Deployment-related trauma infectious complications continue after initial hospital discharge and into Veterans Affairs healthcare (39% developed new post-discharge infection). Injury severity and occurrence of ≥1 inpatient infection were associated with a shorter time to development of a new post-discharge infection.
Keywords: trauma-related infections, combat-related infections, military health, veterans affairs
Abstract
Background
Infectious complications related to deployment trauma significantly contribute to the morbidity and mortality of wounded service members. The Trauma Infectious Disease Outcomes Study (TIDOS) collects data on US military personnel injured in Iraq and Afghanistan in an observational cohort study of infectious complications. Patients enrolled in TIDOS may also consent to follow-up through the Department of Veterans Affairs (VA). We present data from the first 337 TIDOS enrollees to receive VA healthcare.
Methods
Data were collected from the Department of Defense (DoD) Trauma Registry, TIDOS infectious disease module, DoD and VA electronic medical records, and telephone interview. Cox proportional hazard analysis was performed to identify predictors of post-discharge infections related to deployment trauma.
Results
Among the first 337 TIDOS enrollees who entered VA healthcare, 111 (33%) had 244 trauma-related infections during their initial trauma hospitalization (2.1 infections per 100 person-days). Following initial discharge, 127 (38%) enrollees had 239 trauma-related infections (170 during DoD follow-up and 69 during VA time). Skin and soft-tissue infections and osteomyelitis were predominant during and after the initial trauma hospitalization. In a multivariate model, a shorter time to development of a new infection following discharge was independently associated with injury severity score ≥10 and occurrence of ≥1 inpatient infection during initial trauma hospitalization.
Conclusions
Incident infections related to deployment trauma continue well after initial hospital discharge and into VA healthcare. Overall, 38% of enrolled patients developed a new trauma-related infection after their initial hospital discharge, with 29% occurring after the patient left military service.
Infectious complications after combat-related traumatic injuries have been well described throughout history [1–6]. Although risk varies based on the mechanism and pattern of injury, infections have impacted approximately a third of US military personnel wounded in the wars in Iraq and Afghanistan [2–6]. To date, much of the focus has been on infections that arise in the days to weeks following traumatic injury. Significantly less is known about the medium-term (6–9 months) and long-term (≥12 months) risk of infection among wounded military personnel beyond their initial trauma hospitalization, and even less is understood about infectious complications of trauma developing after wounded warriors have left active-duty military service.
The Trauma Infectious Disease Outcomes Study (TIDOS), a multicenter Department of Defense (DoD)–Department of Veterans Affairs (VA) observational cohort study, began enrolling military personnel after deployment-related traumatic injury in June 2009 in order to assess short- and long-term infectious complications of those injuries [5]. As part of TIDOS, DoD healthcare data are prospectively collected on participants both during and after their initial trauma hospitalization. Participants who leave the military and enroll in VA healthcare are given the option of consenting to additional collection of VA healthcare data for inclusion in TIDOS databases. The combination of DoD and VA healthcare data provides near-continuous infectious disease surveillance for the large subset of TIDOS participants who transition to VA healthcare.
Previous analyses of TIDOS data prior to the incorporation of VA data into the databases have shown a heavy burden of infection after deployment-related traumatic injury in the DoD. An analysis of the first 3 years of TIDOS data (2009–2012) found an early onset infection rate during initial hospitalization of 31% among military personnel with deployment-related traumatic injury sustained in Iraq or Afghanistan who were admitted to Landstuhl Regional Medical Center (LRMC; Germany) and transferred to a participating DoD hospital in the United States [7]. Variables related to the pattern and severity of injury (eg, traumatic amputations and volume of blood transfused within 24 hours of injury) were found to be independent risk factors for infectious complications after traumatic injury. In this subset of TIDOS enrollees (TIDOS–VA cohort), we report the incidence and natural history of infections that span from the time of initial deployment-related injury through military discharge and into civilian life.
METHODS
Study Population
A full description of the TIDOS project design has been previously published [5]. Patients were eligible for inclusion in the TIDOS cohort if they were active duty personnel aged ≥18 years, sustained a deployment-related traumatic injury, and required medical evacuation to LRMC before transfer to a participating DoD hospital in the National Capital Region (National Naval Medical Center and Walter Reed Army Medical Center; later merged to be Walter Reed National Military Medical Center) or San Antonio, Texas (Brooke Army Medical Center; renamed San Antonio Military Medical Center). The analyses herein are specific to the subset of the TIDOS population who enrolled in VA healthcare and subsequently consented to additional follow-up through review of VA electronic medical records (EMRs; TIDOS–VA cohort). The Infectious Disease Institutional Review Board (IRB) of the Uniformed Services University of the Health Sciences in Bethesda, Maryland, and the IRB of the VA St. Louis Health Care System approved the study.
Eligible patients were first approached and consented for enrollment during their initial trauma hospitalization at a participating US hospital between 1 June 2009 and 31 January 2015. Participants who subsequently left military service and entered the VA healthcare system were again contacted to obtain a second informed consent, allowing data abstraction of relevant VA healthcare records.
