses of their associated tissues, to efficiently survey the antigen landscape of the whole organism.
Our understanding of lymphocyte responses has broadened significantly in the past decade by the successive discovery of many non-circulating lymphocyte populations. These lymphocytes predominantly reside in non-lymphoid tissues in stark contrast to naïve adaptive lymphocytes, which constantly recirculate between secondary lymphoid organs. In fact, it is now well-appreciated that many, if not all, non-lymphoid organs harbor a sizable population of tissue-resident lymphocytes. These include tissue-resident memory T (TRM) cells; unconventional T cells such as invariant natural killer T (iNKT) cells, intraepithelial lymphocytes (IEL), and γδ T cells; and a diverse family of innate lymphocytes. This property of tissue residency spans across the innate-adaptive spectrum and may be essential for the tissue-specific functions of its respectively resident lymphocyte populations. In this review, we introduce the defining features of tissue-resident lymphocytes, provide an overview of their characteristic features, summarize recent findings on their ontogeny, and discuss their functions in the context of cancer.
Defining tissue-resident lymphocytes
The defining feature of tissue-resident lymphocytes is their distinct migration pattern. In contrast to naïve adaptive lymphocytes which frequently travel between secondary lymphoid organs, tissue-resident lymphocytes constitutively reside in non-lymphoid tissues and generally do not re-circulate through blood (6, 7). This blood-tissue disequilibrium can be conveniently approximated by intravascular staining (8–10). Intravenous administration of fluorescently-conjugated antibody labels vasculature-associated cell populations in a short period of time. Unlabeled cells are thus presumed to reside in the tissue parenchyma and are unlikely to re-circulate. The tissue resident property is most formally demonstrated by parabiosis experiments in which the circulatory systems of two animals are surgically joined, allowing for free exchange of their cell populations (11). Over time, half of the re-circulating lymphocyte compartment in one animal will be derived from its parabiont (6, 11). In contrast, the non-circulating compartment remains dominated by endogenous lymphocyte populations with little to no input from the parabiont (6, 11). This restricted migratory pattern of tissue-resident lymphocytes is often associated with their lack of lymphoid tissue homing chemokine receptors and elevated expressions of several adhesion molecules (7, 12). The sphingosine-1-phosphate receptor (S1PR1) and the chemokine receptor CCR7, whose ligands, S1P, and CCL19/21 are abundantly found in the blood and secondary lymphoid organs, respectively, facilitate re-circulation of lymphocytes and are downregulated as part of the tissue residency program (13–15). On the contrary, CD69, which antagonizes S1PR1 signaling, is reciprocally upregulated (16, 17). In addition, increased expression of integrin molecules, such as CD49a (encoded by Itga1) and CD103 (encoded by Itgae), whose ligands are collagen and E-cadherin, respectively, promotes interaction with tissue constituents, further reinforcing retention of lymphocytes (18, 19). Whereas, the downregulation of CCR7 and S1PR1 seems to be universal for tissue-resident lymphocytes, the usage of integrin molecules is more diverse. CD103 is specifically found on lymphocytes associated with epithelial tissues, such as the small intestine epithelium and ductal epithelium in glandular organs (20–23). CD49a and CD69 also have their own tissue-restricted expression patterns (18, 24–26). These observations highlight the substantial heterogeneity within the tissue-resident lymphocyte compartment. Thus, defining tissue-resident populations solely based on phenotypic markers may not reliably identify all cells. Instead, parabiosis experiments remain the gold standard to properly define tissue residency.
Overview of tissue-resident lymphocyte populations
So far, tissue-resident populations have been identified for all known types of lymphocyte across the innate-adaptive spectrum (6), strongly suggesting that the acquisition of the tissue residency program represents a state of differentiation rather than commitment to a distinct lineage. Resident lymphocyte populations are hypothesized to sense in their home organs tissue disturbances stemming from infection, stress and other deviations from the norm. In turn, they initiate the necessary immune responses to restore homeostasis. Below we briefly describe the characteristic features of various tissue-resident lymphocyte populations and their functions in maintaining tissue integrity.
Innate lymphocytes
Innate lymphocytes are characterized by their lack of functionally re-arranged antigen receptors. This population includes the prototypic member, natural killer (NK) cells, and the emerging family of innate lymphoid cells (ILCs) (27, 28). Under steady-state conditions, NK cells are recirculating while ILCs are not (6). Emerging evidence suggest that ILCs can be further parsed based on their cytotoxic potential into two subsets: helper ILCs, which are IL-7R-expressing cytokine producers, and killer ILCs, which express cytotoxic molecules but have little to no IL-7R expression (28). Helper ILCs are enriched at mucosal sites and include ILC1, ILC2, and ILC3, each of which produces signature cytokines not unlike their helper T cell subset counterparts (27). The killer ILCs, on the other hand, are mostly found in the liver and epithelium of glandular tissues, such as the salivary, prostate, and mammary glands, and can mediate direct cytolysis of target host cells through granzyme secretion or Fas ligand engagement (23, 29–31).
The exact function of tissue-resident type 1 innate lymphocytes remains contentious. Because of their striking resemblance to NK cells at the phenotypic level, studies aiming to test NK cell functions by depleting NK marker-expressing populations through antibodies or diphtheria toxin system may have inadvertently eliminated type 1 ILCs as well. Hence it is difficult to pinpoint which population mediates the observed phenotypes. This caveat has only been recognized recently but nevertheless precipitated the development of new genetic tools to selectively target either populations. For instance, a recent study utilized animals deficient for the transcription factor Zfp683, or Hobit, to specifically reduce the number of liver ILCs, leaving the NK compartment intact (32). In these animals, control of early viral replication in the liver was impaired, supporting the idea that resident type 1 ILCs function as first line defenders.
Type 2 ILCs are the most homogenous among the innate lymphocytes and produce signature cytokines of the type 2 response, such as IL-5, IL-13, and amphiregulin, in a transcription factor Gata3-, Bcl11b, and Rora-dependent manner (33–35). ILC2s control normal immune responses through cross-talk between stroma and other immune cell types. For instance, during helminth infection, intestinal tuft cell-derived IL-25 activates ILC2s to secrete IL-13, which feedbacks on the epithelium to promote tuft cell differentiation (36). The alarmin IL-33 produced upon tissue injury also stimulates IL-5 production by ILC2s, which in turn recruits eosinophils and enhance their innate effector functions (37). This pathway can be antagonized by a secretory product of the helminth H. polygyrus, HpARI, which prevents the release of IL-33 by tethering it to necrotic cells (38), further demonstrating the evolutionary benefit of ILC2-dependent responses.
Group 3 ILCs are highly complex and can be roughly unified by their dependency on the transcription Rorc for development and function (39). Upon activation by IL-23, a subset of ILC3s produce IL-22, which in turn triggers the anti-microbial peptide production by intestinal epithelium (40–42). Mice with an impairment in the IL-23-ILC3-IL-22 axis succumb to infection by Citrobacter rodentium, a gut effacing bacterium (42–44). Furthermore, IL-22 in concert with IL-18 is essential for control of murine norovirus infection (45). Together, these data demonstrate a critical role for ILC3s in maintaining gut homeostasis.
Innate-like T cells
Innate-like or unconventional T cells express functionally re-arranged T cell receptors (TCRs) of limited diversity. In contrast to conventional T cells whose TCRs strictly recognize peptides in the context of classical polymorphic major histocompatibility molecules (MHCs), the mode of antigen recognition by innate-like T cells is diverse, with TCRs recognizing antigen in the context of canonical MHCs, non-classical non-polymorphic MHC-like molecules, or even independently of MHCs altogether (46). The most well-characterized members of this family of lymphocytes include IELs, iNKT cells, and γδ T cells.
