First-line raltegravir-containing combination antiretroviral therapy in 30 patients living with HIV-2 was well tolerated and yielded a median CD4 gain at 1 year of 87 cells/µL, and HIV-2 RNA <5 copies/µL in 13 of 15 participants.
Keywords: first line-cART, HIV-2, integrase inhibitor
Abstract
Background
New options for first-line treatment of human immunodeficiency virus type 2 (HIV-2) infection are needed. We evaluated an integrase inhibitor (raltegravir)–containing regimen.
Methods
Antiretroviral therapy (ART)–naive adults with symptomatic infection by HIV-2 only, CD4 count <500 cells/μL or CD4 decrease >50 cells/μL/year over the past 3 years, or a confirmed plasma HIV-2 RNA (pVL) load ≥100 copies/mL were eligible for this noncomparative trial. The composite primary endpoint was survival at 48 weeks without any of the following: CD4 gain from baseline <100 cells/μL, confirmed pVL ≥40 copies/mL from week 24, raltegravir permanent discontinuation, or incident B or C event. HIV-2 ultrasensitive pVL (uspVL) and total DNA were assessed using in-house polymerase chain reaction (PCR) assays.
Results
Baseline median CD4 count of 30 enrolled individuals (67% women) was 436 cells/µL (interquartile range [IQR], 314–507 cells/µL); pVL was ≥40 copies/mL in 67% of them, uspVL was ≥5 copies/mL in 92%, and total DNA was >6 copies by PCR in 32%. At week 48, the composite endpoint of success was reached in 40% [95% confidence interval, 22.7%–59.4%]. Failure was mainly (50%) due to CD4 gain <100 cells/µL; uspVL was <5 copies/mL in 87% and total DNA >6 copies by PCR in 12% of participants. Median CD4 gain was 87 cells/µL (IQR, 38–213 cells/µL; n = 28). No serious adverse reactions were reported.
Conclusions
Raltegravir-containing ART is a safe option for first-line treatment of HIV-2 infection, yielding a comparable success rate to protease inhibitors.
Clinical Trials Registration
Like human immunodeficiency virus type 1 (HIV-1), human immunodeficiency virus type 2 (HIV-2) causes AIDS-defining events, yet immunodeficiency and disease progression is far slower [1, 2]. Indeed, based on the definition of long-term nonprogressors used in HIV-1 infection, the proportion of people living with HIV-2 (PLWH2) meeting this profile was reported to be up to 6%, as compared to <1% among people living with HIV-1 (PLWH1) [3]. Moreover, HIV-2 is characterized by a lower replication capacity than HIV-1, that is, approximately 30 times less as shown by modeling [4–7], yielding 9% of patients meeting definition criteria for elite controllers [3].
In terms of treatment, HIV-2 is naturally resistant to 2 antiretroviral drug classes commonly used to treat HIV-1 infection, namely, nonnucleoside reverse transcriptase inhibitors and fusion inhibitors [8]. Furthermore, phenotypic studies suggest that HIV-2 susceptibility to protease inhibitors (PIs) varies according to the specific drug tested [9]. Thus, the limited armamentarium of drugs available to manage HIV-2 infection calls for evaluation of every innovation when it becomes available in HIV-1 infection.
Geographically, the HIV-2 epidemic is restricted mainly to West Africa, Angola, Mozambique, and Europe (mostly Portugal or France, due to migratory flows from affected African regions) as HIV-2 is less efficiently transmitted than HIV-1, essentially through sexual or mother-to-child transmission [10–12]. In this context, high level of evidence for the treatment of HIV-2 remains scarce and powerful designs such as randomized clinical trials are difficult to implement.
In addition, while disease profile includes traditional determinants of progression such as clinical stage, CD4 lymphocyte count, and plasma viral load (pVL), the latter is much lower on average than in HIV-1 infection and below the detection threshold in very large proportions of untreated PLWH2 [10, 13–15]. Therefore, evaluation of treatment innovations cannot predominantly rely on the measurement of pVL for eligibility and outcome, and CD4 cell response should also be considered within a composite endpoint [16].
Given the lack of randomized trials, observational studies currently provide the best available evidence for treatment guidelines, including a combination of 2 nucleoside reverse transcriptase inhibitors (NRTIs) and 1 PI as recommended first-line combination antiretroviral therapy (cART) since 2012 [17–21].
Current first-line cART yields lower response in terms of CD4 cell count in PLWH2 than in PLWH1, even with regimens including recently approved PIs [14, 19, 22–26].
More recently, 2 series of observations raised a specific interest for the evaluation of raltegravir in antiretroviral-naive PLWH2. First, phenotypic virological studies showed that the optimal inhibitory concentration 50% (IC50) for HIV-2 were those of lopinavir, darunavir, and raltegravir [9, 27]. Second, raltegravir was associated with dramatic immune recovery in some heavily pretreated patients experiencing virological failure [28–32].