Study Design and Data Collection
After initial consent to participate in TIDOS, data elements obtained from the DoD Trauma Registry [8] provided information about demographics, injury circumstances and patterns, trauma history, injury severity, and elements of early trauma care prior to arrival at LRMC. Data regarding clinical care at LRMC and US DoD hospitals during initial trauma hospitalization were abstracted from medical records using the supplemental TIDOS infectious disease module [5]. To account for potential documentation delays/omissions from multiple transitions of care, prophylactic antibiotics received on day of injury (DOI) or DOI plus 1 day (DOI+1) were included.
Study participants were contacted at prespecified intervals following their initial hospital discharge (ie, 1 month, 3 months, 6 months, 12 months, 18 months, 2 years, 3 years, 4 years, and 5 years). Methods used to obtain follow-up data included telephonic interviews and review of DoD EMRs. After VA consent was obtained, study personnel at the VA St. Louis Health Care System using the VA’s Compensation and Pension Records Interchange abstracted VA healthcare data from the patients’ medical records. Trained study personnel collected data using a comprehensive data collection manual, with consultation from infectious diseases specialists as needed. Data definitions between DoD and VA were identical. The VA study data were entered directly into TIDOS study databases through a secure electronic interface.
Infectious disease events during follow-up were only collected if they were considered complications of the initial traumatic injury and included skin and soft-tissue infections (SSTIs), osteomyelitis, bloodstream infections, sepsis, pneumonia, intraabdominal infections, central nervous system infections, urinary tract infections (UTIs), sinusitis, and Clostridium difficile infections. UTIs were included if there was a traumatic injury to the pelvic/genital region or a severe head/spinal injury that necessitated urinary catheterization. Intrathoracic infections, including empyema and pneumonia, were included if there was a penetrating thoracic injury or severe head injury that required use of mechanical ventilation. As previously described [5], infections were identified based on clinical findings, laboratory test results, and imaging studies and were classified in accordance with standardized definitions. Infectious disease events were included in the analysis if there was a clinical diagnosis of infection associated with directed antimicrobial treatment (duration ≥5 days) even if the a priori definitions were not met. Conversely, if an alternate noninfectious diagnosis was documented and antimicrobial therapy was discontinued, candidate events were excluded. An infection that occurred at an identical anatomic site was considered to be a new infection if the previous treatment was completed, along with an absence of signs and symptoms of infection following discontinuation of treatment.
Statistical Analyses
Characteristics of patients with documented post-discharge infections related to their initial trauma were compared using χ2 testing for categorical indicators and nonparametric testing for continuous characteristics. A Cox proportional hazard model was used to identify associations between potential risk factors and the time to first infection following initial hospital discharge. Variables significantly associated with time to first infection or development of any infection in the unadjusted univariate models were evaluated with stepwise selection for inclusion in the adjusted multivariate model. Time to first infection in relation to various factors was analyzed in Kaplan-Meier plots (assessed with log-rank and Wilcoxon χ2). Analysis was conducted with SAS version 9.3 (SAS, Cary, North Carolina). Statistical significance was defined as P < .05.
RESULTS
Study Population
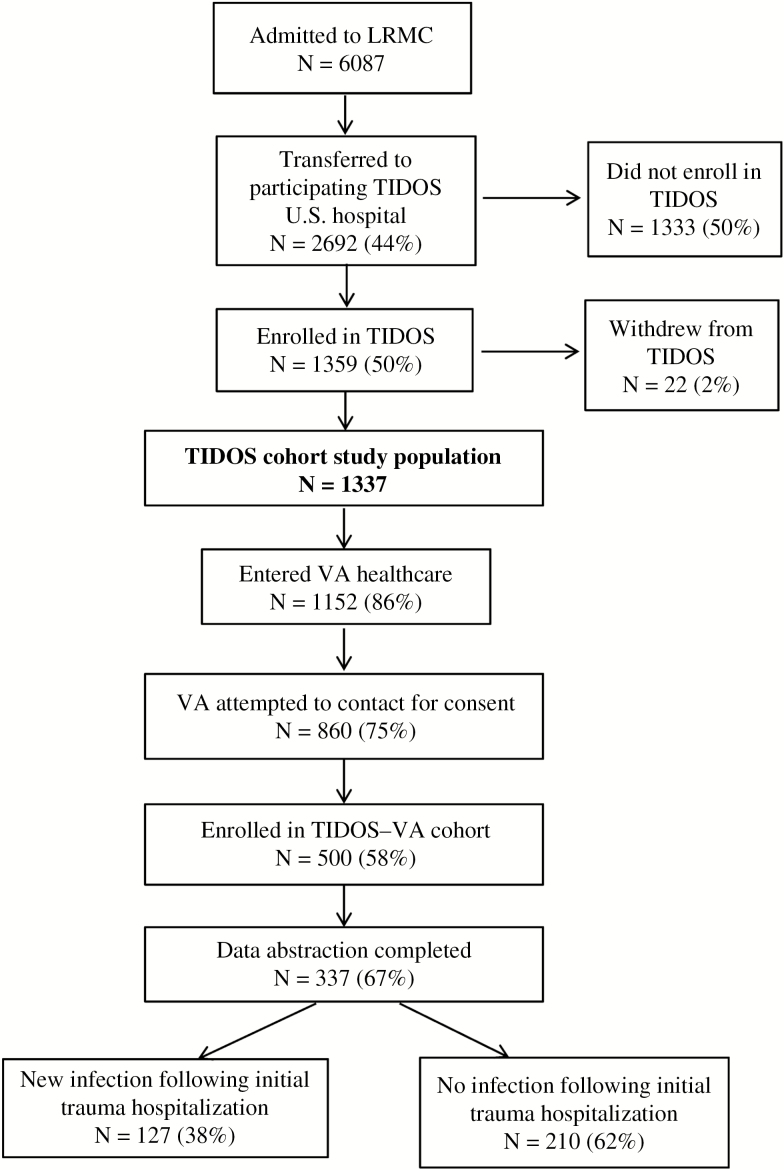
A total of 1359 wounded military personnel enrolled in TIDOS between 1 June 2009 and 31 January 2015 (end of enrollment period; Figure 1). As of 31 March 2015, 1152 enrollees (86%) had registered at a VA facility and 500 had enrolled in the TIDOS–VA cohort (enrollment remains ongoing and is currently 638). Data abstraction has been completed for the first 337 patients enrolled in the cohort, which is the study population discussed herein.
