Protease inhibitors were independent predictors of cerebrospinal fluid (CSF) escape in antiretroviral therapy (ART)–experienced human immunodeficiency virus–infected adults. M184V/I combined with thymidine analog mutations were more frequent in adults with CSF escape compared to no escape. These findings suggest optimizing ART may reduce likelihood of CSF escape.
Keywords: CSF viral escape, HIV-1, protease inhibitor, drug resistance mutations, antiretroviral therapy
Abstract
Background
Cerebrospinal fluid (CSF) viral escape occurs in 4%–20% of human immunodeficiency virus (HIV)–infected adults, yet the impact of antiretroviral therapy (ART) on CSF escape is unclear.
Methods
A prospective study of 1063 participants with baseline plasma viral load (VL) ≤400 copies/mL between 2005 and 2016. The odds ratio (OR) for ART regimens (protease inhibitor with nucleoside reverse transcriptase inhibitor [PI + NRTI] vs other ART) and CSF escape was estimated using mixed-effects models.
Results
Baseline mean age was 46 years, median plasma VL, and CD4 count were 50 copies/mL, and 424 cells/μL, respectively. During median follow-up of 4.4 years, CSF escape occurred in 77 participants (7.2%). PI + NRTI use was an independent predictor of CSF escape (OR, 3.1; 95% confidence interval, 1.8–5.0) in adjusted analyses and models restricted to plasma VL ≤50 copies/mL (P < .001). Regimens that contained atazanavir (ATV) were a stronger predictor of CSF viral escape than non-ATV PI + NRTI regimens. Plasma and CSF M184V/I combined with thymidine-analog mutations were more frequent in CSF escape vs no escape (23% vs 2.3%). Genotypic susceptibility score–adjusted central nervous system (CNS) penetration-effectiveness (CPE) values were calculated for CSF escape with M184V/I mutations (n = 34). Adjusted CPE values were low (<5) for CSF in 27 (79%), indicating suboptimal CNS drug availability.
Conclusions
PI + NRTI regimens are independent predictors of CSF escape in HIV-infected adults. Reduced CNS ART bioavailability may predispose to CSF escape in patients with M184V/I mutations.
Contemporary antiretroviral therapy (ART) is effective at reducing plasma and cerebrospinal fluid (CSF) human immunodeficiency virus type 1 (HIV-1) RNA viral loads (VL) to below detectable levels and preventing severe forms of HIV-associated cognitive impairment [1–4]. In some individuals, HIV RNA can be detected in CSF despite plasma ART suppression, an event termed CSF viral escape [2, 5]. Neurologically symptomatic CSF escape frequently occurs in the setting of drug resistance mutations (DRMs) [5–7], while asymptomatic CSF escape has been detected when lumbar punctures are obtained for research [8, 9]. The long-term implications of CSF escape remain unclear.
CSF escape occurs in approximately 4%–20% of ART-experienced HIV-infected adults worldwide [10]. Low nadir CD4+ T-cell count, duration of HIV infection, and low-level viremia are risk factors for CSF escape [6, 7]. ART penetration into the central nervous system (CNS) varies by medication, with lower-penetrance ART regimens associated with higher CSF HIV-1 RNA [11, 12]. In contrast to other ART classes, protease inhibitors (PI), particularly atazanavir (ATV), do not consistently achieve in vitro 50% inhibitory concentrations (IC50) for wild-type HIV-1 in CSF, raising the possibility that CNS concentrations of certain PI regimens may be subtherapeutic in some individuals [13, 14]. The relationship between ART drug classes and likelihood of CSF escape has not been investigated in longitudinal studies, in part, due to low prevalence of CSF escape and need for large-scale studies to evaluate risk posed by specific ART regimens [15].
Our aim in this study was to determine the impact of ART on the probability of CSF escape in a prospective study of 1063 ART-experienced HIV-infected participants enrolled in US-based, multisite cohorts from 2005–2016. Given the known association of CSF escape with HIV-1 resistance mutations, the frequency of DRMs in reverse transcriptase (RT) and protease genes from a merged cohort of participants and published studies was also examined.
METHODS
Participants
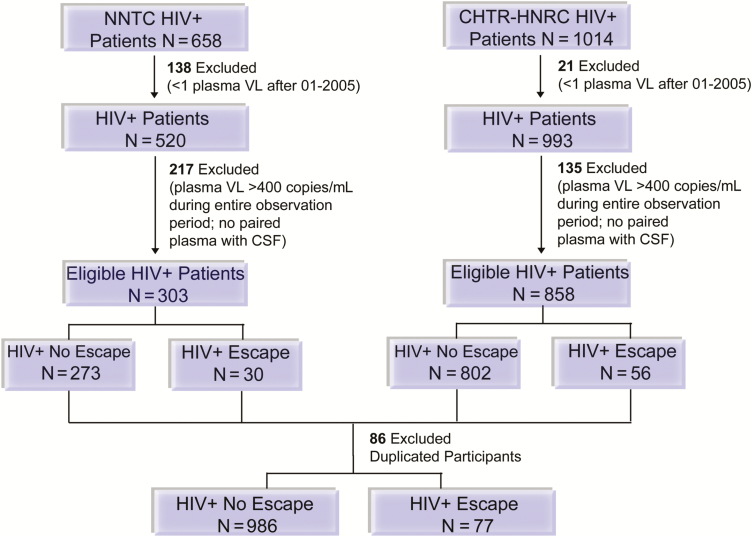
This prospective cohort included data from the National NeuroAIDS Tissue Consortium (NNTC), CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER), and HIV Neurobehavioral Research Center (HNRC) between January 2005 and September 2016. Criteria and data collection for individual cohorts have been described [16–18]. Eligible participants were age ≥18 years with at least 1 paired plasma and CSF VL measurement (Figure 1). Participants were followed from the first date after 2005 with plasma VL <400 copies/mL until the participant’s final recorded visit or second consecutive visit with plasma VL ≥1000 copies/mL, considered to represent virologic failure. Subsequent analyses were right censored to the last paired plasma and CSF VL in follow-up. CSF escape was defined as paired CSF VL > plasma VL (98% within 24 hours). If plasma VL was undetectable by clinical diagnostic assay (85% with lower limit of quantification of 50 copies/mL), CSF VL ≥51 copies/mL was denoted as CSF escape. The institutional review board at the NNTC, CHARTER, and HNRC clinical sites approved the research, and participants signed a written statement of informed consent.