We postulated that a combination of raltegravir plus 2 NRTIs could yield a better CD4 response than the average response reported with PI-containing regimens, along with a good safety profile.
METHODS
Trial Design and Participants
The French National Agency for Research on AIDS and Viral Hepatitis (ANRS) 159 HIV-2 study was a noncomparative multicenter, nationwide, 1-step, phase 2 clinical trial, conducted in 18 hospital centers in France, from July 2012 to December 2015.
We used the setting of the French ANRS CO5 HIV-2 cohort, involving >1000 adults infected with HIV-2 only in 120 clinical sites, to identify potentially eligible patients, and investigators invited them to participate in the study. Eligibility criteria for the trial were the following: antiretroviral-naive PLWH2 with a history of Centers for Disease Control and Prevention group B or C–defining event, CD4 count <500 cells/μL, or CD4 decrease >50 cells/μL/year over the last 3 years, with a last value of ±10% of CD4 nadir or confirmed HIV-2 RNA pVL ≥100 copies/mL. Pneumocystis prophylaxis, combined with toxoplasmosis prophylaxis in the presence of specific antibodies, was an additional eligibility criteria for participants with CD4 count <200 cells/μL. By April 2013, the scientific committee of the trial recommended that women who had started a antiretroviral therapy (ART) other than an integrase inhibitor (INSTI)–containing combination during pregnancy for prevention of mother-to-child transmission and stopped it at the time of delivery could be further included if they met other eligibility criteria.
Noneligibility criteria were absence of effective contraception method in women of childbearing age; pregnancy or planning a pregancy; breastfeeding; progressive opportunistic infection with curative treatment not compatible with that of the trial; malignancy requiring chemotherapy or radiotherapy; chronic hepatitis C with Metavir score F2 or greater, decompensated cirrhosis; uncontrolled insulin-dependent diabetes mellitus; corticosteroid treatment >3 weeks; hemoglobinemia <7 g/dL, polynuclear neutrophil count <500/µL, platelet count <50 000/µL, creatinine clearance <50 mL/minute, aminotransferases or alkaline phosphatases or bilirubin >2.5 times the upper limit of normal laboratory range; contraindication to 1 of the excipients of study treatments; judicial protection; or participation in any other medication trial.
The protocol was approved according to French regulation by an Ethics Committee (Comité de Protection des Personnes de Paris Ile de France XI). The trial was performed in accordance with good clinical practices and the ethical principles of the Declaration of Helsinki. All participants provided signed informed consent. Data were regularly reviewed by an independent data monitoring committee.
Study Treatment, Design, and Procedures
Study treatment consisted of emtricitabine 200 mg/day, tenofovir disoproxil fumarate 300 mg/day, and raltegravir 400 mg twice daily from week (W) 0 to W48.
The primary endpoint was a composite criterion, defined as the proportion of participants in therapeutic success, that is, surviving at W48 without any of the following events from trial start: CD4 gain <100 cells/μL at W48 compared to the baseline CD4 count (mean W –4, W0), pVL ≥40 copies/mL from W24 confirmed within the next 4 weeks, raltegravir permanent discontinuation, or new B or C event validated by a dedicated independent data monitoring committee.
By April 2013, as a pVL quantification assay with a detection threshold of 40 copies/mL became available, the scientific committee of the trial recommended that the detection threshold of pVL used for the virological endpoint of the composite outcome be lowered from 100 copies/mL to 40 copies/mL.
The component related to discontinuation of raltegravir was included to reflect the potential of durability of this first-line regimen, as the number of potential third antiretroviral agents to be associated to NRTIs is very limited in HIV-2 infection.
The main secondary endpoint was the mean change in CD4 lymphocyte count between baseline (mean of W –4 and W0 count) and W12. Other endpoints included evolution of the rate of participants with undetectable ultrasensitive pVL (uspVL), with undetectable total HIV-2 DNA from baseline to W48, respectively; change in CD4 percentage, pVL, and total DNA values from baseline to W48; description of the resistance mutations selected (number and type of mutations in the reverse transcriptase [RT] and the integrase genes) compared to W0, in case of virological failure; rate of clinical progression (from stage A to B, C, or death and from stage B to C or death); adherence; rate of treatment switch or discontinuation (overall and for each study drug); safety (number, nature, severity and time to adverse event [AE]).
Clinical examination and laboratory sampling, from which CD4 cell counts and pVL were performed, were performed at screening (W –4), baseline (W0), W4, W8, W12, W18, W24, W36, and W48.
Ultrasensitive pVL quantification was performed at W0, W24, and W48 using real-time reverse-transcription PCR assay with a threshold between 5 and 10 copies/mL depending on the volume of plasma available (Biocentric, Bandol, France) [33].Total HIV-2 DNA quantification was performed at the same time points using a real-time PCR assay with a threshold of 6 copies developed by the ANRS AC-11 HIV Quantification group [34].