Figure 1.
Flowchart of patients following admission to Landstuhl Regional Medical Center through enrollment in the Veterans Affairs cohort of the Trauma Infectious Disease Outcomes Study, with inclusion of infection outcome information.
Abbreviations: LRMC, Landstuhl Regional Medical Center; TIDOS, Trauma Infectious Disease Outcomes Study; VA, Veterans Affairs.
Patients included in the TIDOS–VA cohort were predominantly male (99%) serving in the US Army (64%) in support of operations in Afghanistan (87%). In addition, the majority of patients sustained blast injuries (73%) and were started on post-trauma antimicrobial prophylaxis (79%). Injury severity on initial presentation was high throughout the cohort, with 55% being assigned an injury severity score (ISS) [9] of >15, indicating severe or critical injuries. Nonetheless, 53% of patients were not admitted to the intensive care unit (ICU). Time to VA registration occurred a median of 424 days (interquartile range [IQR], 267–720) after initial trauma hospitalization, while data abstraction of enrolled patients was a median of 1449 days (IQR, 1330–1578). The total person-days of follow-up was 688214, with a median of 2084 days (IQR, 1884–2249).
Incident Infections
Infections Occurring During Initial Trauma Hospitalization
Of the 337 patients, 111 (33%) were diagnosed with at least 1 infection related to traumatic injury during their initial trauma hospitalization either at LRMC or a participating US DoD hospital. There were 244 unique initial trauma hospitalization infections and a rate of 2.1 infections per 100 person-days (Table 1). A higher rate of infection was found among patients admitted to the LRMC ICU vs the non-ICU ward (P < .001). Patients most frequently had either 1 or 2 infections (43% and 28%, respectively), and the majority of these were diagnosed following admission to hospitals in the United States (80%). The most common infections were SSTIs (43%), osteomyelitis (14%), pneumonia (13%), and bloodstream infections (11%). Moreover, SSTIs and bloodstream infections were most frequent among patients with ≥3 infections (43% and 16%, respectively). Regarding the timing of infections, pneumonia had the shortest duration from injury to diagnosis (median, 4 days), followed by sepsis (median, 5 days), bloodstream infections (median, 8 days), SSTIs (median, 17 days), and osteomyelitis (median, 25 days).
Table 1.
Characteristics of Infections Related to Traumatic Injury Diagnosed During the Initial Trauma Hospitalization Among Wounded Military Personnel Enrolled in the Trauma Infectious Disease Outcomes Study–Veterans Affairs Cohort
| Infection Characteristic | Patients (N = 337) |
|---|---|
| Unique infections, no. | 244 |
| Infections per 100 person-days, no. (95% CI) | 2.1 (1.9–2.4) |
| Patients admitted initially to LRMC ICUa | 2.8 (2.4–3.3) |
| Patients admitted initially to LRMC warda | 1.1 (0.8–1.5) |
| Incidence density rate ratio (95% CI): LRMC ICU vs ward* | 2.5 (1.9–3.5) |
| Patients with ≥1 infection, no. (%) | 111 |
| Infections per patient, no. (%)b | |
| 1 event | 48 (43.2) |
| 2 events | 31 (27.9) |
| 3 events | 14 (12.6) |
| ≥4 events | 18 (16.2) |
| Level of care location for infection, no. (%)b | |
| LRMC only | 7 (6.3) |
| US hospital only | 89 (80.2) |
| Both LRMC and US hospital | 15 (13.5) |
| Type of Infection, No. (%)c | |
| Skin and soft-tissue infections | 104 (42.6) |
| Osteomyelitis | 34 (13.9) |
| Pneumonia | 31 (12.7) |
| Bloodstream infection | 26 (10.7) |
| Urinary tract infection | 12 (4.9) |
| Sepsis (excluding systemic inflammatory response system) | 8 (3.3) |
Abbreviations: CI, confidence interval; ICU, intensive care unit; LRMC, Landstuhl Regional Medical Center.
aTotal number of patients initially admitted to LRMC ICU and ward was 147 and 190, respectively.