Figure 1.
Selection of the human immunodeficiency virus-infected (HIV+) study cohort, 2005–2016. The sequential application of inclusion and exclusion criteria to define the study population is shown. If cerebrospinal fluid escape was not present during the observation period, the participant was defined as a control. If participants were enrolled in both studies, data from the CHARTER-HNRC cohort was used to prevent data duplication. Abbreviations: CHTR-HNRC, CHARTER-HIV Neurobehavioral Research Center; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; N, number of participants; NNTC, National NeuroAIDS Tissue Consortium; VL, viral load.
Calculation of DRMs and CNS Penetration Values
In a combined dataset of cohort participants with available genotypes (n = 50) and genotypes from published CSF escape cases (n = 49; [5]), frequencies of CSF and plasma mutations in RT and protease genes were analyzed. Frequencies of individual HIV-1 mutations were calculated for participants with and without CSF escape. The effectiveness of CNS ART penetration was estimated for each regimen using the CNS penetration effectiveness (CPE) value [19]. Genotypic susceptibility scores (GSS) were calculated using CSF or plasma genotypes and assigning a score of 0 (resistant), 0.5 (intermediate resistance), or 1 (susceptible) to each drug [20]. GSS-adjusted CPE scores were calculated by multiplying the CPE value by the GSS for each ART drug and summing scores.
Statistical Analyses
Given repeated plasma and CSF VL measures within the same individual, the probability of CSF escape was estimated using generalized mixed-effects models for repeated binary outcome in PROC GLIMMIX (SAS 9.4 Institute, Cary, North Carolina). The primary covariate of interest was PI with 2 nucleoside reverse transcriptase inhibitors (PI + NRTI) vs other ART regimens. Regimens that combined a PI with a non-NRTI (NNRTI) and NRTI were classified as other ART. Adjusted continuous covariates included duration of HIV infection based on self-report, nadir CD4+ T-cell count, age at study visit, and number of lumbar punctures to adjust for sampling bias; modeled binary covariates included plasma VL (suppressed [≤50 copies/mL] vs nonsuppressed) and cohort indicator (NNTC vs CHARTER and HNRC combined). Of 11 622 total person-visits, 300 visits (2.6%) were among participants on monotherapy or nonstandard drug regimens that did not include at least 2 NRTIs in combination with a third active ART drug from another class. Monotherapy is associated with virologic failure and development of DRMs [21]; therefore, we excluded monotherapy or nonstandard regimens from mixed-effects models as they may falsely overestimate probability of CSF viral escape (see Supplementary Methods).
RESULTS
A total of 1063 participants met eligibility criteria, of which 77 (7.2%) developed CSF escape during median follow-up of 4.4 years. Participants were 81.6% men, 48.4% white, 31.2% black, ranged in age from 19 to 79 years (mean = 46.2, standard deviation = 8.87 years), and the majority entered observation during 2005–2008 (Table 1). At baseline, the majority of participants with CSF escape had been HIV infected for more than 10 years (79%) and had low median nadir CD4+ T-cell counts (50 cells/µL); 65% reported being on a PI-based regimen.
Table 1.