Resistance-associated mutations to NRTIs and INSTIs were assessed at screening and at time of virological failure, defined as the first pVL value ≥40 copies/mL (confirmed within the following 4 weeks) after a value <40 copies/mL, by sequencing the RT and integrase genes from plasma specimens using the consensus technique of the ANRS AC11 Resistance Group (www.hivfrenchresistance.org). HIV-2 resistance mutations were identified using the list generated by the Collaborative HIV and Anti-HIV Drug Resistance Network [33, 35].
Adherence to treatment was assessed by the ANRS self-administered questionnaire of adherence at W4, W12, W24, W36, and W48, by trough plasma concentrations of antiretroviral drug measurement in case of virological failure, and by checking the number of treatment trial pills at each dispensing visit.
Clinical and laboratory AEs were graded using the ANRS Scale for Grading Adult Adverse Events (grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening).
Statistical Methods
The primary efficacy analysis was an intention-to-treat analysis, which included all participants who received at least 1 dose of emtricitabine/tenofovir and raltegravir, regardless of treatment changes during the trial. Missing data were considered as failure. We also performed the analysis on available data. Data were analyzed with SAS software (version 9.2 and higher).
RESULTS
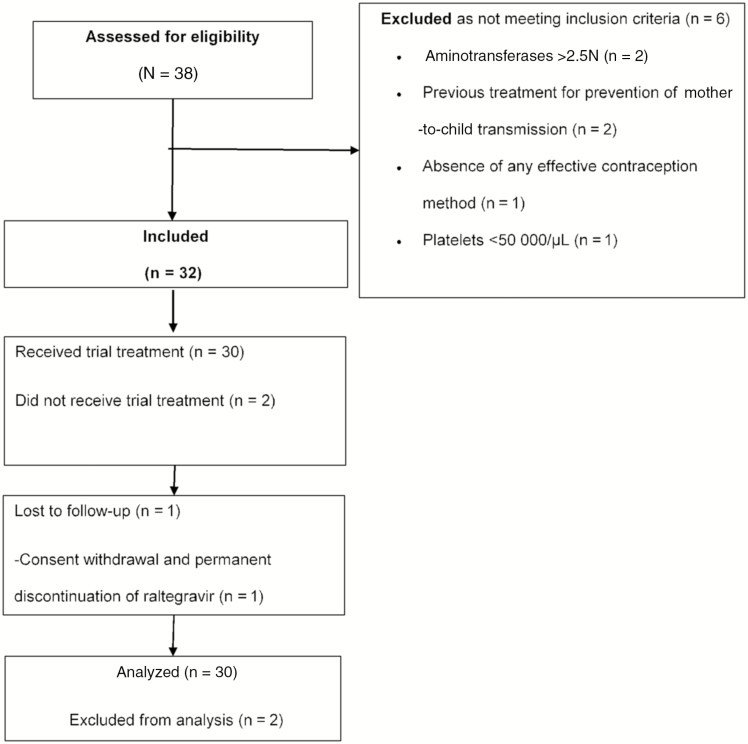
Among the 32 participants included in the study, 30 received at least 1 dose of the study drugs and were included in the intention-to-treat analysis. Twenty-eight participants (93%) completed the W48 follow-up: 1 participant withdrew consent (while reporting a permanent discontinuation of raltegravir) and 1 participant was lost to follow-up (Figure 1).
Figure 1.
Flow diagram of the ANRS (France REcherche Nord&Sud Sida-Hiv Hépatites) 159 HIV-2 trial.
Baseline Characteristics
Two-thirds of participants were women and most of them originated from West Africa. They had a long history with HIV-2 infection while remaining at the asymptomatic stage with rather high baseline CD4 nadir. Baseline median CD4 count was >400 cells/µL and reached at least 500 cells/µL in one-quarter of the participants. Median pVL was 2.5 log10 copies/mL in 20 participants with pVL ≥40 copies/mL (Table 1).
Table 1.