* P < .001.
bThe total number of patients with ≥1 infection was used to calculate percent.
cThe total number of unique infections was used to calculate percent. Does not include miscellaneous infections, such as sinusitis and central nervous system infections.
Infections Occurring After Discharge From Initial Trauma Hospitalization
Following initial hospitalization, 127 patients (38%) developed a total of 239 new infections related to their traumatic injury (Table 2). Patients diagnosed with a new infection post-discharge were more likely to have had an ISS ≥10 during their initial trauma hospitalization compared to those who did not (83% vs 64%; P = .002). In addition, more patients with new post-discharge infections received large-volume (≥10 units) transfusions of blood products within 24 hours post-injury (28% vs 15%; P = .004) and prophylactic antimicrobial therapy (86% vs 75%; P = .020). Patients who developed a new infection post-discharge were also more than twice as likely to have had at least 1infection during their initial trauma hospitalization compared to those who did not develop a later infection (47% vs 24%; P < .001). Approximately 66% of patients with ≥3 infections during initial hospitalization developed a new infection post-discharge.
Table 2.
Characteristics of Wounded Military Personnel by Presence or Absence of Infection Related to Traumatic Injury After Discharge From Initial Trauma Hospitalization
| Characteristic | Total (N = 337) | Infection Following Discharge (N = 127) | No Infection Following Discharge (N = 210) | P Value |
|---|---|---|---|---|
| Operational theater, no. (%) | .989 | |||
| Iraq | 45 (13.3) | 17 (13.3) | 28 (13.3) | … |
| Afghanistan | 292 (86.6) | 110 (86.6) | 182 (86.6) | … |
| Male, no. (%) | 333 (98.8) | 125 (98.4) | 208 (99.0) | .630 |
| Age at time of injury, median (IQR) | 24 (22, 29) | 24 (22, 29) | 24 (22, 28) | .106 |
| Branch of service, no. (%) | .608 | |||
| Army | 215 (63.7) | 85 (66.9) | 130 (61.9) | … |
| Marine | 104 (30.8) | 35 (27.5) | 69 (32.8) | … |
| Air Force/Navy | 18 (5.3) | 7 (5.5) | 11 (5.2) | … |
| Blast mechanism of injury, no. (%) | 247 (73.2) | 97 (76.3) | 150 (71.4) | .320 |
| Dismounted at time of injury, no. (%)a | 106 (31.4) | 45 (35.4) | 61 (29.0) | .412 |
| Injury severity score,b no. (%) | .002 | |||
| 0–9 (minor) | 97 (28.7) | 21 (16.5) | 76 (36.1) | … |
| 10–15 (moderate) | 54 (16.0) | 23 (18.1) | 31 (14.7) | … |
| 16–25 (severe) | 74 (21.9) | 31 (24.4) | 43 (20.4) | … |
| ≥26 (critical) | 112 (33.2) | 52 (40.9) | 60 (28.5) | … |
| ICU admission, no. (%)c | .540 | |||
| LRMC only | 43 (12.7) | 15 (11.8) | 28 (13.3) | … |
| US hospital ± LRMC | 115 (34.1) | 48 (37.7) | 67 (31.9) | … |
| Non-ICU | 179 (53.1) | 64 (50.3) | 115 (54.7) | … |
| Blood transfusion within 24 hours (units) | .004 | |||
| Zero or missing unitsd | 187 (55.5) | 56 (44.1) | 131 (62.4) | … |
| 1–9 | 84 (24.9) | 36 (28.3) | 48 (22.8) | … |
| 10–20 | 47 (13.9) | 23 (18.1) | 24 (11.4) | … |
| >20 | 19 (5.6) | 12 (9.4) | 7 (3.3) | … |
| Received prophylactic antimicrobial therapy DOI/ DOI + 1 | 267 (79.2) | 109 (85.8) | 158 (75.2) | .020 |
| Had an inpatient infection | 111 (32.9) | 60 (47.2) | 51 (24.2) | <.001 |
| Total hospitalization, median days (IQR) | 23 (13, 43) | 30 (19, 55) | 18 (11, 35) | <.001 |
Abbreviations: DOI/DOI + 1, day of injury or day of injury plus 1 day; ICU, intensive care unit; IQR, interquartile range; LRMC, Landstuhl Regional Medical Center.
aMounted status data are missing/unknown for 130 patients.
bInjury severity score provides an overall score of injury severity based on anatomic regional values [9].
cAdmission is recorded within the first week of care at each facility.
dEight patients had zero units and 179 had missing data. Missing blood transfusion data are not randomly distributed. Patients with missing blood data are characterized by lower injury severity scores and shock indices. In addition, the majority of patients with missing blood data did not sustain a traumatic amputation and were not admitted to the LRMC ICU.