Baseline Cohort Characteristics of Cerebrospinal Fluid Viral Escape in National NeuroAIDS Tissue Consortium and CHARTER-HRNC, 2005–2016
| Characteristic | Cerebrospinal Fluid Escape (n = 77) | Total Cohort (n = 1063) |
|---|---|---|
| Years of observation, median (IQR) | 5.82 (2.92, 8.88) | 4.39 (1.59, 7.65) |
| Baseline age (years), mean (SD) | 45.79 (8.16) | 46.18 (8.87) |
| Male gender, n (%) | 64 (83.1) | 867 (81.6) |
| Race, n (%) | ||
| White | 45 (58.4) | 514 (48.4) |
| Black | 21 (27.3) | 332 (31.2) |
| Other | 11 (14.3) | 217 (20.4) |
| Years of education (>12 years), n (%) | 44 (57.1) | 575 (54.1) |
| Baseline year, n (%) | ||
| 2005–2008 | 66 (85.7) | 896 (84.3) |
| 2009–2012 | 10 (13.0) | 148 (13.9) |
| 2013–2016 | 1 (1.3) | 19 (1.8) |
| Baseline HIV disease characteristics | ||
| Duration of HIV infection (>10 years), n (%) | 61 (79.2) | 633 (60.9) |
| Plasma viral load (copies/mL), median (IQR) | 50 (50, 109) | 50 (50, 50) |
| Nadir CD4+ T-cell count (cells/µL), median (IQR) | 50 (5, 183) | 89 (14, 200) |
| CD4+ T-cell count (cells/µL), median (IQR) | 364 (187, 539) | 424 (253, 626) |
| CD4/CD8 ratio, median (IQR) | 0.36 (0.20, 0.67) | 0.49 (0.28, 0.75) |
| Baseline antiretroviral therapy regimen, n (%) | ||
| PI + NRTIs | 50 (64.9) | 514 (48.4) |
| Other ART | ||
| Integrase inhibitor + NRTIs | 2 (2.6) | 60 (5.6) |
| NNRTI + NRTIs | 10 (13.0) | 289 (27.2) |
| PI + NNRTI + NRTIs | 4 (5.2) | 63 (5.9) |
| Monotherapy/nonstandard therapya | 2 (2.6) | 37 (3.5) |
| Specific drugs in regimen | ||
| Atazanavir | 24 (31.2) | 241 (22.7) |
| Lopinavir | 10 (13.0) | 185 (17.4) |
| Darunavir | 8 (10.4) | 66 (6.2) |
| Efavirenz | 10 (13.0) | 255 (24.0) |
| Abacavir | 15 (19.5) | 233 (21.9) |
| Central nervous system penetration-effectiveness value (mean (SD)) | 7.8 (2.2) | 8.0 (2.0) |
| Neurological coinfections, n (%) | ||
| Number of patients with coinfectionsb | 26 (33.8) | 270 (25.4) |
| Neurosyphilis, n (%) | 9 (11.7) | 114 (10.7) |
| Herpesc | 10 (13.0) | 126 (11.9) |
| Progressive multifocal leukoencephalopathy | 5 (6.5) | 27 (2.5) |
| Cryptococcus and/or Toxoplasmosis | 3 (3.9) | 40 (3.8) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
aNonstandard therapy: regimen inconsistent with 2 NRTIs in combination with a third active antiretroviral therapy drug from another class.
bCentral nervous system coinfections were counted if infection occurred within 2 years prior or 1 year after cerebrospinal fluid (CSF) escape or last CSF visit. Multiple coinfections were reported in some patients.
cIncludes varicella zoster virus encephalitis or vasculopathy and/or cytomegalovirus encephalitis (Supplementary Table 1).
CSF Escape
Among 77 participants with CSF escape, median plasma VL was 50 copies/mL, CD4+ T-cell count was 394 cells/µL, and the CD4-to-CD8 ratio was 0.44 at first instance of escape (Table 2). Symptomatic neurocognitive deficits were more frequent in participants with CSF escape (35% vs 20%), while 40% were neurocognitively unimpaired. The distribution of ART regimens is shown in Table 2; 66% were on a PI-based regimen with ATV, darunavir, and lopinavir as the most frequent PIs, 22% were on a NNRTI-based regimen, and 3 were on an integrase inhibitor (raltegravir) at the time of first escape. The mean CPE value did not differ between groups (P = .3). Sixty-five participants (84%) had been on other ART regimens before the first CSF escape. Twenty-five of 30 participants with available adherence data and CSF escape (83%) reported adherence to ART, while 5 (17%) were nonadherent within 1 year of their first CSF escape. These estimates were comparable to those for participants without CSF escape within 1 year of their last paired CSF visit (adherent, 310 participants [90%]; nonadherent, 33 participants [9.6%]). The proportion of missing adherence data was similar for participants with and without CSF escape (61% vs 65%), respectively. CSF escape was observed at 135 visits, with 26 participants (34% of those with escape; Supplementary Table 2) demonstrating multiple instances of higher HIV RNA levels in CSF compared to plasma. Participants with repeated CSF escape were younger (P = .01) and more likely to be on a PI-containing regimen (88% vs 55%, P = .03) than those with only 1 escape episode (Supplementary Table 3).
Table 2.
Factors Associated With First Cerebrospinal Fluid (CSF) Viral Escape or Last Paired CSF-Plasma Viral Load Visit
| Characteristic | CSF Viral Escape (n = 77) | No CSF Viral Escape (n = 986) | P Value |
|---|---|---|---|
| HIV disease characteristics, median (IQR) | |||
| Plasma viral load (copies/mL) | 50 (40, 115.50) | 50 (40, 50) | .24 |
| Nadir CD4+ T-cell count (cells/µL) | 46 (4, 180) | 94 (15, 203) | <.01 |
| CD4+ T-cell count (cells/µL) | 394 (220, 578) | 497.5 (311, 715) | <.01 |
| CD4/CD8 ratio | 0.44 (0.25, 0.71) | 0.62 (0.36, 0.91) | .01 |
| CSF studies, median (IQR) | |||
| Viral load (copies/mL) | 300 (141, 1780) | 50 (40, 50) | <.01 |
| White blood cell count (cells/µL) | 5 (2, 13) | 2 (1, 3) | <.01 |
| Protein (mg/dL) | 45 (36, 57) | 38 (29, 49) | <.01 |
| No. CSF viral load analyses/subject, median (IQR)a | 5 (3, 9) | 4 (2, 6) | <.01 |
| Neurocognitive status,b n (%) | .01 | ||
| Symptomatic neurocognitive impairment | 27 (35.1) | 198 (20.4) | … |
| HIV-associated dementia | 7 (9.1) | 52 (5.4) | … |
| Mild neurocognitive disorder | 13 (17.1) | 99 (10.2) | … |
| Neuropsychiatric impairment–other | 7 (9.1) | 47 (4.8) | … |
| Asymptomatic neurocognitive impairment | 17 (22.1) | 297 (30.6) | … |
| Neurocognitively unimpaired | 33 (42.9) | 475 (49.0) | … |
| ART Regimens, n (%) | |||
| PI + NRTIs | 51 (66.2) | 464 (47.1) | <.01 |
| Other ART | 20 (26.0) | 491 (49.8) | … |
| Integrase inhibitor + NRTIs | 3 (3.9) | 153 (15.5) | … |
| NNRTI + NRTIs | 13 (16.9) | 287 (29.1) | … |
| PI + NNRTI + NRTIs | 4 (5.2) | 51 (5.2) | … |
| Monotherapy/nonstandard therapyc | 4 (5.2) | 17 (1.7) | … |
| Specific drugs in regimen | |||
| Atazanavir | 27 (35.1) | 226 (22.9) | .02 |
| Lopinavir | 9 (11.7) | 135 (13.7) | .75 |
| Darunavir | 10 (13.0) | 143 (14.5) | .84 |
| Efavirenz | 13 (16.9) | 224 (22.7) | .3 |
| Abacavir | 16 (20.8) | 221 (22.4) | .85 |
| Central nervous system penetration-effectiveness value (mean (SD)) | 7.7 (2.1) | 7.9 (1.8) | .3 |
Bold indicates significant after Bonferroni correction (P ≤ .01).