Baseline Characteristics of the Participants in the ANRS (France REcherche Nord&Sud Sida-Hiv Hépatites) 159 HIV-2 Trial (N = 30)
| Characteristic | No. (%) |
|---|---|
| Female sex | 20 (66.7) |
| Age, y, median (IQR) | 49 (46.0–52.6) |
| <35 | 1 (3.3) |
| 35–44 | 5 (16.7) |
| 45–54 | 17 (56.7) |
| 55–64 | 4 (13.3) |
| ≥65 | 3 (10.0) |
| Place of birth | |
| West Africa | 26 (86.7) |
| France | 4 (13.3) |
| HIV-2 transmission mode | |
| Heterosexual contact | 23 (76.7) |
| Transfusion | 4 (13.3) |
| Unknown | 3 (10.0) |
| Time since HIV-2 diagnosis, y, median (IQR) | 11 (7.5–13.9) |
| CDC stage (week –4) | |
| A | 26 (86.7) |
| B | 2 (6.7) |
| C | 2 (6.6) |
| Nadir CD4 count, cells/µL, median (IQR) | 351 (286.0–455.0) |
| CD4 count, cells/µL (week –4, week 0), median (IQR) | 436 (314–507) |
| <200 | 3 (10.0) |
| 200–349 | 6 (20.0) |
| 350–499 | 13 (43.3) |
| ≥500 | 8 (26.7) |
| CD4/CD8 ratio (week 0), median (IQR) | 0.9 (0.6–1.2) |
| Plasma HIV-2 RNA ≥40 copies/mL (week 0) | 20 (66.7) |
| Plasma HIV-2 RNA ≥5 copies/mL (week 0) | 23 (92.0) |
| Plasma HIV-2 RNA (week 0), log10 copies/mL, median (IQR) | 2.5 (1.8–3.2) |
| Total HIV-2 DNA (week 0) >6 copies (by PCR) | 8 (32.0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CDC, Centers for Disease Control and Prevention; HIV-2, human immunodeficiency virus type 2; IQR, interquartile range; PCR, polymerase chain reaction.
Outcomes
The proportion of participants declaring moderate to good adherence between W4 and W48 ranged from 76% to 83%.
At W48, the composite endpoint of success was reached in 12 of 30 participants (40% [95% confidence interval {CI}, 22.7%–59.4%]). Failure was mainly due to not reaching the immunological criterion of the endpoint, that is, CD4 gain <100 cells/µL from baseline (n = 15), then to virological failure, that is, pVL ≥40 copies/mL (n = 1) or to withdrawal before W48 (n = 2).
In terms of immunological response, the median CD4 count change in the 15 patients with a gain <100 cells/µL was +38 cells/µL; a decrease in CD4 cell count was observed in 5 of them (overall in 16.7% of participants).
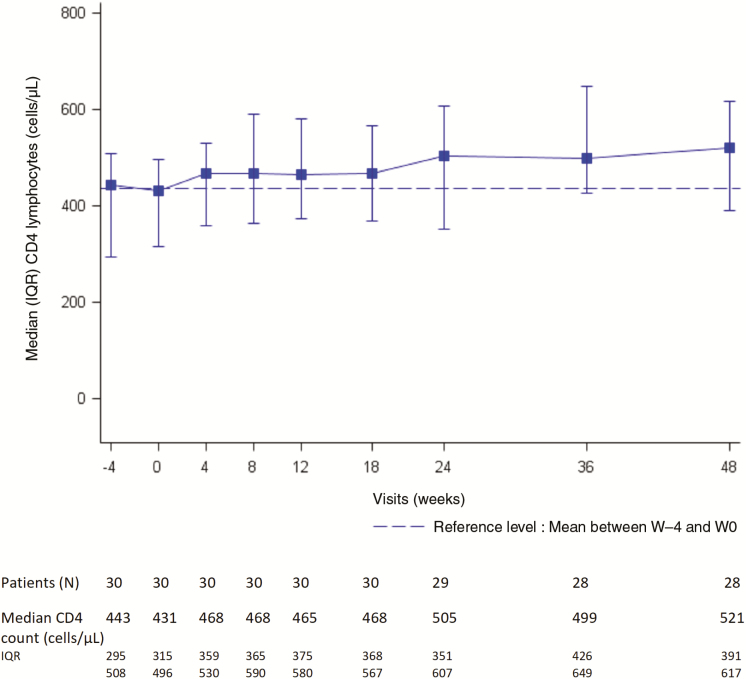
Among the 22 participants with baseline CD4 count <500 cells/µL and the 8 with CD4 count ≥500 cells/µL, 36% (95% CI, 17.2%–59.3%]) and 50% (95% CI, 15.7%–84.3%]) experienced treatment success, respectively. Overall, median CD4 count change at W12 and W48 was +73 cells/µL (IQR, +2 to +143 cells/µL; n = 30) and +87 cells/µL (IQR, +38 to +213 cells/µL; n = 28), respectively (Figure 2).
Figure 2.
Median CD4 cell count from week (W) 0 to week 48, ANRS (France REcherche Nord&Sud Sida-Hiv Hépatites) 159 HIV-2 trial. Median CD4 change at W12 and W48: +73/µL (interquartile range [IQR], +2 to +143) (n = 30) and +87/µL (IQR, +38 to +213) (n = 28), respectively. At W48, median CD4 change: +115/µL and +70/µL in those with baseline plasma viral load ≥40 and <40 copies/mL, respectively. Mean CD4 count between W –4 and W0 was 437 cells/µL and is represented by the dotted horizontal line.