Many epithelial tissues contain resident IEL populations (47). The most studied are the small intestinal IELs, which consist of both TCRαβ- and TCRγδ-expressing subsets (48). The TCRαβ+ IELs can be further divided into two major populations based on the surface expression of CD8αβ heterodimer. CD8αβ− IELs, typically expressing the CD8αα homodimer, develop early in life, but its population dwindles as the animal ages and is progressively replaced by CD8αβ+ IELs (48). Thus, the CD8αα+ subsets are often termed “natural” or “unconventaionl” IELs, to distinguish them from the more conventional CD8αβ+ subsets, or the “induced” IELs. In addition to the TCR, CD8αα+ IELs also express panoply of activating and inhibitory receptors typically found on innate lymphocytes. These include the Ly49 and other NK receptor family members (49–51). Recently, another subset of IELs, characterized by the expression of both CD4 and CD8αβ co-receptors was identified (52, 53). A series of experiments demonstrate that these CD4+CD8αβ+ IELs are in fact converted from conventional CD4+ T cells by intestinal tissue-specific signals, such as TGFβ (54). So far, the exact functions of IELs remain elusive, although in specific settings, IELs contribute to anti-pathogen responses in the gut (52, 53, 55, 56).
iNKT cells express an invariant TCRα chain paired with a TCRβ chain of limited diversity (46, 57). Distinct from other TCRαβ+ T cells, iNKT cells recognize lipid antigens presented in the context of the MHC class I-like molecule, CD1d (58–60). The synthetic glycolipid, alpha-galactosylceramide, has been one of the prototypic stimulators of iNKT cells (61). Since then, a plethora of structurally homologous lipids capable of activating iNKT cells have been identified (62). These range from foreign substances, such as certain bacterial cell wall components (63–65) to endogenous sources, such as intermediates in lipid metabolism (66, 67), although the latter is often only transiently present, rare, and less potent. Nevertheless, sensing of endogenous lipid ligands may be the major mechanism by which iNKT cells detect a breach of tissue integrity. Two studies demonstrate an essential role for iNKT cells in controlling infection by pathogens that lack potent agonist ligands (68, 69), supporting the idea that iNKT cells may primarily survey host cells for altered metabolism as a result of pathogen invasion. Similar to ILCs, iNKT cell subsets analogous to the TH1, TH2, and TH17 conventional CD4 T cells have been described (70). Not unlike these T helper cells, each iNKT cell subset produces its signature cytokines driven by distinct master transcription factors (70).
T cells expressing the TCRγδ are present at barrier sites with a particular enrichment at the skin and intestinal epithelium (71, 72). In mice, rearrangement of the TCRγ locus follows a strict temporal order, resulting in the sequential appearances of distinct γδ T cells bearing monoclonal or oligoclonal TCRs that seed various epithelial tissues during fetal development (71–73). For instance, dendritic epithelial T cells (DETC), characterized by their monoclonal TCR composed of Vγ3 and Vδ1, develop between embryonic days 14 and 16 (73, 74). In contrast, intestinal Vγ7+ γδ T cells arise between 2 and 3 weeks after birth (75). It is conceivable that developmental stage-dependent tissue-derived signals permit temporally ordered colonization by distinct γδ T clones. In support of this, two studies demonstrate that Skint1 and Btnl molecules, which are expressed by epithelium during specific stages of development, induce the maturation and potentiate the responses of Vγ5+ DETCs and Vγ7+ intestinal γδ T cells, respectively (75, 76). The cognate antigens for γδ TCRs are still elusive. Whether MHC molecules are involved in γδ TCR recognition is also unresolved. Similar to innate lymphocytes, γδ T cells rapidly produce cytokines, including interferon gamma (IFNγ) and IL-17, when activated (77). A recent study revealed an unconventional role of skin resident γδ T cells in antagonizing carcinogen-induced melanoma (78). In an IL-4-dependent manner, these γδ T cells promote extrafollicular production of autoreactive IgE, which in turn activate basophils.
Tissue-resident memory T (TRM) cells
The term tissue-resident memory T cells specifically describe populations of conventional T cells that acquire tissue-resident properties. Both CD4 and CD8 T cells can adopt tissue-resident phenotypes (12). Because the CD8+ subset has been better characterized, TRM hereafter refers to CD8+ TRM cells unless noted otherwise. TRM cells have been commonly regarded as first line of defense in peripheral tissues especially against previously encountered threats (79–81). They are hypothesized to provide timely control of tissue threats before the participation of circulatory memory populations. For instance, a report showed that pre-existing herpes simplex virus (HSV) 2 antigen-specific TRM cells at the vaginal mucosa protect hosts from lethal HSV-2 challenge by restricting viral replication at the site of infection as well as preventing the spread of virus to the peripheral nervous system (81). TRM cells engage in diverse effector functions to mediate host protection. As CD8+ T cells can directly lyse infected target cells through the release of granzymes and perforin, several studies reported granzyme B expression in TRM cells as well (19, 23, 82, 83). Notably, TRM cells in the brain can lyse antigen-loaded targets in situ (84), suggesting their cytotoxic potential and direct killing as their means of immunosurveillance. By contrast, lung TRM cells protect hosts from influenza virus infection through a process involving IFNγ rather than cytotoxicity (85). More strikingly, recent studies highlighted the innate-like effector property of TRM cells (83, 86, 87). Local activation of TRM cells resulted in their chemokine production, which potently recruited non-antigen specific T cells and initiated an innate immune cascade. Such a bystander response resulted in near-sterilizing immunity against antigentically unrelated pathogens. Thus, in this context, TRM cells can serve as alarm-sounders rather than front line defenders.
Origin of innate and innate-like tissue-resident lymphocytes
Adaptive lymphocytes are naturally circulatory and only acquire tissue residency program upon activation. In contrast, innate and innate-like lymphocytes migrate directly to their home tissues after exiting sites of development, bypassing this recirculatory step. We postulate that this difference in trafficking between adaptive and innate/innate-like lymphocytes is imprinted during their development. The developmental pathway of thymocytes to mature T cells is punctuated by several checkpoints, one of which occurs at the double-positive (DP) stage (Figure 1). Here, DP thymocytes test their functionally assembled TCRs for reactivity against self-derived antigens in the context of MHC molecules (88). Strong self-reactivity instructs DP thymocytes to adopt innate-like T cells fates whereas weakly reactive clones are diverted into conventional T cell lineages (88). For instance, thymocytes expressing a transgenic TCR predominantly develop into unconventional IELs when its cognate ligand is expressed in the thymus, but into conventional T cells when otherwise. This process of agonist selection instructs a phenotypic change on DP thymocytes characterized by the downregulation of both CD4 and CD8 co-receptors and the concomitant upregulation of PD-1 (89–92). This population, when adoptively transferred into lymphopenic recipients, exclusively become CD8αα+ unconventional IELs, and is thus named IEL progenitor (IELp; Figure 1) (89). Consistently, thymocytes expressing TCRs isolated from natural IELs also adopt the IELp phenotypes (90, 91). In a similar fashion, the endogenous agonist selection ligand, isoglobotrihexosylceramide (iGb3), which strongly stimulates the invariant NKT TCR, drives the lineage commitment of DP thymocytes into iNKT cells (Figure 1) (93). The homotypic interaction between SLAM family receptors is also essential for iNKT development, presumably by complementing TCR-driven selection signals (94, 95). Thus, strong self-reactivity underlies the innate-like T cell fate choice.
Figure 1.

Ontogeny of tissue-resident lymphocytes. All lymphocytes develop from the common lymphoid progenitor (CLP). In the bone marrow, an early innate lymphoid progenitor (EILp) can give rise to natural killer (NK) cells and innate lymphoid cells (ILCs). Whereas, the identity of an NK-restricted progenitor (NKp) remains unknown, a committed innate lymphoid cell progenitor (ILCp), which can give rise to all helper ILCs (ILChs), but not NK cells has been described. Less understood, ILCs with cytotoxic potential, or killer ILCs (ILCk) may arise from a hypothetical killer ILC progenitor (ILCkp) that have lost ILCh and NK potential. While ILCs are inherently tissue-resident, NK cells recirculate. Whether NK cells can acquire tissue-resident features remains unknown. Thus, the term tissue-resident NK (trNK) cells is better kept until such a possibility can be unequivocally ruled out. Beside innate lymphocytes, CLP also gives rise to T lineage-committed progenitors that complete their differentiation in the thymus. The vast majority of TCRαβ-expressing T cells undergo a double positive (DP) stage, during which MHC-based selection takes place. DP thymocytes bearing strongly self-reactive TCRs develop into unconventional intraepithelial lymphocyte (IEL) and natural killer T (NKT) cell lineages through agonist selection, while those with weakly self-reactive TCRs are diverted into single positive (SP) thymocytes, which subsequently give rise to conventional T (conv. T) cells. Whereas, IELs and NKT cells are naturally tissue-resident, conventional T cells recirculate but can become tissue-resident (TRM) upon activation.