Abbreviations: ART, antiretroviral therapy; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
aNumber of CSF analyses performed during follow-up.
bNearest available neurocognitive status (n = 1047; 98.5% cohort). Two-way analysis of variance comparing symptomatic neurocognitive impairment, asymptomatic neurocognitive impairment, and neurocognitively unimpaired.
cNonstandard therapy; regimen inconsistent with 2 NRTIs in combination with a third active ART drug from another class.
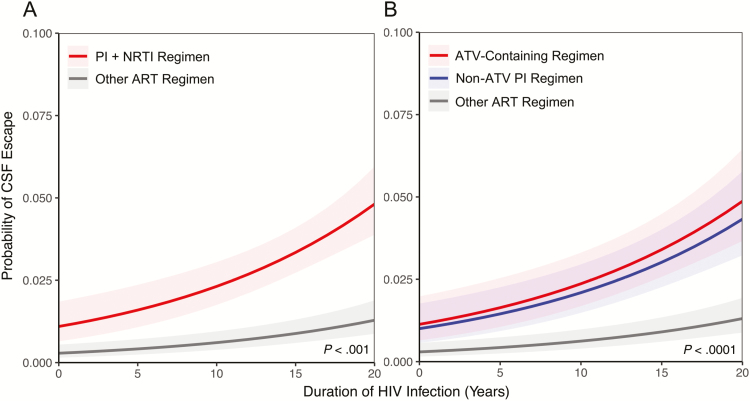
Analysis of visits with paired CSF and plasma VL showed a 3.7-fold greater odds of CSF escape in participants on PI + NRTI regimens compared to other ART regimens in univariate analysis (Table 3). In multivariable mixed-effects models, there was a 3.9-fold increased odds of CSF escape (95% confidence interval [CI], 2.5–6.2) on PI + NRTI regimens after accounting for years of HIV infection; the predicted mean probability of CSF escape over 20 years of HIV infection is shown in Figure 2A. In models adjusted for nadir CD4+ T-cell count, years of HIV infection, age, plasma viral suppression, number of CSF analyses, and cohort, the association between PI use and CSF escape remained significant, with an estimated 3.1-fold odds of CSF escape on PI-based therapy (95% CI, 1.9–5.1) at 15 years of HIV infection (Table 3). Lower nadir CD4+ T-cell count (P = .03), younger age (P < .0001), and plasma VL >50 copies/mL (P = .01) were additional independent risk factors associated with CSF escape. Participants in the NNTC cohort were more likely to have CSF escape than those in CHARTER–HNRC cohorts, which may reflect more advanced stages of HIV disease in the NNTC cohort. Cohort was not a factor when the dataset was restricted to visits with suppressed plasma VL (Supplementary Table 4) [16]. The number of CSF analyses was also not associated with increased odds of CSF escape (P = .7).
Table 3.
Predictors Associated With Cerebrospinal Fluid Viral Escape in Mixed-Effects Logistic Regression Models
| Predictor | PIs vs Other ART Regimens | Atazanavir-Containing, Non–Atazanavir-Containing PI vs Other ART Regimens | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Model (n = 5732) | Time Adjusted Model (n = 5663) | Covariate Adjusted Model (n = 5663) | Time Adjusted Model (n = 5663) | Covariate Adjusted Model (n = 5663) | ||||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| ART regimen | ||||||||||
| PI + NRTIs | 3.7 (2.4–5.8) | <.01 | 3.9 (2.5–6.2) | <.01 | 3.1 (1.9–5.1) | <.01 | … | … | … | … |
| Other ART | 1.0 (Ref.) | … | 1.0 (Ref.) | … | 1.0 (Ref.) | … | … | … | … | … |
| ART regimen | ||||||||||
| Atazanavir-containing | ... | ... | ... | ... | ... | ... | 3.9 (2.4–6.3) | <.01 | 3.1 (1.9–5.3) | <.001 |
| Non-atazanavir-containing PI | ... | ... | ... | ... | ... | ... | 3.4 (2.1–5.6) | <.01 | 2.7 (1.6–4.5) | <.01 |
| Other ART | ... | ... | ... | ... | ... | ... | 1.0 (Ref.) | ... | 1.0 (Ref.) | ... |
| Estimated years of human immunodeficiency virus infectiona | ... | ... | 1.5 (1.3–1.7) | <.01 | 1.7 (1.4–2.0) | <.01 | 1.5 (1.3–1.7) | <.01 | 1.7 (1.4–2.0) | <.01 |
| Nadir CD4+ T-cell countb | ... | ... | ... | ... | 1.2 (1.0–1.5) | .03 | ... | ... | 1.3 (1.0–1.6) | .02 |
| Age at study visitc | ... | ... | ... | ... | 0.9 (0.9–1.0) | <.01 | ... | ... | 0.9 (0.9–1.0) | <.01 |
| Plasma viral load | ||||||||||
| Nonsuppressed (>50 copies/mL) | ... | ... | ... | ... | 1.7 (1.2–2.6) | .01 | ... | ... | 1.7 (1.2–2.6) | <.01 |
| Suppressed (≤50 copies/mL) | ... | ... | ... | ... | 1.0 (Ref.) | ... | ... | ... | 1.0 (Ref.) | ... |
| Number of lumbar punctures | ... | ... | ... | ... | 1.0 (1.0-1.0) | .77 | ... | ... | 1.0 (1.0-1.0) | .67 |
| Cohort indicator | ||||||||||
| National NeuroAIDS Tissue Consortium | ... | ... | ... | ... | 1.7 (1.1–2.7) | .02 | ... | ... | 1.7 (1.1–2.7) | .02 |
| CHARTER-HIV Neurobehavioral Research Center | ... | ... | ... | ... | 1.0 (Ref.) | ... | ... | ... | 1.0 (Ref.) | ... |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; NRTI, nucleoside reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor; Ref., reference.