At W48, median CD4 count change was +115 cells/µL and +70 cells/µL in participants with baseline pVL ≥40 and <40 copies/mL, respectively. Median baseline CD4 count was 465 cells/µL (IQR, 406–658 cells/µL) in participants gaining at least 100 CD4 cells/µL at W48 vs 414 (IQR, 292–480 cells/µL) in those with immunologic failure. Furthermore, among the 28 participants followed up to W48, the CD4 cell count increased to ≥500 cells/µL in 8 of the 21 with baseline count <500 cells/µL at baseline, and to ≥350 cells/µL in 4 of the 9 with baseline CD4 count <350 cells/µL. Median CD4 percentage was 33% (IQR, 24.5%–37%) at baseline and 36% (IQR, 29%–42%) at W48, resulting in a median change of +3% (IQR, +4.5% to +5%).
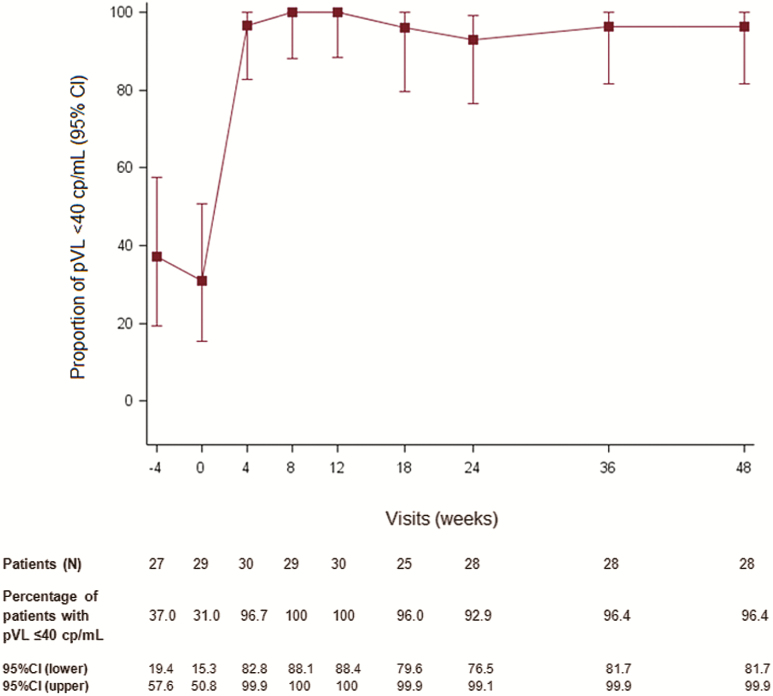
In terms of virological response, at W48, 27 of 28 participants who completed the 48-week follow-up had pVL <40 copies/mL (Figure 3). Virological failure was reported in 1 patient, with a pVL of 3.3 log10 copies/mL at W18 who reported good adherence in dedicated self-questionnaire. Antiretroviral plasma concentrations measured on W18 and W20 samples were in the expected range, but the number of pills brought back to hospital was smaller than expected, suggesting incorrect adherence between W8 and W12. At time of virological failure, drug resistance mutations were evidenced in the integrase region (E92Q, T97A, and Y143C/G/H/R), but no resistance mutation was detected in the RT region.
Figure 3.
Proportion of participants with plasma human immunodeficiency virus type 2 RNA <40 copies/mL from week –4 to week 48, ANRS (France REcherche Nord&Sud Sida-Hiv Hépatites) 159 HIV-2 trial. Abbreviations: CI, confidence interval; lower, lower bound; pVL, plasma viral load; upper, upper bound.
In the analysis considering only participants with available data at W48 (n = 29), treatment success was reported in 12 of 29 (41.4% [95% CI, 23.5%–61.1%]).
At W48, among those participants with available data for these specific secondary endpoints, uspVL was <5 copies/mL in 13 of 15 participants (87%) and HIV-2 total DNA was >6 copies by PCR in 3 of 26 (12%). In the sole case of virological failure, DNA was unchanged at the time of failure (W24) as compared to W0.
Fifty-six AEs were reported in 20 participants and were mainly mild to moderate; 50% of these AE were reported in 3 participants. Among the 5 grade 3 clinical AEs (4 participants; hysterectomy [1]; treatment trial overdosing without toxicity [1]; bronchopulmonary carcinoma [1]; intestinal functional disorder [1]; gastrointestinal disorder [1]), only 1 AE was related to study treatment: gastrointestinal disorder at day 1, leading to a switch from emtricitabine/tenofovir to lamivudine/abacavir, followed by complete resolution. A third of clinical AEs (n = 17 [30.4%]) were gastrointestinal disorders: nausea (5), abdominal pain (2), others (10) (1 each). None of the participants experienced grade 4 AEs. Four severe AEs occurred in 3 participants: lacunar ischemic stroke (n = 1), Helicobacter pylori gastritis/Escherichia coli cystitis/functional colopathy (n = 1), treatment trial overdosing without toxicity (n = 1), and suspected recurrence of bronchopulmonary cancer (n = 1), but none was related to antiretrovirals. There was no death nor new B or C event reported over the trial duration. One pregnancy was reported in a participant who never received the study treatments.