Because innate lymphocytes do not express antigen receptors, their self-reactivity is difficult to gage. However, there exist several striking parallels between innate lymphocyte and T cell development. All innate lymphocytes appear to arise from an early innate lymphoid progenitor (EILp; Figure 1). One defining feature of EILp is downregulation of IL-7 receptor (IL-7R), which also occurs in DP thymocytes, presenting a peculiar similarity between the two progenitors among the otherwise IL-7R-dependent intermediates during lymphopoeisis (96, 97). Just as agonist selection signals drive PD1 expression, a PD1-expressing innate lymphoid cell progenitor (ILCp) downstream of EILp has been identified (Figure 1) (35). Like NKT cells, ILCp expresses the transcription factor PLZF and can differentiate into all subsets of helper ILCs (98). The transient upregulation of PD1 on ILCp suggests that all ILCp-derived ILCs engage in a brief but strong stimulation during their development, which parallels the autoreactive TCR-mediated signals that drive IEL commitment. Notably, NK potential is lost in ILCp, although a dedicated NK progenitor remains unidentified (Figure 1) (98). The default circulatory behavior of NK cells aligns them more with the conventional T cells than ILCs. Conceivably, NK cells, like conventional CD8 T cells, may not have experienced a PD1high state during development. In fact, the lack of PD1 expression may help distinguish such NK-dedicated progenitors from their ILC-committed counterparts. The developmental path of cytotoxic ILCs is less understood. In contrast to IL-7R-expressing helper ILCs, which require the transcription factor Gata3 and Nfil3 for development, cytotoxic ILCs in the salivary gland are marginally affected upon loss of either transcription factors (29, 31, 99–101). Furthermore, while the vast majority of IL-7R-expressing ILCs develop from the PLZF-expressing ILCP, a substantial fraction of cytotoxic ILCs in the salivary gland do not (102). Additionally, whereas conventional NK cells are critically dependent on Eomes and Nfil3, cytotoxic ILCs again are not (103–105). These genetic data suggest the existence of yet another innate lymphocyte lineage, which is distinct from both the ILCh and conventional NK cells, and is tentatively named ILCk (Figure 1). ILCks in fact resemble IEL in their constitutive expression of cytotoxic molecules and inherent tissue-resident nature (23). Provocatively, ILCk progenitor may develop from EILp and assume IELp-like phenotypes such as high PD1 but little PLZF expression.
Acquisition of tissue resident program by circulating lymphocytes
Best exemplified by TRM cells, re-circulating lymphocytes can acquire tissue resident properties upon activation. The exact time point at which the tissue-resident program is launched during the activation history of a T cell is still unknown. Several lines of evidence suggest that tissue tropism of an activated T cells can be imprinted by dendritic cells (DCs) during priming. For instance, T cells activated by DCs isolated from peripheral lymph nodes upregulate E- and P-selectin while those primed by DCs from mesenteric lymph nodes express gut-homing molecules, such as α4β7 integrin and CCR9 (106, 107). Furthermore, the expression of skin- and gut-homing receptors can be enhanced by metabolites specific to these two tissues, such as retinoic acid (108, 109). These data collectively suggest that activated T cells acquire tissue tropism and specific homing capacity during priming. Contrary to this model, recent studies demonstrated that T cell migration is rather promiscuous during the effector phase of the immune response. In fact, T cells primed at any site can access almost every tissue in the organism. For instance, priming of T cells during systemic LCMV infection leads to the migration of antigen-specific T cells to many peripheral tissues (110). More strikingly, intranasal immunization with Sendai virus also results in the migration of antigen-specific T cells to other peripheral tissues (110). Further examination revealed that T cells primed in any secondary lymphoid organs can in fact upregulate homing receptors for non-lymphoid tissues (111). Thus, the entry of a T cell into non-lymphoid tissues can be instated regardless of priming locations. Once inside the tissue, local signals then orchestrate the tissue resident program. Indeed, adoptive transfer of in vitro activated CD8 T cells into the dermis is sufficient to induce their differentiation into long-lived CD103+CD69+ TRM cells, phenotypically indistinguishable from those generated in vivo (18). These data suggest that entry into the tissue is a stochastic but pivotal event that marks the initiation of tissue resident program. Recently, fate-mapping experiments using KLRG1-Cre revealed further heterogeneity within the TRM population with contribution from both KLRG1-fate mapped and non-fate mapped precursors (112). This is in contrast to previous studies where KLRG1+ CD8 T cells fail to give rise to CD103+ TRM when adoptively transferred (18). The discrepancy may be caused by the use of different infection models. Interestingly, although both KLRG1-fate mapped and non-fate mapped precursors lost KLRG1 expression when entering the tissue, the progeny of the two exhibits nuanced but discernable differences in effector functions (112), suggesting that other events before tissue entry can impact the functional capacity of TRM.
Often deemed as the counterpart to conventional CD8 T cells, whether NK cells can acquire tissue resident features like TRM differentiation is less understood. In one study, adoptive transfer of hepatic DX5+ conventional NK cells into lymphopenic mice did not result in their upregulation of tissue resident markers, such as CD49a in the liver (105). In contrast, when transferred into tumor-bearing lymphopenic recipients, DX5+ cells infiltrate the tumor and assume tissue resident phenotypes in a TGFβ-dependent manner (113). These results suggest that re-circulating conventional NK cells possess the tissue resident potential, but its manifestation requires tissue-specific signals. Further studies, such as fate-mapping experiments, are needed to formally test this hypothesis.
Maintenance of tissue resident lymphocytes
Long-term parabiosis experiments revealed that under steady-state conditions, tissue resident lymphocytes are long-lived and replenish their population predominantly by local expansion (6). Consistently, other studies in mice and rhesus macaques showed that the tissue memory CD8 T cell populations are stable for 300–700 days, with little to no input from the circulatory memory pool (114–116). These observations suggest that while the concerted actions of adhesion molecules and chemokine receptors enforce tissue retention, additional cell-extrinsic signals promote the maintenance of tissue resident lymphocytes.
IL-7 and IL-15, both of which signal through the common gamma chain (γc), have pleiotropic roles during lymphocyte development and maintenance. While mice deficient for γc (encoded by Il2rg) lack B, T, NK, and ILCs, innate lymphocyte progenitors, such as EILp and ILCp were minimally affected (97), suggesting that the depletion of NK and ILCs in Il2rg−/− mice most likely stem from defective maintenance of the mature populations. In the absence of IL-7, bone marrow ILC2p, intestinal ILC2 and ILC3, but not ILC1 are drastically reduced (97, 117–119). In contrast, IL-15 deficiency predominantly impairs ILC1 in the liver, salivary glands, and the small intestine lamina propria (29, 119, 120), although intestinal NKp46+ ILC3 are dually dependent on IL-7 and IL-15 (119, 120). While the NK-restricted progenitor remains elusive, a Lin−CD127+CD122+ population has been identified to contain NK cell precursors and develop normally in the absence of Il2rg (121). The profound ablation of mature CD127− NK cells in these animals are attributed to the lack of IL-15 signaling as IL-15, but not other γc cytokines, deficiency can solely recapitulate this defect (121–123). In the thymus, a minute population of CD127+NK1.1+ innate lymphocytes, currently called thymic NK cells, require IL-7 for development (124).