aOR per 5 years of estimated years of human immunodeficiency virus infection.
bOR per 100 cell decrement in nadir CD4+ T-cell count.
cOR per year. For mixed models, all values must be present to be modeled. Models were fit using Proc GLIMMIX for logistic regression for repeated measures with cerebrospinal fluid escape as the dependent variable and predictors as fixed effects. Adjusted models were analyzed with random intercept and time.
Figure 2.
Protease inhibitor use is associated with higher probability of cerebrospinal fluid (CSF) escape in human immunodeficiency virus (HIV)–infected adults on combination antiretroviral therapy (ART). A, Estimated mean probability of CSF escape according to protease inhibitor with nucleoside reverse transcriptase inhibitors (PI+NRTI) use vs other ART regimens over the estimated duration of HIV infection. B, PI use is associated with increased probability of CSF escape when compared to other ART multidrug regimens (P < .001). Atazanavir-containing regimens account for a portion of the increased probability of CSF escape among PI-containing regimens compared to other ART regimens (P < .0001). CSF escape is independently associated with longer duration of HIV infection in both models (P < .001). Abbreviations: ART, antiretroviral therapy; ATV, atazanavir; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The association between PI + NRTI regimens and CSF escape remained significant in sensitivity analyses limited to visits with plasma VL ≤50 copies/mL (odds ratio [OR], 3.8 [2.0–7.6]; Supplementary Table 4). Stability of results was further confirmed in multivariable models limited to the first CSF escape event or last paired CSF and plasma VL (PI + NRTI vs other ART; OR, 2.2; 95% CI, 1.2–4.0) and in multivariable models using months in follow-up as a proxy for time (PI + NRTI vs other ART; OR, 3.1; 95% CI, 2.0–4.8); months in follow-up was not significantly associated with escape (P = .84). Adjusted mixed-models were fit using 10 imputed datasets, and pooled estimates demonstrated similar associations between PI + NRTI regimens vs other ART (3.1, OR; 95% CI, 2.6–3.6; data not shown) and CSF escape, suggesting that missingness is unlikely to account for the relationship between PI-based regimens and CSF escape.
ATV and CSF Escape
ATV was the most commonly reported PI. ATV achieves lower CSF concentrations than other PI drugs and does not consistently achieve the IC50 in CSF [13]. After adjusting for duration of HIV infection in mixed-effects models, ATV-containing and non–ATV-containing regimens had a 3.9-fold (95% CI, 2.4–6.3) and 3.4-fold (95% CI, 2.1–5.6) increased odds for CSF escape compared to other ART regimens, respectively (Table 3). The probability of CSF escape was not significantly different between ATV-containing compared to non–ATV-containing regimens and CSF escape, suggesting that while regimens with ATV pose an increased likelihood of CSF escape, it is not the only PI-based regimen associated with CSF escape (Figure 2B). In fully adjusted models, ATV-containing regimens (OR, 3.1; 95% CI, 1.9–5.3) remained a stronger independent predictor for CSF escape than nadir CD4+ T-cell count (OR, 1.3; 95% CI, 1.0–1.6) or years of HIV infection (OR, 1.7; 95% CI, 1.4–2.0).