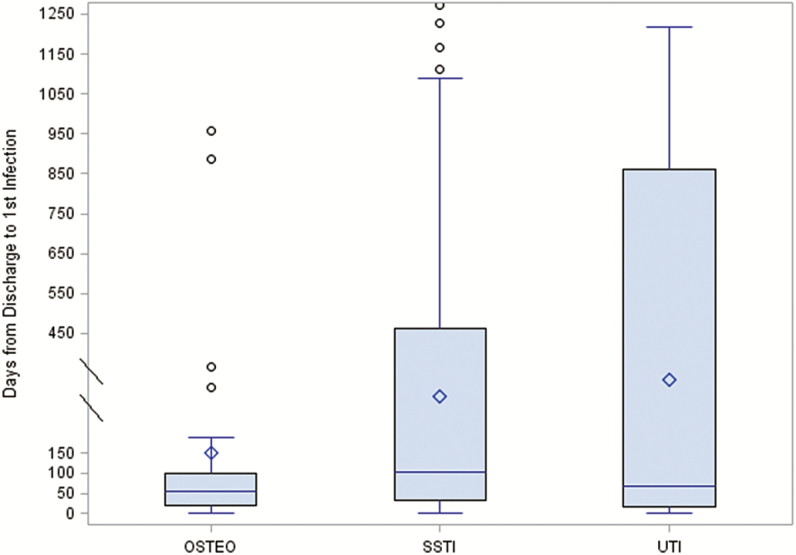
Of the 239 unique infections after initial hospital discharge, 170 (71%) were identified through DoD medical records and follow-up and 69 (29%) via VA EMRs (Table 3). Similar to infections that occurred during the initial trauma hospitalization, the most common infections after initial discharge were SSTIs (68%) and osteomyelitis (13%), as well as UTIs (7%). The time from initial hospital discharge to diagnosis of incident infections was a median of 88 days (IQR, 19–351) for any infection, 104 days (IQR, 33–462) for SSTIs, 54 days (IQR, 20–99) for osteomyelitis, and 69 days (IQR, 15–860) for UTIs (Figure 2). Using time following hospital discharge to diagnosis of first infection, the incidence density rate for SSTIs was 0.018 per 100 person-days (95% confidence interval [CI], 0.015–0.023), while it was 0.004 (95% CI, 0.002–0.005) for osteomyelitis and 0.002 (95% CI, 0.0008–0.003) for UTIs.
Table 3.
Distribution of Infections Related to Traumatic Injury Following Initial Trauma Hospitalization Among Wounded Military Personnel Enrolled in the Trauma Infectious Disease Outcomes Study–Veterans Affairs Cohort
| Data Source, No. (%) | |||||
|---|---|---|---|---|---|
| Department of Defense | Veterans Affairs | ||||
| Type of Infection | Number of Infectionsa | N | Time to Diagnosis, Median (IQR)b | N | Time to Diagnosis, Median (IQR)b |
| Skin and soft-tissue infections | 161 | 117 (73) | 170 (48–411) | 44 (27) | 829 (187–1292) |
| Osteomyelitis | 31 | 23 (74) | 97 (26–315) | 8 (26) | 81 (41–910) |
| Urinary tract infections | 16 | 4 (25) | 119 (43–666) | 12 (75) | 95 (17–748) |
| Sinusitis and mastoiditis | 11 | 11 (100) | 266 (162–423) | 0 | NA |
| Pneumonia/tracheobronchitis | 6 | 6 (100) | 422 (291–507) | 0 | NA |
| Intraabdominal infection | 6 | 6 (100) | 211 (9–493) | 0 | NA |
| Bloodstream infection | 5 | 3 (60) | 180 (36–409) | 2 (40) | 401 (0–802) |
| Clostridium difficile | 2 | 0 | NA | 2 (100) | 1260 (1253–1267) |
| Otitis media | 1 | 0 | NA | 1 (100) | 1578 (NA) |
| Total | 239 | 170 (71) | … | 69 (29) | … |
Abbreviations: IQR, interquartile range; NA, not applicable.
aPatients may have more than 1 infection, so sum is greater than patient total.
bDays from initial hospital discharge to infection diagnosis.
Figure 2.
Time to new infection related to traumatic injury following initial trauma hospital discharge for the most common infections (skin and soft-tissue infections, osteomyelitis, urinary tract infections).
Abbreviations: OSTEO, osteomyelitis; SSTI, skin and soft-tissue infection; UTI, urinary tract infection.
Risk Factor Analysis
Risk factors were examined in a Cox proportional hazard model using time to first infection following discharge from the initial US hospitalization as the primary event (Table 4). Volume of blood products transfused within 24 hours post-injury, ISS, length of initial trauma hospitalization, number of infections during initial hospitalization, number of operating room visits within 2 weeks of injury, receipt of prophylactic antibiotics, and sustaining a traumatic or surgical amputation were significantly associated with a shorter time to infection in the univariate model. Upon multivariate analysis, an ISS of ≥10 (greatest with ISS 10–15; hazard ratio [HR], 2.72; 95% CI, 1.45–5.11) and having at least 1 infection during initial hospitalization (greatest with ≥3 infections; HR, 2.66; 95% CI, 1.50–4.73) remained statistically significant independent risk factors for a shorter time to first infection following the initial trauma hospitalization (Table 4).