The critical roles of homeostatic cytokines IL-7 and IL-15 for the maintenance of re-circulating naïve and memory T cells, respectively have been long appreciated. The dependency on IL-15 for TRM varies by their locations. TRM in the non-lymphoid tissues, such as the skin, are critically dependent on IL-15 (18) whereas those in the secondary lymphoid organs are not (125). Like TRM, CD8αα+ intestinal IELs are also maintained by IL-15 and enterocyte-expressed IL-15 in an otherwise IL-15-deficient animal is sufficient to restore unconventional IELs (126), suggesting that IL-15 critically sustains mature IELs rather than their precursors. In support of this, PD1+ IEL progenitors develop independent of IL-15 in the thymus (127). While TRM are induced in an antigen-dependent manner, they can be maintained in the absence of cognate antigen in the skin, reproductive tract, and salivary glands (18, 19, 21). In other tissues, persisting antigens contribute to TRM differentiation (19, 26, 82, 84, 128). Thus, the requirement for antigen during TRM maintenance may be tissue-specific. Lastly, given the similar requirement for IL-7 and IL-15 during their homeostasis, resident lymphocytes may occupy overlapping tissue niche. Pinpointing the source of these cytokines in the tissue may help elucidate the redundant and non-redundant roles of each resident lymphocyte population in maintaining tissue integrity.
Tissue-resident cytotoxic lymphocyte responses in cancer
The vertebrate immune system has evolved to exquisitely distinguish self from non-self, thereby achieving effective anti-pathogen responses while curbing autoreactivity. Cancer presents a unique challenge to this fine-tuned system as transformed cells are pathogenic agents derived from the host itself. Yet prevailing evidence has demonstrated that the immune system exerts constant pressure on tumors (129). These observations underlie the preponderant concept of cancer immunosurveillance (130–132). Mechanistically, increased somatic mutation as a result of genomic instability in transformed cells may generate neo-epitopes that can be recognized by conventional adaptive lymphocytes (133, 134). Although these T cells often exhibit “exhausted” phenotypes, their effector functions may be restored by checkpoint blockade therapies (134–136) (Figure 2). Targeting this mode of immunosurveillance certainly has been fruitful. However, not all cancer types sustain high mutation burden (137, 138). In such cases, CD8 T cell responses elicited by unmutated self-antigen often fail to restrict tumor growth (139, 140). These findings thus highlight the need to explore other immunosurveillance mechanisms for effective cancer immunotherapies.
Figure 2.
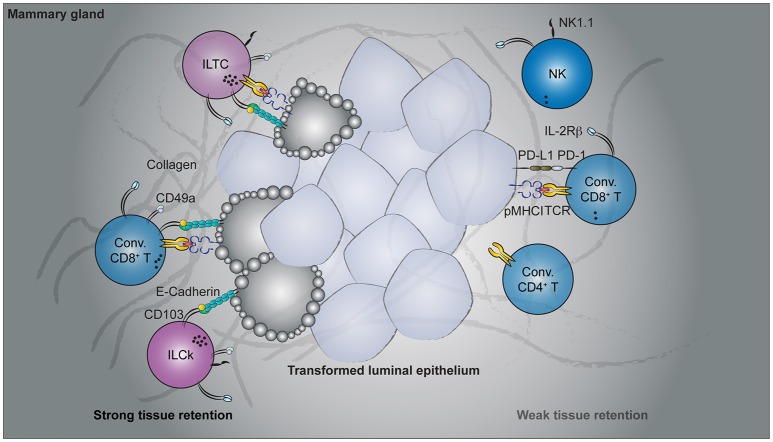
Cancer immunosurveillance by tissue-resident lymphocytes. Spontaneous oncogene-driven breast tumors are infiltrated by group 1 innate lymphocytes, conventional, and unconventional T cells. Parabiosis experiments revealed the tissue-resident nature of CD49a- and CD103-co-expressing lymphocytes, including the innate-like T cells (ILTCs), killer innate lymphoid cells (ILCks), and some conventional (Conv.) CD8+ T cells. In contrast, natural killer (NK) cells, PD1-expressing conventional CD8+ T cells recirculate through blood. Functionally, CD49a+CD103+ tissue-resident lymphocytes abundantly express lytic granules and can potently lyse transformed target cells. Despite their cytotoxicity, therapies targeting these tissue-resident populations are lacking while rapid advancement has been made to target conventional NK and T cells.
Just as pre-existing TRM populations are essential for restraining previously encountered pathogens, prophylactically induced TRM cells by cancer vaccines provide superior control of tumor growth over re-circulating memory T cells (141, 142). In fact, the presence of circulating tumor antigen-specific CD8 T cells alone is not sufficient to control tumor growth (141, 143), highlighting the potential therapeutic benefit of targeting tissue-resident lymphocytes. Strategies to enhance the differentiation and maintenance of these vaccine-induced TRM cells may decrease the relapse rate as well as restrict metastasis. However, prophylactic vaccination with tumor-associated antigen may not always be feasible in clinical settings, as it requires knowing the antigen ahead of time when patients who seek medical attention often have developed tumors already. Notwithstanding, tumorigenesis does naturally elicit tissue-resident lymphocyte responses (23, 144–148). Importantly, a substantial fraction of participating lymphocyte populations appear to have cytotoxic potential (23, 145, 148). These include conventional T cells of the CD8 lineage as well as more recently identified unconventional T cells and group 1 innate lymphocytes (Figure 2). Below, we summarize the latest findings on their characterization and potential cancer immunosurveillance functions.
Conventional and unconventional αβT cells
In many murine tumor models, αβ T cells can make up a substantial fraction of infiltrating lymphocytes. Among them, populations expressing tissue-resident markers are abundantly found (23, 144, 145, 148–152). These include T cells of both the conventional and unconventional lineages.
Our understanding of tissue-resident T cell responses in the context of cancer has only begun to advance in recent years. Much of the foundation is in fact built upon extrapolating observations from TRM cells in infectious settings. While these studies provide an invaluable conceptual framework to start with, cancer and acute infection differ fundamentally. Tumorigenesis is a continuous process without a defined time course. In contrast to acute infections where the pathogen load peaks and wanes within a week's time, tumor-associated antigen is continuously present and, in most oncogene-driven cancer models, persist until the endpoint of disease. Thus, there is no well-defined memory phase in the context of cancer and the term “tissue-resident memory T cells” seems to be a misnomer. In a sense, tumorigenesis is more analogous to chronic than acute infections. Indeed, the induction and accumulation of dysfunctional cytotoxic T lymphocytes (CTLs) by persistent antigen stimulation is a shared feature in both settings (153). To what extent the PD1hi CTLs are tissue-resident remains to be determined. Beside the PD1hi population, which appears to dominate in multiple cancer types, tumor-infiltrating CD8+ T cells that express tissue-resident markers have also been reported in several mouse cancer models (Figure 2). In a B16-F10 mouse melanoma transplantable tumor model, a fraction of antigen-specific tumor-infiltrating CD8 T cells acquired CD69 and CD103 expression 3 weeks after tumor engraftment (149). Furthermore, administration of blocking antibodies against CD103 resulted in a slight but significant acceleration in tumor growth (149), implying a CD103-dependent cancer immunosurveillance mechanism by these putative tissue-resident tumor-infiltrating lymphocytes (TILs). Using a similar transplantable melanoma model, another study demonstrated a CD8 T cell-intrinsic requirement for the transcription factor Runx3 in the development of tumor-resident CTL responses (144). CD8 T cells with reduced levels of Runx3 expression failed to constrain tumor growth (144), further implicating a tumor surveillance role for tissue-resident CTLs. In a spontaneous oncogene-driven breast tumor model, a proportion of intratumoral CD8+ T cells co-express CD49a and CD103 (23). Unlike in the transplantable tumor models, some CD49a+CD103+ T cells co-express natural killer (NK) receptors, such as NK1.1 and have innate-like features (Figure 2). These NK1.1+CD49a+CD103+CD8+ T cells are distinct from iNKT cells as they developed in the absence of CD1d, and thus represent a novel tissue-resident T cell population with no currently known counterpart in the TRM field (23). For this, NK1.1+CD49a+CD103+CD8+ T cells are termed innate-like T cells (ILTCs) to distinguish them from their NK1.1− counterparts. Parabiosis experiments confirmed the tissue-resident property of both ILTCs and NK1.1− tumor-infiltrating T cells, with the former being significantly less circulatory (23). Further studies demonstrated that these ILTCs produce little to no IFNγ, but abundantly express the cytotoxic molecule granzyme B (23). Indeed, ILTCs exhibit potent cytotoxicity toward transformed target cells in vitro, suggesting their potential role in anti-tumor responses (23). Thus, using infection-induced TRM cells as a template, these seminal works demonstrated the presence of tissue-resident cytotoxic T cells in mouse tumor models and implicated their immunosurveillance functions.