HIV-1 Genotyping and DRMs
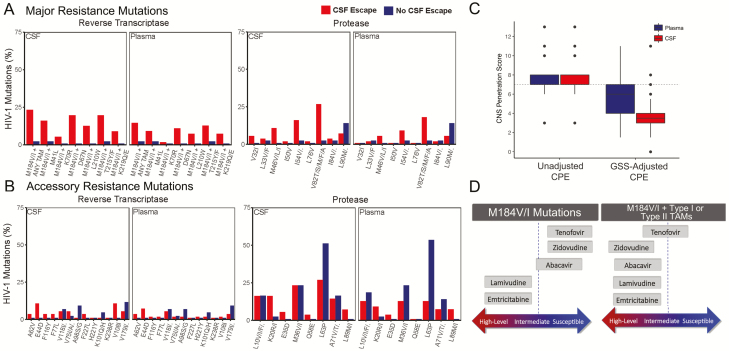
DRMs are associated with worse clinical outcomes [22] and reported in neurologically symptomatic CSF escape [5, 6]. We hypothesized that ART regimens with incomplete viral suppression in the CNS could lead to development of DRMs. Among participants with CSF escape using the combined dataset of cohort participants and published CSF cases, M184V or M184I (M184V/I) mutations were detected in CSF and plasma in 61% (34/56) and 30% (16/55) of samples, respectively, while M184V/I mutations in CSF and plasma were detected in only 7% (3/43) of samples from participants without escape (Supplementary Table 5). Given the high frequency of M184V/I detected in all samples (28%, 56/197), these mutations were combined with thymidine-analog mutations (TAMs; ie, M41L, L210W, T215Y, D67N, K70R, T215F, K219Q/E) for analysis. In 23% (13/56) of CSF escape cases, M184V/I mutation was detected with at least 1 TAM in CSF compared to 15% (8/55) in plasma, potentially lowering the threshold for accumulation of other mutations that lead to NRTI cross resistance in the CNS (Figure 3D) [23, 24]. Major but not accessory resistance mutations were more frequent in CSF among those with CSF escape compared to participants without CSF escape (Figure 3A and 3B).
Figure 3.
Frequency of major resistance mutations and adjusted central nervous system penetration-effectiveness (CPE) values in plasma and cerebrospinal fluid (CSF) among participants with CSF escape. The frequencies of resistance mutations in CSF and plasma in reverse transcriptase and protease genes are shown by human immunodeficiency virus (HIV)–infected participants with CSF escape (red) and no CSF escape (blue) during the study period. For simplicity, M184V/I mutations were combined with thymidine analog mutations (TAMs). Major resistance mutations (A), but not accessory resistance mutations (B), were more frequent in those with CSF escape compared to those without CSF escape. HIV-infected adults with CSF escape and M184V/I mutations on genotypic resistance tests in CSF and/or plasma were identified from published studies (n = 34). Median CPE values were 7 (interquartile range [IQR], 7–8), but genotypic susceptibility score–adjusted CPE values were 6 (IQR, 4–7) in plasma and 3.5 (IQR, 3–4) in CSF (C). Schematic diagram showing the predicted resistance to nucleoside reverse transcriptase inhibitor with M184V/I mutations [24]. Abbreviations: CNS, central nervous system; CPE, central nervous system penetration effectiveness; CSF, cerebrospinal fluid; GSS, genotypic susceptibility scores; HIV, human immunodeficiency virus; TAM, thymidine analog mutation.
M184V/I Mutations and CPE Values
M184V/I mutations reduce HIV-1 viral fitness [25], can delay emergence of TAMs [23], and are frequently present in ART-experienced individuals [6, 26]. While ART drug regimens with higher CPE values are associated with lower CSF VL [11, 27], CPE values do not reflect resistance mutations. We therefore identified ART-experienced participants with CSF escape and M184V/I mutations on genotypic resistance tests in CSF and/or plasma from published studies (n = 34 [5]) and calculated CPE values based on reported ART drug regimens at CSF escape. GSS was calculated by assigning a value to each ART drug, and the composite score accounting for viral resistance and CNS penetration was calculated (GSS-adjusted CPE), as described [20]. Among 34 HIV-infected participants with M184V/I, the median CPE value was 7 (interquartile range [IQR], 7–8), consistent with median CPE values in published studies [20, 28], while GSS-adjusted CPE values were 6 (IQR 4–7) in plasma and 3.5 (IQR 3–4) in CSF (Figure 3C). Although there are no clinically recommended CPE cutoffs, regimens with CPE values of <5 are generally considered suboptimal CNS penetration of ART [11, 20, 27, 29]. In participants with CSF escape and M184V/I mutations, GSS-adjusted CPE values were <5 for CSF and plasma in 27 (79%) and 13 (38%) of 34 participants, respectively, indicating that the majority of those with M184V/I mutations and CSF escape have a low estimated ART penetration in the CNS (Supplementary Table 6).
DISCUSSION
In this prospective multisite study of 1063 ART-experienced HIV-infected participants during 2005–2016, PI regimens were associated with 3-fold higher odds of CSF escape compared to other ART regimens in adjusted mixed-effects models. Additionally, individuals on ATV had increased likelihood of CSF escape compared to non–PI-based regimens. Lower nadir CD4+ T-cell count and longer duration of HIV infection remained associated with CSF escape despite plasma suppression, which is consistent with previous studies [6, 10, 30]. To our knowledge, these data represent the largest longitudinal analyses of CSF escape predictors among HIV-infected adults on ART. These analyses also show that CSF escape cases with M184V/I are likely to harbor variants with major DRMs that confer resistance to at least 1 ART drug. These findings highlight ART drug classes and/or individual drugs as important predictors of CSF escape, potentially through mechanisms that involve suboptimal CNS penetration, incomplete control of viral replication, and DRM accumulation.
ART regimens have highly variable penetration in tissue compartments including the CNS, leading to lower drug concentrations compared to peripheral blood [14, 19, 31, 32]. In nonhuman primate models, low viral RNA levels were detected in brain despite suppressive ART and were correlated with reduced drug concentrations in non-CNS tissues compared to peripheral blood mononuclear cells [31, 32]. PIs penetrate the CNS less effectively than other ART classes, as evidenced by CSF concentrations <1% of those in plasma [11, 13, 33]. In 13 boosted, protease-inhibitor monotherapy, randomized, controlled, clinical trials, there were 6 cases of detectable CSF VL, all in the boosted-PI monotherapy arm [15]. Our mixed-effects analyses excluded visits while on monotherapy and showed that combination PI-based regimens still pose a 3-fold higher odds of CSF escape compared to other ART regimens. The association with PI-based ART regimens remained significant when only the first episode of detectable CSF VL was analyzed, was stronger when repeated events were considered, and suggests PI-based therapy may not adequately control virus in the CNS despite achieving plasma suppression [31].