Table 4.
Cox Proportional Hazard Analysis of Factors Associated With the Time to First Infection Related to Traumatic Injury Following Initial Trauma Hospitalization
| Risk Factor | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) | P Value |
|---|---|---|---|
| Operational theater | |||
| Afghanistan | Reference | … | … |
| Iraq | 1.08 (0.65–1.81) | … | … |
| Combat-related injury | 1.13 (0.46–2.76) | 0.56 (0.20–1.56) | .265 |
| Mechanism of injury | |||
| Non-blast | Reference | … | … |
| Blast | 1.12 (0.75–1.69) | … | … |
| Injury severity score | |||
| 0–9 (minor) | Reference | Reference | … |
| 10–15 (moderate) | 2.66 (1.45–4.88) | 2.72 (1.45–5.11) | .002 |
| 16–25 (severe) | 2.67 (1.53–4.67) | 2.65 (1.45–4.84) | .002 |
| ≥26 (critical) | 2.66 (1.57–4.51) | 2.40 (1.24–4.62) | .009 |
| Blood transfusion within 24 hours (units) | |||
| Zeroa | Reference | … | … |
| 1–9 | 1.59 (1.04–2.41) | … | … |
| 10–20 | 1.85 (1.14–3.02) | … | … |
| >20 | 2.15 (1.15–4.01) | … | … |
| Total initial hospitalization (days) | |||
| ≤14 | Reference | … | … |
| 15–30 | 1.86 (1.12–3.09) | … | … |
| 31–60 | 2.32 (1.37–3.95) | … | … |
| >60 | 3.13 (1.80–5.43) | … | … |
| Received prophylactic antimicrobial therapy DOI/DOI + 1 | 1.88 (1.11–3.17) | 1.71 (0.95–3.11) | .076 |
| ICU admission, no. (%) | |||
| Non-ICU | Reference | Reference | … |
| LRMC only | 0.97 (0.55–1.70) | 0.58 (0.31–1.10) | .094 |
| US hospital ± LRMC | 1.19 (0.82–1.73) | 0.65 (0.40–1.05) | .079 |
| Infections diagnosed during initial hospitalization | |||
| None | Reference | Reference | |
| 1–2 | 1.91 (1.29–2.84) | 1.81 (1.16–2.82) | .009 |
| ≥3 | 2.73 (1.67–4.46) | 2.66 (1.50–4.73) | <.001 |
| Operating room visits with 2 weeks of injury | |||
| None or 1 | Reference | … | … |
| 2–3 | 1.70 (1.02–2.84) | … | … |
| ≥4 | 2.16 (1.34–3.48) | … | … |
| Mechanical ventilation during initial hospitalization | |||
| None | Reference | … | … |
| LRMC only | 1.28 (0.82–2.00) | … | … |
| LRMC and US hospital –first week | 1.22 (0.69–2.15) | … | … |
| LRMC and US hospital ≥second week | 1.29 (0.67–2.50) | … | … |
| Traumatic amputationb | 1.58 (1.07–2.33) | … | … |
| Surgical amputation/revision of amputationc | 1.82 (1.16–2.83) | … | … |
Abbreviations: CI, confidence interval; DOI/DOI + 1, day of injury or day of injury plus 1 day; ICU, intensive care unit; LRMC, Landstuhl Regional Medical Center.
aIf patients were missing blood transfusion data, they were included in “zero” category.
bAmputation recorded prior to or at LRMC.
cAmputation recorded at US hospital.
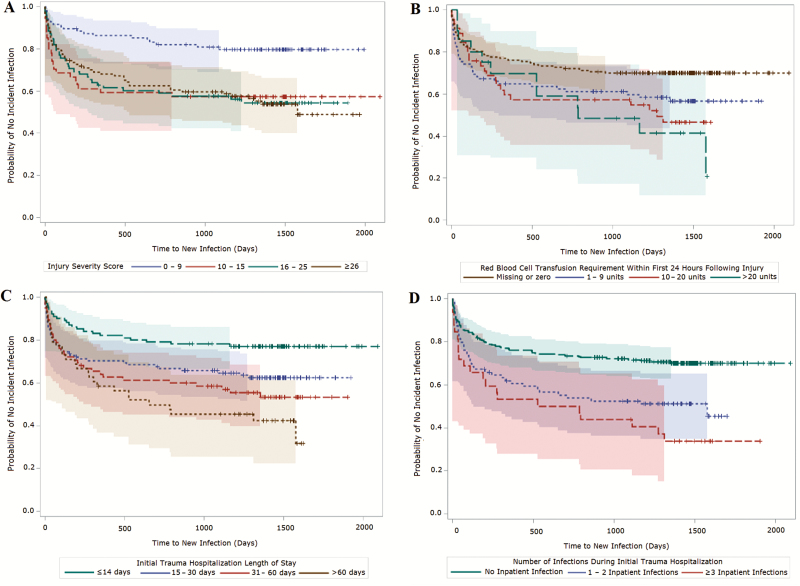
The associations of time to new infection with ISS, volume of blood transfusion in the first 24 hours post-injury, length of initial trauma hospitalization, and number of infections during initial trauma hospitalization were also examined in Kaplan-Meier plots (Figure 3). The likelihood of developing an infection was greatest within the first year following hospital discharge. Associations between time to new infection and all 4 examined variables were statistically significant in Kaplan-Meier analyses.