In human patients, CD103-expressing tumor infiltrating CD8+ T cells are abundantly present in multiple types of epithelium-derived cancers (145–147). In many cases, the accumulation of intratumoral CD103+CD8+ T cells is associated with favorable prognosis (145–147, 154, 155). Although the exact mechanisms by which these TILs contribute to restraining cancer progression remains elusive, emerging evidence unveil their similarity to TRM cells and suggest cytotoxicity as their mechanism of immunosurveillance. Whether CD103+ TILs are indeed tissue-resident cannot be easily established in humans. Nonetheless, whole genome transcriptome analysis reveals that these TILs share a gene expression program typically associated with pathogen-induced TRM cells and tumor-elicited CD49a+CD103+ TILs in mouse models (23, 142, 148, 156). For instance, CD103+ TILs from non-small cell lung carcinoma co-express CD49a and CD69, but little to no S1PR1 and the lymphoid tissue homing receptor CCR7 (145, 148). In addition to potentially increased tissue retention, CD103+ TILs appear to be in a distinct activation state compared to their CD103− counterparts. Not only do more CD103+ TILs exit quiescence, as measured by Ki67 expression (148), they also express higher levels of granzymes (148) and possess increased degranulation potential relative to CD103− TILs in response to stimulation (145). When incubated with autologous tumor cells, CD103+ TILs potently induced cytolysis of target cells (145). Whether this CD103+ population also contains innate-like T cells, such as the ILTCs found in mice, remains an outstanding question although NK receptor-expressing CD8+ T cells in human cancer patients have been documented (157–159). Nevertheless, these data demonstrate that the tissue-resident cytotoxic T cell response is a conserved cancer immunosurveillance mechanism between mouse and human and represents a promising target for tumor immunotherapy.
Group 1 innate lymphocytes
The protective role of group 1 innate lymphocytes against tumors has been repeatedly demonstrated in chemically-induced sarcoma and transplantable tumor models (160–163). However, most of these seminal works were done before the distinction between NK cells and ILCs was recognized. Most studies in this genre made use of depleting antibodies against NK1.1 or genetic systems in which diphtheria toxin is specifically expressed in NKp46+ cells. These approaches effectively eliminated NK cells, but also depleted ILC1s and ILCks as they too express NK1.1 and NKp46. Thus, one cannot conclude which of the affected population contributes to the reported phenotype (164). Having recognized this ambiguity, some studies further subset the NK1.1+NKp46+ innate lymphocyte populations with a set of markers conventionally used to distinguish between NK cells and ILC1s/ILCks (113, 165). Adoptive transfer of each subset into tumor-bearing lymphopenic hosts then allowed them to identify the population responsible for the protective phenotypes. In these studies, most anti-tumor activity appears to reside within the conventional NK cell compartment (75, 113). Non-NK tissue-resident innate lymphocytes, on the other hand, were shown to dampen anti-tumor immune responses (113). This is in contrast to their roles in oncogene-driven spontaneous tumor models (23, 166). For example, in a breast tumor model, early control of tumor progression is critically dependent on innate lymphocytes, as IL-15 deficient animals, which lack group 1 innate lymphocytes showed accelerated tumor growth (23). However, conventional NK cells were dispensable for this innate lymphocyte-dependent anti-tumor responses because Nfil3-deficient mice, which have profoundly diminished NK cell compartment, did not exhibit accelerated tumor growth (23). These data collectively imply that non-NK group 1 innate lymphocytes, most likely ILCks, assume a dominant role in early anti-tumor responses (Figure 2). Despite these tumor model-specific discrepancies, the immunosurveillance potential of tumor-infiltrating group 1 innate lymphocytes has garnered much therapeutic interest in recent years.
Many types of human solid tumors are also infiltrated by group 1 innate lymphocytes. Although collectively called NK cells, they in fact consist of two populations distinguished by the makers CD56 and CD16 (167–170). The CD56brightCD16− subset outnumbers their CD56dimCD16+ counterpart in tissues, both at steady state and during inflammation. In contrast, the CD56dimCD16+ population is far more abundant in the blood. Not surprisingly, the CD56brightCD16− innate lymphocytes express several tissue-resident markers as well as a defining gene expression program for tissue residency (169, 170). Under the current paradigm, both populations belong to the NK lineages and are related in a linear developmental pathway, namely, CD56brightCD16− cells give rise to CD56dimCD16+ in a process of differentiation (171, 172). However, it is also possible that the two populations are in fact of disparate lineages, a distinction not unlike the one seen between mouse NK cells and ILC1s/ILCks. While this debate awaits, if possible, a resolution, some clinical evidence suggest a potential anti-tumor role for type 1 innate lymphocytes. For example, in clear cell renal carcinoma, enrichment of type 1 innate lymphocyte-associated transcripts in the tumor mass correlates with favorable prognosis (173). Similarly, for gastrointestinal stroma tumors, the number of CD56-expressing infiltrating lymphocytes is associated with better overall survival (174). For patients with non-small cell lung carcinoma however, the presence of CD56-expressing lymphocytes does not correlate with clinical outcomes, presumably because their cytokine production and cytotoxicity are inhibited by the tumor microenvironment (175). Overcoming immunosuppression strategies deployed by tumor cells may re-invigorate these innate lymphocytes (176–178). A recent study devised an antibody that stabilizes the expression of a stress-induced ligand for the NK activating receptor, NKG2D on the tumor cell surface (179). Administration of this therapeutic agent enhances innate lymphocyte-dependent anti-tumor responses (179). Collectively, tumor-resident cytotoxic innate lymphocytes present a promising target for therapeutic intervention in addition to conventional CD8 T cells, for which a plethora of checkpoint blockade modalities are already in place.
Concluding remarks
Originally defined in the T cell field, the tissue residency program has now been found to be used by nearly all known lymphocyte lineages across the hematopoietic tree. Intriguingly, the vast majority of innate and innate-like lymphocytes (with the exception of NK cells) are inherently tissue-resident whereas the more recently evolved adaptive lymphocytes are not, suggesting an ancient origin of the tissue residency program. Since strong self-reactivity during lymphocyte development appears to be a key selection factor for gaining tissue-homing capacity, it is reasonable to assume that the most primordial function of tissue-resident lymphocytes is in fact to detect stress in host cells rather than to sense pathogen or its derivatives. Further extrapolation of this idea would provocatively suggest that the MHC-based selection mechanisms originally served to generate self-reactive T cells. Positive selection, templated on the extant agonist selection mechanisms, evolved later in vertebrate evolution.
Author contributions
CC and ML conceived the ideas. CC wrote the manuscript, and ML edited it.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Briana G. Nixon, Emily R. Kansler, and Efstathios Stamatiades for valuable discussions and critical reading of the manuscript.
Footnotes
Funding. This work was supported by the National Institute of Allergy and Infectious Diseases (R01 CA198280-01 to ML), the Howard Hughes Medical Institute (Faculty Scholar Award to ML), and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). CC is a Cancer Research Institute Irvington Fellow supported by the Cancer Research Institute.