The CPE method remains a topic of controversy, with some studies suggesting improved neurocognitive scores or lower CSF HIV-1 RNA with higher CPE values [11, 27, 28, 34], while others show no association or worse outcomes [35, 36]. When an adjustment was factored into CPE values to account for drug resistance, Peluso et al found that the adjusted CPE value was thought to be a more accurate reflection of ART penetration in CSF escape [6]. A similar strategy was used to determine impact of ART regimens on neurocognitive impairment and showed improved correlations with cognitive scores compared to unadjusted CPE values [20]. In our study, CPE values were similar between those with and without CSF escape, suggesting that unadjusted values alone may not discriminate those at risk for CSF escape. However, when resistance mutations were factored into CPE values among participants with common NRTI M184V/I mutations, median adjusted CPE scores in CSF were low, suggesting that accumulation of resistance mutations in the CNS may lead to ineffective viral control despite regimens with high unadjusted CPE values. Participants in our study cohort had historical immunosuppression, as evidenced by low median nadir CD4+ T-cell counts, and the majority had long duration of HIV infection. We hypothesize that a sequential process rather than a single driver is necessary for CSF escape, with several factors that include accumulated CNS DRMs and insufficient ART penetration leading to periodic CNS viral replication.
This study has several limitations, including potential selection bias for participants enrolled in research studies. While the estimated prevalence of CSF escape is consistent with those listed in other publications of CSF escape [5, 7–9], some participants were enrolled in neurological substudies that may be subject to referral bias and therefore may overestimate prevalence of CSF escape compared to the general HIV-infected population. Additionally, choice of ART regimen is not random in ART-experienced patients; thus, potential channeling biases cannot be fully accounted for in these analyses. The majority of CSF escape cases were exposed to prior ART regimens. While reasons for ART switch were not available, plasma VL, duration of HIV infection, and nadir CD4 count were adjusted for in analyses, and the follow-up period was restricted to visits with plasma VL <1000 copies/mL on consecutive visits to reduce biases that result from virological failure. ART adherence is a critical factor in maintaining durable viral suppression [37] and could not be fully assessed due to missing data at the time of CSF escape. However, subanalyses of visits with suppressed plasma VL as a proxy for ART suppression showed that PI use remained an independent predictor of CSF escape. Additional limitations include the limited number of participants on integrase inhibitors at the time of CSF escape. Last, only a subset of participants had available HIV-1 genotypes, and samples were not available to perform resistance testing at CSF escape episodes.
The search for a functional cure is a top priority for HIV research [38]. Latently infected cells that harbor integrated HIV DNA persist within the CNS, [39] are not cleared with suppressive ART and represent a potential source of viral rebound. Analyses in this study suggest that individuals on PI-based regimens are at elevated risk for CSF escape, and that risk may extend to individuals with historic M184V/I mutations and CSF accumulation of major DRMs. Understanding viral and host factors associated with incomplete suppression of CSF HIV-1 RNA during ART is an important step toward identifying patients with CNS reservoirs and has clinical implications, particularly in those with neurological symptoms, regarding optimal ART regimens for ART-experienced patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to Rajesh Gandhi and Robert Parker for review of the data and manuscript.
Financial support. This work was supported by the National Institutes of Health (NIH) grants to D. G. (R01MH097659, R01MH110259, R01DA040391). Training and educational support for S. S. M was provided by NIH (T32AG000222 and K23MH115812). Additional support for S. S. M included Biostatistical Consultation, which was made possible with help from the Harvard University Center for AIDS Research, an NIH-funded program (grant P30AI060354). NNTC and CHARTER sites were supported by NIMH and the National Institute of Neurological Disorders and Stroke (NINDS) (grants U01 MH083501, R24 MH59724, U01 MH083507, R24 NS45491, U01 MH083500, R24 NS38841, U01 MH083506, R24 MH59745, U01 MH083545, N01 MH32002, and N01 MH22005). The HIV Neurobehavioral Research Center (HNRC) is supported by a Center Award from NIMH (P30 MH062512), and the San Diego HNRC group is affiliated with the University of California–San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System. S. L. was also supported by K24 MH097673.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: NIH NeuroHIV Symposium, 21–22 October 2017, Bethesda, MD and Conference on Retroviruses and Opportunistic Infections, 7 March 2018, Boston, MA. Abstract 123.