Figure 3.
Kaplan-Meier survival plots (with 95% Hall-Wellner bands) of time to new infection following initial trauma hospital discharge. A, Plot stratified by injury severity score. Log-rank χ2, 16.8 (P < .001); Wilcoxon χ2, 16.2 (P = .001). B, Plot stratified by volume of blood transfusion within 24 hours of injury. Log-rank χ2, 11.2 (P = .011); Wilcoxon χ2, 7.4 (P = .060). C, Plot stratified by length of inpatient hospitalization. Log-rank χ2, 18.7 (P < .001); Wilcoxon χ2, 15.7 (P = .001). D, Plot stratified by number of inpatient infections. Log-rank χ2, 21.8 (P < .001); Wilcoxon χ2, 18.2 (P < .001).
DISCUSSION
While many trauma-related infections present during the initial phase of care after a combat-related injury, the burden of infectious complications persists long after hospital discharge for many wounded military personnel. The TIDOS project is the first study to assess trauma-related infections among deployed military personnel with respect to rates, syndromes, outcomes, and risk factors from the time of initial care through military service and into civilian life. The merging of DoD and VA data for this study provides a unique opportunity to understand the burden and natural history of infection in this population. Our data show that approximately 38% of enrolled patients developed a new trauma-related infection after their initial hospital discharge and that 29% of such infections occurred after leaving military service.
The results of our risk factor analysis corroborate prior investigations that identified an independent association between ISS and infection risk [3, 10]. The high injury severity is likely a reflection of the high rate of blast injuries that occurred in Afghanistan, where 87% of our study population sustained injuries. These injuries were frequently characterized by traumatic amputations and often required large-volume blood transfusions within 24 hours of injury [11]. It is noteworthy that risk factors independently associated with a shorter time to development of a new infection following initial hospital discharge are not variables that can be easily modified by clinicians but could increase awareness and close follow-up during this period.
SSTIs and osteomyelitis were the most common types of infection identified within the TIDOS–VA cohort both during initial trauma hospitalization and in subsequent DoD and VA healthcare, consistent with what is expected from blast injuries with contaminated wounds. Both infection syndromes are well-described sequelae of open lower extremity fractures sustained by military personnel after blast injury in Afghanistan and Iraq [12–17]. While most of these infections occurred within the first 30 days of injury in our cohort, we identified a second peak in SSTIs and, to a lesser extent, osteomyelitis 6 to 12 months later. This is not surprising given the often chronic and recurrent nature of these types of infections, and it is likely that the VA healthcare system will shoulder the majority of care of these patients as they leave active-duty service. Interestingly, UTIs were more frequently diagnosed in VA healthcare compared to the DoD in our cohort and may represent an underrecognized and potentially chronic complication of perineal trauma, which is frequently seen after blast-related injury in dismounted military personnel.
This study provides the first longitudinal survey of infectious complications in combat-injured US military personnel spanning DoD and VA healthcare. The use of DoD and VA healthcare system EMRs allows for the comprehensive capture of clinical data from most, if not all, health encounters within each respective system. It is possible that TIDOS–VA participants may have also received healthcare at civilian clinical sites outside of the DoD and VA system. While telephonic interviews are a component of TIDOS data collection, such outside events could have been missed due to recall inaccuracies or lack of disclosure by the participant. By virtue of our reliance on EMRs for clinical data, another limitation of this study is that all determinations of infectious disease events were made based on the strength of clinical documentation. Therefore, it is plausible that minor or ambiguous infections could have been excluded from our database. As all TIDOS–VA participants had to have sustained trauma severe enough to require medical evacuation to Germany before being transferred back to the United States, our cohort does not include patients with minor trauma treated exclusively within the combat zone. If we had included these patients, it is possible that we might have identified even more trauma-related infections, particularly SSTIs, given potential for wound contamination with improvised explosive devices and other blast mechanisms.
It is possible that key risk factors for infection related to modifiable elements of early care were not included in this analysis. Future analyses focusing on specific infectious syndromes, such as SSTI and osteomyelitis, may elucidate such early care elements, which may be adjusted in order to improve outcomes. In addition, of particular interest to us is the impact of mental health and social support on infection recurrence. We have collected longitudinal data for this cohort on depression, post-traumatic syndrome disorder, substance use, and social support during VA healthcare time. Future analyses focusing on the risk of infection during VA care will incorporate these data elements and help determine whether these potentially modifiable risk factors impact late infection risk.