References
- 1.Medzhitov R, Janeway C, Jr. Innate immunity. N Engl J Med. (2000) 343: 338–44. 10.1056/NEJM200008033430506 [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Approaching the asymptote: 20 years later. Immunity (2009) 30:766–75. 10.1016/j.immuni.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Boehm T. Evolution of vertebrate immunity. Curr Biol. (2012) 22:R722–32. 10.1016/j.cub.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 4.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. (2000) 343:1020–34. 10.1056/NEJM200010053431407 [DOI] [PubMed] [Google Scholar]
- 5.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu Rev Immunol. (2010) 28:275–94. 10.1146/annurev-immunol-030409-101253 [DOI] [PubMed] [Google Scholar]
- 6.Gasteiger G, Fan XY, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science (2015) 350:981–5. 10.1126/science.aac9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity (2014) 41:886–97. 10.1016/j.immuni.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. (2005) 115:3473–83. 10.1172/JCI24482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. (2012) 189:2702–6. 10.4049/jimmunol.1201682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. (2014) 9:209–22. 10.1038/nprot.2014.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science (2001) 294: 1933–6. 10.1126/science.1064081 [DOI] [PubMed] [Google Scholar]
- 12.Mackay LK, Kallies A. Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol. (2017) 38:94–103. 10.1016/j.it.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 13.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8(+) T cells. Nat Immunol. (2013) 14:1285–93. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. (2005) 6:895–901. 10.1038/ni1240 [DOI] [PubMed] [Google Scholar]
- 15.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. (2005) 6:889–94. 10.1038/ni1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature (2006) 440:540–4. 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- 17.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. (2010) 285:22328–37. 10.1074/jbc.M110.123299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. (2013) 14:1294–301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- 19.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. (2012) 188:4866–75. 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. (2010) 207:553–64. 10.1084/jem.20090858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA. (2012) 109:7037–42. 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortez VS, Cervantes-Barragan L, Robinette ML, Bando JK, Wang Y, Geiger TL, et al. Transforming growth factor-beta signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity (2016) 44:1127–39. 10.1016/j.immuni.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell (2016) 164:365–77. 10.1016/j.cell.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. (2009) 10:524–30. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 25.Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, et al. The collagen binding alpha 1 beta 1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity (2004) 20:167–79. 10.1016/S1074-7613(04)00021-4 [DOI] [PubMed] [Google Scholar]
- 26.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity (2013) 39:687–96. 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klose CSN, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. (2016) 17:765–74. 10.1038/ni.3489 [DOI] [PubMed] [Google Scholar]
- 28.Chou C, Li MO. Re(de)fining Innate Lymphocyte Lineages in the Face of Cancer. Cancer Immunol Res. (2018) 6:372–7. 10.1158/2326-6066.CIR-17-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol. (2014) 192:4487–91. 10.4049/jimmunol.1303469 [DOI] [PubMed] [Google Scholar]
- 30.Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang YM, Durum SK, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. (2015) 16:306–17. 10.1038/ni.3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sojka DK, Plougastel-Douglas B, Yang LP, Pak-Wittel MA, Artyomov MN, Ivanova Y, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife (2014) 3:e01659. 10.7554/eLife.01659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weizman OE, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, et al. ILC1 confer early host protection at initial sites of viral infection. Cell (2017) 171:795–808.e12. 10.1016/j.cell.2017.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity (2012) 37:634–48. 10.1016/j.immuni.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor ROR alpha is critical for nuocyte development. Nat Immunol. (2012) 13:229–36. 10.1038/ni.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Tsang JCH, Wang C, Clare S, Wang JX, Chen X, et al. Single-cell RNA-seq identifies a PD-1(hi) ILC progenitor and defines its development pathway. Nature (2016) 539:102–6. 10.1038/nature20105 [DOI] [PubMed] [Google Scholar]
- 36.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature (2016) 529:221–5. 10.1038/nature16161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Gonzalez I, Steer CA, Takei F. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. (2015) 36:189–95. 10.1016/j.it.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 38.Osbourn M, Soares DC, Vacca F, Cohen ES, Scott IC, Gregory WF, et al. HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity (2017) 47:739–51.e5. 10.1016/j.immuni.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cording S, Medvedovic J, Cherrier M, Eberl G. Development and regulation of ROR gamma t(+) innate lymphoid cells. Febs Lett. (2014) 588:4176–81. 10.1016/j.febslet.2014.03.034 [DOI] [PubMed] [Google Scholar]
- 40.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. ROR gamma t and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46(+) cells. Nat Immunol. (2009) 10:83–91. 10.1038/ni.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, et al. ROR gamma t(+) innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. (2011) 12:320–6. 10.1038/ni.2002 [DOI] [PubMed] [Google Scholar]
- 42.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. (2008) 14:282–9. 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]
- 43.Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. (2013) 14:937–48. 10.1038/ni.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rankin LC, Girard-Madoux MJ, Seillet C, Mielke LA, Kerdiles Y, Fenis A, et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol. (2016) 17:179–86. 10.1038/ni.3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang BY, Chassaing B, Shi ZD, Uchiyama R, Zhang Z, Denning TL, et al. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science (2014) 346:861–5. 10.1126/science.1256999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. (2015) 16:1114–23. 10.1038/ni.3298 [DOI] [PubMed] [Google Scholar]
- 47.Olivares-Villagomez D, Van Kaer L. Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. Trends Immunol. (2018) 39:264–75. 10.1016/j.it.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. (2011) 11:445–56. 10.1038/nri3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shires J, Theodoridis E, Hayday AC. Biological insights into TCR gamma delta(+) and TCR alpha beta(+) intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity (2001) 15:419–34. 10.1016/S1074-7613(01)00192-3 [DOI] [PubMed] [Google Scholar]
- 50.Denning TL, Granger S, Mucida D, Graddy R, Leclercq G, Zhang WG, et al. Mouse TCR alpha beta(+)CD8 alpha alpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. (2007) 178:4230–9. 10.4049/jimmunol.178.7.4230 [DOI] [PubMed] [Google Scholar]
- 51.GuyGrand D, CuenodJabri B, MalassisSeris M, Selz F, Vassalli P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T cell intraepithelial lineages. Eur J Immunol. (1996) 26:2248–56. 10.1002/eji.1830260942 [DOI] [PubMed] [Google Scholar]
- 52.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat Immunol. (2013) 14:271–80. 10.1038/ni.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reis BS, Hoytema van Konijnenburg DP, Grivennikov SI, Mucida D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity (2014) 41:244–56. 10.1016/j.immuni.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. (2013) 14:281–9. 10.1038/ni.2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller S, Buhler-Jungo M, Mueller C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J Immunol. (2000) 164:1986–94. 10.4049/jimmunol.164.4.1986 [DOI] [PubMed] [Google Scholar]
- 56.Lepage AC, Buzoni-Gatel D, Bout DT, Kasper LH. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J Immunol. (1998) 161:4902–8. [PubMed] [Google Scholar]
- 57.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. (2010) 11:197–206. 10.1038/ni.1841 [DOI] [PubMed] [Google Scholar]
- 58.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. (1997) 15:535–62. 10.1146/annurev.immunol.15.1.535 [DOI] [PubMed] [Google Scholar]
- 59.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. (2005) 23:877–900. 10.1146/annurev.immunol.23.021704.115742 [DOI] [PubMed] [Google Scholar]
- 60.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. (2007) 25:297–336. 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- 61.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science (1997) 278:1626–9. 10.1126/science.278.5343.1626 [DOI] [PubMed] [Google Scholar]
- 62.Joyce S, Girardi E, Zajonc DM. NKT cell ligand recognition logic: molecular basis for a synaptic duet and transmission of inflammatory effectors. J Immunol. (2011) 187:1081–9. 10.4049/jimmunol.1001910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li XM, et al. Invariant natural killer T cells recognize glycolipids from pathogenic gram-positive bacteria. Nat Immunol. (2011) 12:966–74. 10.1038/ni.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MREI, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. (2006) 7:978–86. 10.1038/ni1380 [DOI] [PubMed] [Google Scholar]