References
- 1. Saylor D, Dickens AM, Sacktor N, et al. . HIV-associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat Rev Neurol 2016; 12:234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr HIV/AIDS Rep 2015; 12:280–8. [DOI] [PubMed] [Google Scholar]
- 3. Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis 2006; 194:1686–96. [DOI] [PubMed] [Google Scholar]
- 4. Ellis RJ, Gamst AC, Capparelli E, et al. . Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology 2000; 54:927–36. [DOI] [PubMed] [Google Scholar]
- 5. Mukerji SS, Misra V, Lorenz D, et al. . Temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J Acquir Immune Defic Syndr 2017; 75:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peluso MJ, Ferretti F, Peterson J, et al. . Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012; 26:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nightingale S, Geretti AM, Beloukas A, et al. . Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J Neurovirol 2016; 22:852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edén A, Fuchs D, Hagberg L, et al. . HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 2010; 202:1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edén A, Nilsson S, Hagberg L, et al. . Asymptomatic cerebrospinal fluid HIV-1 viral blips and viral escape during antiretroviral therapy: a longitudinal study. J Infect Dis 2016; 214:1822–5. [DOI] [PubMed] [Google Scholar]
- 10. Joseph J, Cinque P, Colosi D, et al. . Highlights of the global HIV-1 CSF Escape Consortium Meeting, 9 June 2016, Bethesda, MD, USA. J Virus Erad 2016; 2:243–50. [PMC free article] [PubMed] [Google Scholar]
- 11. Cusini A, Vernazza PL, Yerly S, et al. ; Swiss HIV Cohort Study Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr 2013; 62:28–35. [DOI] [PubMed] [Google Scholar]
- 12. Letendre SL, Mills AM, Tashima KT, et al. ; Extended ING116070 Study Team ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis 2014; 59:1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Best BM, Letendre SL, Brigid E, et al. ; CHARTER Group Low atazanavir concentrations in cerebrospinal fluid. AIDS 2009; 23:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antinori A, Perno CF, Giancola ML, et al. . Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis 2005; 41:1787–93. [DOI] [PubMed] [Google Scholar]
- 15. Arribas JR, Girard PM, Paton N, et al. . Efficacy of protease inhibitor monotherapy vs. triple therapy: meta-analysis of data from 2303 patients in 13 randomized trials. HIV Med 2016; 17:358–67. [DOI] [PubMed] [Google Scholar]
- 16. Morgello S, Gelman BB, Kozlowski PB, et al. . The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol 2001; 27:326–35. [DOI] [PubMed] [Google Scholar]
- 17. Heaton RK, Clifford DB, Franklin DR Jr, et al. ; CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heaton RK, Franklin DR, Ellis RJ, et al. ; CHARTER Group; HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammond ER, Crum RM, Treisman GJ, et al. ; CHARTER Group The cerebrospinal fluid HIV risk score for assessing central nervous system activity in persons with HIV. Am J Epidemiol 2014; 180:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fabbiani M, Grima P, Milanini B, et al. . Antiretroviral neuropenetration scores better correlate with cognitive performance of HIV-infected patients after accounting for drug susceptibility. Antivir Ther 2015; 20:441–7. [DOI] [PubMed] [Google Scholar]
- 21. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Section accessed: Regimen Switching in the Setting of Virologic Suppression. Accessed 26 March 2018. [Google Scholar]
- 22. Godfrey C, Thigpen MC, Crawford KW, et al. . Global HIV antiretroviral drug resistance: a perspective and report of a National Institute of Allergy and Infectious Diseases Consultation. J Infect Dis 2017; 216:798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wensing AM, Calvez V, Günthard HF, et al. . 2017 update of the drug resistance mutations in HIV-1. Top Antivir Med 2017; 24:132–3. [PMC free article] [PubMed] [Google Scholar]
- 24. Shafer RW, Schapiro JM. HIV-1 drug resistance mutations: an updated framework for the second decade of HAART. AIDS Rev 2008; 10:67–84. [PMC free article] [PubMed] [Google Scholar]
- 25. Paredes R, Sagar M, Marconi VC, et al. . In vivo fitness cost of the M184V mutation in multidrug-resistant human immunodeficiency virus type 1 in the absence of lamivudine. J Virol 2009; 83:2038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le T, Chiarella J, Simen BB, et al. . Low-abundance HIV drug-resistant viral variants in treatment-experienced persons correlate with historical antiretroviral use. PLoS One 2009; 4:e6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Letendre S, Marquie-Beck J, Capparelli E, et al. ; CHARTER Group Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carvalhal A, Gill MJ, Letendre SL, et al. ; Centre for Brain Health in HIV/AIDS Central nervous system penetration effectiveness of antiretroviral drugs and neuropsychological impairment in the Ontario HIV Treatment Network Cohort Study. J Neurovirol 2016; 22:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ciccarelli N, Fabbiani M, Colafigli M, et al. . Revised central nervous system neuropenetration-effectiveness score is associated with cognitive disorders in HIV-infected patients with controlled plasma viraemia. Antivir Ther 2013; 18:153–60. [DOI] [PubMed] [Google Scholar]
- 30. Rawson T, Muir D, Mackie NE, Garvey LJ, Everitt A, Winston A. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect 2012; 65:239–45. [DOI] [PubMed] [Google Scholar]
- 31. Estes JD, Kityo C, Ssali F, et al. . Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 2017; 23:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clements JE, Li M, Gama L, et al. . The central nervous system is a viral reservoir in simian immunodeficiency virus–infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J Neurovirol 2005; 11:180–9. [DOI] [PubMed] [Google Scholar]
- 33. Kim RB, Fromm MF, Wandel C, et al. . The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 1998; 101:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marra CM, Zhao Y, Clifford DB, et al. ; AIDS Clinical Trials Group 736 Study Team Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 2009; 23:1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caniglia EC, Cain LE, Justice A, et al. ; HIV-CAUSAL Collaboration Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 2014; 83:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. . Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 2011; 76:1403–9. [DOI] [PubMed] [Google Scholar]
- 37. Konstantopoulos C, Ribaudo H, Ragland K, Bangsberg DR, Li JZ. Antiretroviral regimen and suboptimal medication adherence are associated with low-level human immunodeficiency virus viremia. Open Forum Infect Dis 2015; 2:ofu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deeks SG, Lewin SR, Ross AL, et al. ; International AIDS Society Towards a Cure Working Group International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22:839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamers SL, Rose R, Maidji E, et al. . HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy-treated patients with undetectable viral loads. J Virol 2016; 90:8968–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.