The unique nature of our data, obtainable only through DoD–VA collaboration, highlights the vital importance of interagency research collaboration. While many research groups have successfully combined DoD and VA healthcare data for research, it is notable that many daunting barriers to carrying out such research persist, most prominently related to sharing data and research funding. Efforts by both agencies to streamline processes of collaboration should continue.
In conclusion, our analysis of the TIDOS–VA cohort demonstrates that many wounded US military personnel from the wars in Iraq and Afghanistan remain at significant risk for trauma-related infection for many years, long past the point of hospital discharge and even after leaving military service. Although our study was restricted to severely wounded service members, our findings may be applicable to generalized military and civilian populations in circumstances that involve serious traumatic injuries, particularly those involving a blast mechanism. In the coming years, more US veterans of the wars in Iraq and Afghanistan will transition to VA healthcare. As TIDOS–VA enrollment and data collection are presently ongoing, the size and statistical power of the cohort will continue to grow. Future analyses of this cohort will include examination of specific infectious syndromes, risk of late/recurrent infection, and impact of mental health and social support on infection.
Notes
Acknowledgments. We are indebted to the Infectious Disease Clinical Research Program, The Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
Disclaimer. The views expressed are those of the authors and do not reflect the official views or policies of the Uniformed Services University of the Health Sciences, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, Walter Reed National Military Medical Center, Landstuhl Regional Medical Center, US Army Medical Department, the US Army Office of the Surgeon General, the Department of Defense, and the Departments of the Army, Navy, and Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Financial support. This work (IDCRP-024) was supported by the Infectious Disease Clinical Research Program, a DoD program executed through the Uniformed Services University of the Health Sciences, Department of Preventive Medicine and Biostatistics. This project was funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health (interagency Agreement Y1-AI-5072), and the Department of the Navy under the Wounded, Ill, and Injured Program (HU001-10-1-0014).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Murray CK, Hinkle MK, Yun HC. History of infections associated with combat-related injuries. J Trauma 2008; 64:S221–31. [DOI] [PubMed] [Google Scholar]
- 2. Murray CK. Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. J Trauma 2008; 64:S232–8. [DOI] [PubMed] [Google Scholar]
- 3. Petersen K, Riddle MS, Danko JR, et al. . Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007; 245:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murray CK, Wilkins K, Molter NC, et al. . Infections in combat casualties during Operations Iraqi and Enduring Freedom. J Trauma 2009; 66:S138–44. [DOI] [PubMed] [Google Scholar]
- 5. Tribble DR, Conger NG, Fraser S, et al. . Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 2011; 71:S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murray CK, Wilkins K, Molter NC, et al. . Infections complicating the care of combat casualties during Operations Iraqi Freedom and Enduring Freedom. J Trauma 2011; 71:S62–73. [DOI] [PubMed] [Google Scholar]
- 7. Weintrob AC, Murray CK, Xu J, et al. . Infections complicating the care of casualties from Iraq and Afghanistan. Surg Infect 2018; 19:286–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB. Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2006; 61:1366–72; discussion 1372–3. [DOI] [PubMed] [Google Scholar]
- 9. Linn S. The injury severity score—importance and uses. Ann Epidemiol 1995; 5:440–6. [DOI] [PubMed] [Google Scholar]
- 10. Dickens CP, Kilcoyne CK, Kluk CM, et al. . Risk factors for infection and amputation following open, combat-related calcaneal fractures. J Bone Joint Surg Am 2013; 95: e241–8. [DOI] [PubMed] [Google Scholar]
- 11. Ficke JR, Eastridge BJ, Butler F, et al. . Dismounted complex blast injury report of the Army Dismounted Complex Blast Injury Task Force. J Trauma Acute Care Surg 2012; 73:S520–34. [Google Scholar]
- 12. Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis 2007; 45:409–15. [DOI] [PubMed] [Google Scholar]
- 13. Yun HC, Branstetter JG, Murray CK. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma 2008; 64:S163–8; discussion S168. [DOI] [PubMed] [Google Scholar]
- 14. Mody RM, Zapor M, Hartzell JD, et al. . Infectious complications of damage control orthopedics in war trauma. J Trauma 2009; 67:758–61. [DOI] [PubMed] [Google Scholar]
- 15. Burns TC, Stinner DJ, Mack AW, et al. ; Skeletal Trauma Research Consortium Microbiology and injury characteristics in severe open tibia fractures from combat. J Trauma Acute Care Surg 2012; 72:1062–7. [DOI] [PubMed] [Google Scholar]
- 16. Huh J, Stinner DJ, Burns TC, Hsu JR; Late Amputation Study Team Infectious complications and soft tissue injury contribute to late amputation after severe lower extremity trauma. J Trauma 2011; 71:S47–51. [DOI] [PubMed] [Google Scholar]
- 17. Napierala MA, Rivera JC, Burns TC, Murray CK, Wenke JC, Hsu JR; Skeletal Trauma Research Education Consortium Infection reduces return-to-duty rates for soldiers with type III open tibia fractures. J Trauma Acute Care Surg 2014; 77:S194–7. [DOI] [PubMed] [Google Scholar]