DISCUSSION
In this noncomparative trial, a first line cART-containing raltegravir yielded a complete success rate of 40% at 1 year, comparable to PI-containing regimens, and was well tolerated. Ultrasensitive pVL and total DNA were undetectable in most participants.
Improving evidenced-based treatment of HIV-2 infection is very challenging in terms of trial designs. First, the best design to inform evidence-based guidelines is controlled randomized trials. Nevertheless, due to the limited number of people living with HIV-2 in western and northern regions of the globe, such trials can only be conducted in West Africa [16, 36], and it is therefore useful to also consider observational studies or pilot trials to inform guidelines for clinical decision or further research. Our pilot trial was indeed the first step of designing an ongoing randomized trial in West Africa within the ANRS network (ANRS 12294, ClinicalTrials.gov identifier NCT02150993).
Second, plasma HIV-2 RNA level above the routine detection limit cannot reasonably be used as the sole or main inclusion criterion as it pVL is quantifiable in fewer PLWH2 than in PLWH1; for example, in the ANRS nationwide HIV-2 cohort, no more than 19% of patients present with pVL >100 copies/mL at enrollment (Sophie Matheron, personal communication). Therefore, other markers of disease progression should be considered, such as clinical staging, or CD4 count. Immunological progression remains slower than that of HIV-1, thus relevant decline of CD4 slope in the context of HIV-2 seems more appropriate [17]. Third, a composite endpoint is useful to optimize the power of trials. Therefore, our composite primary endpoint included clinical progression and CD4 gain in addition to virological outcome. Retention in the first-line cART regimen also seemed highly relevant as the number of potent antiretroviral drugs is far smaller than for HIV-1; moreover, a high level of NRTI cross-resistance impacts this drug class more rapidly [37].
Using a similar composite endpoint, PI-containing cART in antiretroviral-naive patients yielded a global success rate of 45% (95% CI, 31%–60%) at 12 months in an European retrospective study, the immunological component of the composite endpoint being the main cause of failure, with a median increase of 76–88 cells/µL/year after at least 3 months of treatment [24].
Based (1) on available data on raltegravir-containing cART in PLWH1, with a mean CD4 cell increase of 144 cells/µL at W48 [21], (2) on median values of raltegravir IC50 and IC90 reported in 14 PLWH2 (2.4 nM and 12.5 nM, respectively) [27], and (3) on case reports on immunovirological outcomes of this cART regimen in 3 PLWH2 with an history of multiple treatment failure [27, 29, 30, 32], we prioritized this antiretroviral combination to be evaluated in a pilot trial before consideration as experimental arm in a randomized trial.
The median CD4 cell change in response to treatment (+87 cells/µL at 1 year), even if lower than in HIV-1, was close to that recently reported with another first-line INSTI-containing cART in Senegal [38]. In addition, we showed a trend for higher success rate among PLWH2 starting with baseline CD4 count ≥500 cells/µL than with CD4 <200 cells/µL, pleading for an early start of cART. Our results also demonstrate the virological strength of a raltegravir-containing regimen to reach high levels of undetectable pVL and HIV DNA. These results, along with absence of any serious treatment-related side effects, are current arguments for recommending INSTI-containing regimen as first-line cART, at the same level than PI-containing ones. So far, the randomized trial comparing these regimens is ongoing in West Africa and will provide more information on the preferred one.
Notes
Acknowledgments. We thank A. Diallo, A. Dumont, V. Petrov-Sanchez (INSERM-ANRS); B. Autran (Scientific Committee); D. Costagliola, H. Fischer, A.-G. Marcelin, F. Raffi (Independent Committee); M.-L. Chaix, P.-M. Girard, O. Lortholary, A.-M. Taburet (Independent Data Monitoring Committee); N. Chaghil, L. Pinoges, E. Rouch (Clinical Trials Unit). We also thank all of the patients who agreed to participate in this trial, and all the investigators involved in the ANRS CO5 VIH2 cohort.
Financial support. This work was supported by INSERM-ANRS, sponsor of the trial; Merck Sharp Dohme-Chibret; and Gilead Sciences.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
ANRS 159 HIV-2 Trial Study Group. Hôpital Bichat, Paris: S. Matheron; Hôpital Antoine Béclère, Clamart: F. Boue; Hôpital Bicêtre, Paris: C. Goujard; Hôpital Européen Georges Pompidou, Paris: L. Weiss; Hôpital Lariboisière, Paris: A. Rami; Hôpital Louis Mourier, Colombes: E. Mortier; Hôpital Pitié-Salpêtrière, Paris: R. Tubiana; Hôpital Saint Antoine: P. Campa; Hôpital Saint Louis, Paris: D. Ponscarme; Hôpital Bocage, Dijon: L. Piroth; Hôpital la Croix Rousse, Lyon: P. Miailhes; Hôpital Gui de Chauliac, Montpellier: J. Reynes; Centre Hospitalier René Dubos, Pontoise: L. Blum; Hôpital Delafontaine, Saint Denis: M.-A. Khuong; CHI Villeneuve Saint Georges: O. Patey; CHI Créteil: B. Elharrar; C. H. Mulhouse: G. Beck-Wirth; CHU Angers: P. Fialaire. INSERM-ANRS: I. Amri; F. Cardon; L. Marchand.