- 65.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. (2005) 35:1692–701. 10.1002/eji.200526157 [DOI] [PubMed] [Google Scholar]
- 66.Kain L, Webb B, Anderson BL, Deng SL, Holt M, Costanzo A, et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity (2014) 41:543–54. 10.1016/j.immuni.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kain L, Costanzo A, Webb B, Holt M, Bendelac A, Savage PB, et al. Endogenous ligands of natural killer T cells are alpha-linked glycosylceramides. Mol Immunol. (2015) 68:94–7. 10.1016/j.molimm.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, Zhou DP, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature (2005) 434:525–9. 10.1038/nature03408 [DOI] [PubMed] [Google Scholar]
- 69.Cohen NR, Tatituri RV, Rivera A, Watts GF, Kim EY, Chiba A, et al. Innate recognition of cell wall beta-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe (2011) 10:437–50. 10.1016/j.chom.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. (2013) 25:161–7. 10.1016/j.coi.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chien YH, Meyer C, Bonneville M. Gamma delta T cells: first line of defense and beyond. Annu Rev Immunol. (2014) 32:121–55. 10.1146/annurev-immunol-032713-120216 [DOI] [PubMed] [Google Scholar]
- 72.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, et al. Homing of a gamma-delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature (1990) 343:754–7. 10.1038/343754a0 [DOI] [PubMed] [Google Scholar]
- 73.Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science (1991) 252:1430–2. 10.1126/science.1828619 [DOI] [PubMed] [Google Scholar]
- 74.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of gamma-delta-antigen receptor genes of Thy-1+ dendritic epidermal-cells. Cell (1988) 55:837–47. 10.1016/0092-8674(88)90139-0 [DOI] [PubMed] [Google Scholar]
- 75.Barros RD, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia use butyrophilin-like molecules to shape organ-specific gamma delta T cell compartments. Cell (2016) 167:203–218.e17. 10.1016/j.cell.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. (2008) 40:656–62. 10.1038/ng.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen KDC, Su X, Shin S, Li L, Youssef S, Yarnasaki S, et al. Thymic selection determines gamma delta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity (2008) 29:90–100. 10.1016/j.immuni.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crawford G, Hayes MD, Seoane RC, Ward S, Dalessandri T, Lai C, et al. Epithelial damage and tissue gammadelta T cells promote a unique tumor-protective IgE response. Nat Immunol. (2018) 19:859–70. 10.1038/s41590-018-0161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jameson SC, Masopust D. Understanding subset diversity in T cell memory. Immunity (2018) 48:214–26. 10.1016/j.immuni.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. (2015) 21:688–97. 10.1038/nm.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature (2012) 491:463–7. 10.1038/nature11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, et al. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol. (2012) 188:2173–8. 10.4049/jimmunol.1102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science (2014) 346:98–101. 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U.S.A. (2010) 107:17872–9. 10.1073/pnas.1010201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McMaster SR, Gabbard JD, Koutsonanos DG, Compans RW, Tripp RA, Tompkins SM, et al. Memory T cells generated by prior exposure to influenza cross react with the novel H7N9 influenza virus and confer protective heterosubtypic immunity. PLoS ONE (2015) 10:e0115725. 10.1371/journal.pone.0115725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. (2013) 14:509–13. 10.1038/ni.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, et al. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science (2014) 346:101–5. 10.1126/science.1254803 [DOI] [PubMed] [Google Scholar]
- 88.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. (2012) 30:95–114. 10.1146/annurev-immunol-020711-075035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity (2006) 25:631–41. 10.1016/j.immuni.2006.08.018 [DOI] [PubMed] [Google Scholar]
- 90.Mayans S, Stepniak D, Palida SF, Larange A, Dreux J, Arlian BM, et al. Alpha beta T cell receptors expressed by CD4(-)CD8 alpha beta intraepithelial T cells drive their fate into a unique lineage with unusual MHC reactivities. Immunity (2014) 41:207–18. 10.1016/j.immuni.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McDonald BD, Bunker JJ, Ishizuka IE, Jabri B, Bendelac A. Elevated T cell receptor signaling identifies a thymic precursor to the TCR alpha beta(+)CD4(-)CD8 beta(-) intraepithelial lymphocyte lineage. Immunity (2014) 41:219–29. 10.1016/j.immuni.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA. CD8alphaalpha intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol. (2017) 18:771–9. 10.1038/ni.3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science (2004) 306:1786–9. 10.1126/science.1103440 [DOI] [PubMed] [Google Scholar]
- 94.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. (2005) 201:695–701. 10.1084/jem.20042432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borowski C, Bendelac A. Signaling for NKT cell development: the SAP-FynT connection. J Exp Med. (2005) 201:833–6. 10.1084/jem.20050339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Q, Li FY, Harly C, Xing SJ, Ye LY, Xia XF, et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol. (2015) 16:1044–50. 10.1038/ni.3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harly C, Cam M, Kaye J, Bhandoola A. Development and differentiation of early innate lymphoid progenitors. J Exp Med. (2018) 215:249–62. 10.1084/jem.20170832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature (2014) 508:397–401. 10.1038/nature13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity (2014) 40:378–88. 10.1016/j.immuni.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife (2014) 3:e04406. 10.7554/eLife.04406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu W, Domingues RG, Fonseca-Pereira D, Ferreira M, Ribeiro H, Lopez-Lastra S, et al. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep (2015) 10:2043–54. 10.1016/j.celrep.2015.02.057 [DOI] [PubMed] [Google Scholar]
- 102.Erick TK, Anderson CK, Reilly EC, Wands JR, Brossay L. NFIL3 expression distinguishes tissue-resident NK cells and conventional NK-like cells in the mouse submandibular glands. J Immunol. (2016) 197:2485–91. 10.4049/jimmunol.1601099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. (2014) 211:563–77. 10.1084/jem.20131560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pikovskaya O, Chaix J, Rothman NJ, Collins A, Chen YH, Scipioni AM, et al. Cutting edge: eomesodermin is sufficient to direct type 1 innate lymphocyte development into the conventional NK lineage. J Immunol. (2016) 196:1449–54. 10.4049/jimmunol.1502396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gordon SM, Chaix J, Rupp LJ, Wu JM, Madera S, Sun JC, et al. The transcription factors T-bet and eomes control key checkpoints of natural killer cell maturation. Immunity (2012) 36:55–67. 10.1016/j.immuni.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. (2005) 201:303–16. 10.1084/jem.20041645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature (2003) 424:88–93. 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- 108.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. (2009) 21:8–13. 10.1016/j.smim.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 109.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity (2004) 21:527–38. 10.1016/j.immuni.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 110.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. (2004) 172:4875–82. 10.4049/jimmunol.172.8.4875 [DOI] [PubMed] [Google Scholar]
- 111.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity (2006) 25:511–20. 10.1016/j.immuni.2006.06.019 [DOI] [PubMed] [Google Scholar]
- 112.Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, et al. KLRG1(+) effector CD8(+) T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity (2018) 48:716–29.e18. 10.1016/j.immuni.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gao YL, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol. (2017) 18:1004–15. 10.1038/ni.3800 [DOI] [PubMed] [Google Scholar]
- 114.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature (2012) 483:227–31. 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science (2001) 291:2413–7. 10.1126/science.1058867 [DOI] [PubMed] [Google Scholar]
- 116.Kaufman DR, Liu J, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, et al. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. (2008) 181:4188–98. 10.4049/jimmunol.181.6.4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robinette ML, Bando JK, Song W, Ulland TK, Gilfillan S, Colonna M. IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat Commun. (2017) 8:14601. 10.1038/ncomms14601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature (2010) 463:540–4. 10.1038/nature08636 [DOI] [PubMed] [Google Scholar]
- 119.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. (2010) 207:273–80. 10.1084/jem.20092029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klose CSN, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell (2014) 157:340–56. 10.1016/j.cell.2014.03.030 [DOI] [PubMed] [Google Scholar]
- 121.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. (2005) 174:1213–21. 10.4049/jimmunol.174.3.1213 [DOI] [PubMed] [Google Scholar]
- 122.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood (2003) 101:4887–93. 10.1182/blood-2002-11-3392 [DOI] [PubMed] [Google Scholar]
- 123.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. (2000) 191:771–80. 10.1084/jem.191.5.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. (2006) 7:1217–24. 10.1038/ni1395 [DOI] [PubMed] [Google Scholar]
- 125.Schenkel JM, Fraser KA, Masopust D. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol. (2014) 192:2961–4. 10.4049/jimmunol.1400003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J Immunol. (2009) 183:1044–54. 10.4049/jimmunol.0900420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Klose CS, Blatz K, d'Hargues Y, Hernandez PP, Kofoed-Nielsen M, Ripka JF, et al. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8alphaalpha(+) intraepithelial lymphocyte development. Immunity (2014) 41:230–43. 10.1016/j.immuni.2014.06.018 [DOI] [PubMed] [Google Scholar]
- 128.Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol. (2011) 85:4085–94. 10.1128/JVI.02493-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget (2017) 8:7175–80. 10.18632/oncotarget.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Finn OJ. A believer's overview of cancer immunosurveillance and immunotherapy. J Immunol. (2018) 200:385–91. 10.4049/jimmunol.1701302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. (2006) 90:1–50. 10.1016/S0065-2776(06)90001-7 [DOI] [PubMed] [Google Scholar]
- 132.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity (2004) 21:137–48. 10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- 133.Efremova M, Finotello F, Rieder D, Trajanoski Z. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front Immunol. (2017) 8:1679. 10.3389/fimmu.2017.01679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wirth TC, Kuhnel F. Neoantigen targeting-dawn of a new era in cancer immunotherapy? Front Immunol (2017) 8:1848. 10.3389/fimmu.2017.01848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell (2015) 27:450–61. 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359:1350–5. 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) 348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martin SD, Brown SD, Wick DA, Nielsen JS, Kroeger DR, Twumasi-Boateng K, et al. Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS ONE (2016) 11:e0155189. 10.1371/journal.pone.0155189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science (2008) 319:215–20. 10.1126/science.1148886 [DOI] [PubMed] [Google Scholar]
- 140.Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell (2012) 21:402–17. 10.1016/j.ccr.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun. (2017) 8:15221. 10.1038/ncomms15221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol. (2017) 2:eaam6346. 10.1126/sciimmunol.aam6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martinez-Cano S, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) T cells. Nat Commun. (2017) 8:16073. 10.1038/ncomms16073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Milner JJ, Toma C, Yu BF, Zhang K, Omilusik K, Phan AT, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours (vol 552, pg 253, 2017). Nature (2018) 554:393 10.1038/nature25445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, et al. CD8(+) CD103(+) tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. (2015) 194:3475–86. 10.4049/jimmunol.1402711 [DOI] [PubMed] [Google Scholar]
- 146.Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. CD103 and intratumoral immune response in breast cancer. Clin Cancer Res. (2016) 22:6290–7. 10.1158/1078-0432.CCR-16-0732 [DOI] [PubMed] [Google Scholar]
- 147.Komdeur FL, Prins TM, van de Wall S, Plat A, Wisman GBA, Hollema H, et al. CD103+tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology (2017) 6:e1338230. 10.1080/2162402X.2017.1338230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. (2017) 18:940–50. 10.1038/ni.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murray T, Marraco SAF, Baumgaertner P, Bordry N, Cagnon L, Donda A, et al. Very late antigen-1 marks functional tumor-resident cD8 T cells and correlates with survival of melanoma patients. Front Immunol. (2016) 7:573. 10.3389/fimmu.2016.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell (2018) 174:1293–308.e36. 10.1016/j.cell.2018.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. (2018) 24:986–93. 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 152.Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. (2018) 24:978–85. 10.1038/s41591-018-0045-3 [DOI] [PubMed] [Google Scholar]
- 153.Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. (2018) 69:301–18. 10.1146/annurev-med-012017-043208 [DOI] [PubMed] [Google Scholar]
- 154.Koh J, Kim S, Kim MY, Go H, Jeon YK, Chung DH. Prognostic implications of intratumoral CD103(+) tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget (2017) 8:13762–9. 10.18632/oncotarget.14632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Webb JR, Milne K, Watson P, deLeeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. (2014) 20:434–44. 10.1158/1078-0432.CCR-13-1877 [DOI] [PubMed] [Google Scholar]
- 156.Cheuk S, Schlums H, Gallais Serezal I, Martini E, Chiang SC, Marquardt N, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity (2017) 46:287–300. 10.1016/j.immuni.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: more than a marker for cytotoxicity? Front Immunol (2017) 8:892. 10.3389/fimmu.2017.00892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, et al. Systematic characterization of human CD8(+) T cells with natural killer cell markers in comparison with natural killer cells and normal CD8(+) T cells. Immunology (2001) 103:281–90. 10.1046/j.1365-2567.2001.01248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cohavy O, Targan SR. CD56 marks an effector T cell subset in the human intestine. J Immunol. (2007) 178:5524–32. 10.4049/jimmunol.178.9.5524 [DOI] [PubMed] [Google Scholar]
- 160.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. (2001) 13:459–63. 10.1093/intimm/13.4.459 [DOI] [PubMed] [Google Scholar]
- 161.Glasner A, Ghadially H, Gur C, Stanietsky N, Tsukerman P, Enk J, et al. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J Immunol. (2012) 188:2509–15. 10.4049/jimmunol.1102461 [DOI] [PubMed] [Google Scholar]
- 162.Halfteck GG, Elboim M, Gur C, Achdout H, Ghadially H, Mandelboim O. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J Immunol. (2009) 182:2221–30. 10.4049/jimmunol.0801878 [DOI] [PubMed] [Google Scholar]
- 163.Glasner A, Levi A, Enk J, Isaacson B, Viukov S, Orlanski S, et al. NKp46 receptor-mediated interferon-gamma production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity (2018) 48:107–119.e4. 10.1016/j.immuni.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 164.Jiao YH, Huntington ND, Belz GT, Seillet C. Type 1 innate lymphoid cell biology: lessons learnt from natural killer cells. Front Immunol. (2016) 7:426. 10.3389/fimmu.2016.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cortez VS, Ulland TK, Cervantes-Barragan L, Bando JK, Robinette ML, Wang QL, et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat Immunol. (2017) 18:995–1003. 10.1038/ni.3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dadi S, Li MO. Tissue-resident lymphocytes: sentinel of the transformed tissue. J Immunother Cancer (2017) 5:41. 10.1186/s40425-017-0244-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Levi I, Amsalem H, Nissan A, Darash-Yahana M, Peretz T, Mandelboim O, Rachmilewitz J. Characterization of tumor infiltrating natural Killer cell subset. Oncotarget (2015) 6:1–9. 10.18632/oncotarget.3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Carrega P, Ferlazzo G. Natural killers are made not born: how to exploit nk cells in lung malignancies. Front Immunol. (2017) 8:277 10.3389/fimmu.2017.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Michel T, Poli A, Cuapio A, Briquemont B, Iserentant G, Ollert M, et al. Human CD56(bright) NK cells: an update. J Immunol. (2016) 196:2923–31. 10.4049/jimmunol.1502570 [DOI] [PubMed] [Google Scholar]
- 170.Cristiani CM, Palella E, Sottile R, Tallerico R, Garofalo C, Carbone E. Human NK cell subsets in pregnancy and disease: toward a new biological complexity. Front Immunol. (2016) 7:656. 10.3389/fimmu.2016.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. (2013) 4:499. 10.3389/fimmu.2013.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HYC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. (2006) 203:1033–43. 10.1084/jem.20052507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med. (2012) 90:55–66. 10.1007/s00109-011-0806-7 [DOI] [PubMed] [Google Scholar]
- 174.Rusakiewicz S, Perier A, Semeraro M, Pitt JM, von Strandmann EP, Reiners KS, et al. NKp30 isoforms and NKp30 ligands are predictive biomarkers of response to imatinib mesylate in metastatic GIST patients. Oncoimmunology (2017) 6:e1137418. 10.1080/2162402X.2015.1137418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. (2011) 71:5412–22. 10.1158/0008-5472.CAN-10-4179 [DOI] [PubMed] [Google Scholar]
- 176.Lowry LE, Zehring WA. Potentiation of natural killer cells for cancer immunotherapy: a review of literature. Front Immunol. (2017) 8:1061. 10.3389/fimmu.2017.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fang F, Xiao WH, Tian ZG. NK cell-based immunotherapy for cancer. Semin Immunol. (2017) 31:37–54. 10.1016/j.smim.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 178.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. (2016) 17:1025–36. 10.1038/ni.3518 [DOI] [PubMed] [Google Scholar]
- 179.de Andrade LF, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science (2018) 359:1537–42. 10.1126/science.aao0505 [DOI] [PMC free article] [PubMed] [Google Scholar]