Contributor Information
France REcherche Nord&Sud Sida-Hiv Hépatites (ANRS) 159 HIV-2 Trial Study Group:
S Matheron, F Boue, C Goujard, L Weiss, A Rami, E Mortier, R Tubiana, P Campa, D Ponscarme, L Piroth, P Miailhes, J Reynes, L Blum, M -A Khuong, O Patey, B Elharrar, G Beck-Wirth, P Fialaire, I Amri, F Cardon, and L Marchand
References
- 1. Marlink R, Kanki P, Thior I et al. . Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 1994; 265:1587–90. [DOI] [PubMed] [Google Scholar]
- 2. Whittle H, Morris J, Todd J et al. . HIV-2-infected patients survive longer than HIV-1-infected patients. AIDS 1994; 8:1617–20. [DOI] [PubMed] [Google Scholar]
- 3. Thiébaut R, Matheron S, Taieb A, Brun-Vezinet F, Chêne G, Autran B; Immunology Group of the ANRS CO5 HIV-2 Cohort Long-term nonprogressors and elite controllers in the ANRS CO5 HIV-2 cohort. AIDS 2011; 25:865–7. [DOI] [PubMed] [Google Scholar]
- 4. Ekouévi DK, Avettand-Fènoël V, Tchounga BK et al. . IeDEA West Africa Collaboration Plasma HIV-2 RNA according to CD4 count strata among HIV-2-infected adults in the IeDEA West Africa Collaboration. PLoS One 2015; 10:e0129886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacNeil A, Sarr AD, Sankalé JL, Meloni ST, Mboup S, Kanki P. Direct evidence of lower viral replication rates in vivo in human immunodeficiency virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol 2007; 81:5325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Popper SJ, Sarr AD, Guèye-Ndiaye A, Mboup S, Essex ME, Kanki PJ. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J Virol 2000; 74:1554–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simon F, Matheron S, Tamalet C et al. . Cellular and plasma viral load in patients infected with HIV-2. AIDS 1993; 7:1411–7. [DOI] [PubMed] [Google Scholar]
- 8. Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther 2004; 9:57–65. [PubMed] [Google Scholar]
- 9. Desbois D, Roquebert B, Peytavin G et al. . French ANRS HIV-2 Cohort (ANRS CO 05 VIH-2) In vitro phenotypic susceptibility of human immunodeficiency virus type 2 clinical isolates to protease inhibitors. Antimicrob Agents Chemother 2008; 52:1545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell-Yesufu OT, Gandhi RT. Update on human immunodeficiency virus (HIV)-2 infection. Clin Infect Dis 2011; 52:780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottlieb GS, Hawes SE, Agne HD et al. . Lower levels of HIV RNA in semen in HIV-2 compared with HIV-1 infection: implications for differences in transmission. AIDS 2006; 20:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Visseaux B, Damond F, Matheron S, Descamps D, Charpentier C. HIV-2 molecular epidemiology. Infect Genet Evol 2016; 46:233–40. [DOI] [PubMed] [Google Scholar]
- 13. Andersson S, Norrgren H, da Silva Z et al. . Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa: significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch Intern Med 2000; 160:3286–93. [DOI] [PubMed] [Google Scholar]
- 14. Drylewicz J, Matheron S, Lazaro E et al. . Comparison of viro-immunological marker changes between HIV-1 and HIV-2-infected patients in France. AIDS 2008; 22:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Popper SJ, Sarr AD, Travers KU et al. . Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis 1999; 180:1116–21. [DOI] [PubMed] [Google Scholar]
- 16. Matheron S. HIV-2 infection: a call for controlled trials. AIDS 2008; 22:2073–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. French HIV Experts Group. Prise en charge médicale des personnes vivant avec le VIH—recommandations du groupe d’experts. Sous la direction du Pr Philippe Morlat et sous l’égide du CNS et de l’ANRS Available at: http://cns.sante.fr/actualites/prise-en-charge-du-vih-recommandations-du-groupe-dexperts/. Accessed 8 April 2018.
- 18. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: WHO, 2013. [PubMed] [Google Scholar]
- 19. Ekouevi DK, Tchounga BK, Coffie PA et al. . Antiretroviral therapy response among HIV-2 infected patients: a systematic review. BMC Infect Dis 2014; 14:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilleece Y, Chadwick DR, Breuer J et al. . BHIVA Guidelines Subcommittee British HIV Association guidelines for antiretroviral treatment of HIV-2-positive individuals 2010. HIV Med 2010; 11:611–9. [DOI] [PubMed] [Google Scholar]
- 21. Markowitz M, Morales-Ramirez JO, Nguyen BY et al. . Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr 2006; 43:509–15. [DOI] [PubMed] [Google Scholar]
- 22. Balestre E, Ekouevi DK, Tchounga B et al. . International Epidemiological Database to Evaluate AIDS (IeDEA) West Africa Collaboration Immunologic response in treatment-naive HIV-2-infected patients: the IeDEA West Africa cohort. J Int AIDS Soc 2016; 19:20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bénard A, Damond F, Campa P et al. . ANRS CO5 HIV-2 Cohort Study Group Good response to lopinavir/ritonavir-containing antiretroviral regimens in antiretroviral-naive HIV-2-infected patients. AIDS 2009; 23:1171–3. [DOI] [PubMed] [Google Scholar]
- 24. Benard A, van Sighem A, Taieb A et al. . ACHIEV2E Collaboration Study Group Immunovirological response to triple nucleotide reverse-transcriptase inhibitors and ritonavir-boosted protease inhibitors in treatment-naive HIV-2-infected patients: the ACHIEV2E Collaboration Study Group. Clin Infect Dis 2011; 52:1257–66. [DOI] [PubMed] [Google Scholar]
- 25. Matheron S, Damond F, Benard A et al. . ANRS CO5 HIV2 Cohort Study Group CD4 cell recovery in treated HIV-2-infected adults is lower than expected: results from the French ANRS CO5 HIV-2 cohort. AIDS 2006; 20:459–62. [DOI] [PubMed] [Google Scholar]
- 26. Wittkop L, Arsandaux J, Trevino A et al. . CD4 cell count response to first-line combination antiretroviral treatment in HIV-2 compared to HIV-1 positive patients: a multinational multicohort European study. J Antimicrob Chemother 2017; 72:2869–78. [DOI] [PubMed] [Google Scholar]
- 27. Roquebert B, Damond F, Collin G et al. . French ANRS HIV-2 Cohort (ANRS CO 05 VIH-2) HIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvitegravir in vitro. J Antimicrob Chemother 2008; 62:914–20. [DOI] [PubMed] [Google Scholar]
- 28. Damond F, Lariven S, Roquebert B et al. . Virological and immunological response to HAART regimen containing integrase inhibitors in HIV-2-infected patients. AIDS 2008; 22:665–6. [DOI] [PubMed] [Google Scholar]
- 29. Garrett N, Xu L, Smit E, Ferns B, El-Gadi S, Anderson J. Raltegravir treatment response in an HIV-2 infected patient: a case report. AIDS 2008; 22:1091–2. [DOI] [PubMed] [Google Scholar]
- 30. Peterson K, Rowland-Jones S. Novel agents for the treatment of HIV-2 infection. Antivir Ther 2012; 17:435–8. [DOI] [PubMed] [Google Scholar]
- 31. Peterson K, Ruelle J, Vekemans M, Siegal FP, Deayton JR, Colebunders R. The role of raltegravir in the treatment of HIV-2 infections: evidence from a case series. Antivir Ther 2012; 17:1097–100. [DOI] [PubMed] [Google Scholar]
- 32. Wandeler G, Furrer H, Rauch A. Sustained virological response to a raltegravir-containing salvage therapy in an HIV-2-infected patient. AIDS 2011; 25:2306–8. [DOI] [PubMed] [Google Scholar]
- 33. Avettand-Fenoel V, Damond F, Gueudin M et al. . ANRS-CO5 HIV-2 and the ANRS-AC11 Quantification Working Group New sensitive one-step real-time duplex PCR method for group A and B HIV-2 RNA load. J Clin Microbiol 2014; 52:3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bertine M, Gueudin M, Mélard A et al. . New highly sensitive real-time PCR assay for HIV-2 group A and group B DNA quantification. J Clin Microbiol 2017; 55:2850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Charpentier C, Camacho R, Ruelle J et al. . HIV-2EU: supporting standardized HIV-2 drug resistance interpretation in Europe. Clin Infect Dis 2013; 56:1654–8. [DOI] [PubMed] [Google Scholar]
- 36. Gottlieb GS, Eholie SP, Nkengasong JN et al. . A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS 2008; 22:2069–72; discussion 73–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charpentier C, Camacho R, Ruelle J et al. . HIV-2EU-supporting standardized HIV-2 drug-resistance interpretation in Europe: an update. Clin Infect Dis 2015; 61:1346–7. [DOI] [PubMed] [Google Scholar]
- 38. Ba S, Raugi D, Smith RA et al. . A trial of fixed dose combination of elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate for the initial treatment of HIV-2 infection: 48 week results from Senegal, West Africa [abstract MOPEB0294]. In: Program and Abstracts of the Ninth International AIDS Society Conference, Paris, France, 23–27 July 2017. [Google Scholar]