Abstract
Parvimonas micra is an obligate anaerobic bacterium and a known commensal of the human oral cavity and gastrointestinal tract. It is rarely associated with infections outside the oral cavity. Recently it has been isolated as a causative agent in a variety of systemic infections, but it has never been previously identified to cause a hepatic abscess. We report a 90-year-old woman with multiple hepatic abscesses caused by P. micra.
Introduction
Parvimonas micra is a known commensal bacterium of the human oral flora and gastrointestinal tract. It is an obligate anaerobe, identified in 1933 as Peptostreptococcus micros. It was reclassified in 1999 as Mircomonas micros, and then in 2006 as P. micra. Infections with P. micra outside of the oral cavity are uncommon. Some case reports have isolated P. micra from spinal abscesses, while others have found P. micra as causative agents of meningitis, septic arthritis, chest wall abscess, spondylodiscitis, empyema, endocarditis, and brain abscess.1-12 A thorough literature search revealed no reports of hepatic infections with P. micra in current literature.
Case Report
A 90-year-old woman presented to the emergency room with fatigue, which began a month prior along with generalized weakness that slowly worsened. She had decreased appetite, and she reported increased shortness of breath while walking small distances. Her past medical history was significant for ovarian cancer diagnosed 52 years earlier, for which she underwent total abdominal hysterectomy and bilateral salpingo-oopherectomy, malignant melanoma of the right cheek 20 years earlier, colonic polyps, atrial fibrillation, and a left renal mass diagnosed 5 years earlier, for which patient refused to undergo further work-up. She denied fevers, chills, chest pain, hematochezia, melena, hematemesis, constipation, changes in urinary habits, and travel or exposure to sick contacts.
On presentation she had pulse rate 80 beats per minute, respiratory rate 20 breaths per minute, blood pressure 102/50 mm Hg, and oral temperature of 97°F. She was alert, awake, oriented, and in no distress. Inspection of her mouth showed no oral lesions, dental trauma, or signs of acute intra-oral infection. Lung exam revealed wheezing and coarse breath sounds in all lung fields. Her abdomen was soft, and tenderness was noted in the right upper quadrant. Bowel sounds were present without palpable masses or organomegaly.
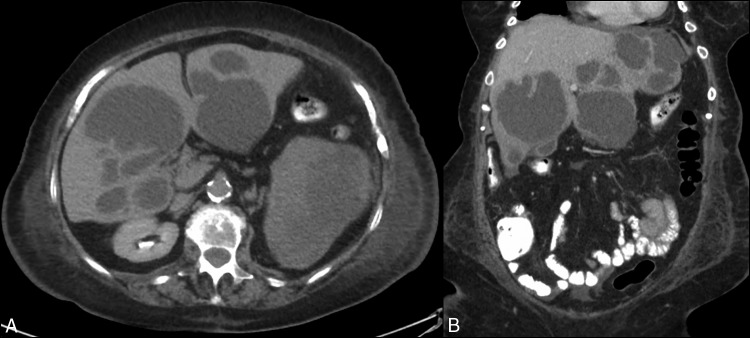
Laboratory studies revealed hemoglobin 11.3 g/dL, white blood cell count 23.6 × 109/L, platelets 334 × 109/L, aspartate aminotransferase 115 U/L, alanine aminotransferase 102 U/L, alkaline phosphatase 329 U/L, total bilirubin 2.1 mg/dL, and serum creatinine 1.93 mg/dL. An ultrasonogram of the liver showed multiple liver masses of varying echogenicity. The 2 largest masses were 11 cm × 8 cm × 8 cm and 11 cm × 9 cm × 7 cm. Computed tomography of liver performed with intravenous contrast demonstrated multiple rim-enhancing lesions in both hepatic lobes with enhancing septations (Figure 1). Blood cultures drawn at admission and on day 6 of hospitalization resulted exhibited no growth. Antibiotic therapy with intravenous vancomycin, ceftriaxone, and metronidazole was initiated for empiric treatment. A surgical consult was requested for drainage of the abscesses, but the patient and her family expressed a desire to perform only minimally invasive procedures.
Figure 1.
Contrast-enhanced abdominal computed tomography: (A) transverse image showing multiple rim-enhancing lesions in both hepatic lobes; (B) coronal image showing thick enhancing septations of hepatic abscesses.
The abscesses were drained using ultrasonography guidance. Drains were left in place, and turbid, purulent yellow drainage was noted. Gram stain of the drainage showed heavy Gram-positive cocci in clusters. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry, P. micra was isolated from the specimen. The antibiotic regimen was de-escalated to ceftriaxone and metronidazole. Cytology study of the fluid did not show any malignant cells. Drains continued to pass 30–45 mL/d for the next few days. Throughout the hospital stay, the patient continued to have sepsis with worsening renal function. Liver transaminase levels decreased initially and later normalized after drainage of abscesses, but leukocytosis worsened. After a few days of antibiotic therapy, in the setting of an undiagnosed renal mass, advanced age, and poor baseline functional status, the patient and her family chose to pursue comfort-focused care. She passed away on day 12 of her hospital stay.
Discussion
Hepatic abscesses can be classified as bacterial, amebic, or fungal, with bacterial accounting for 80% of all cases. Common risk factors include diabetes mellitus and underlying hepatobiliary or pancreatic disease. Abscess formation has several pathogeneses. Bacteria can spread via portal vein circulation, through an infected bowel as seen in appendicitis, or directly through the hepatobiliary system. In healthy liver tissue, Kupfer cells clear any bacteremia. In the immunocompromised population, the lack of this natural defense system can result in infection and abscess formation. In the setting of malignancy, the incidence of hepatic abscess is far more common, as seen in our patient.13 Although cytology of the abscess fluid was negative for malignancy, the possibility of hepatic spread from the undiagnosed renal mass could not be completely ruled out.
The presence of multiple large abscesses also makes our case rare in presentation. The presence of a clear focus of infection could not be established despite work-up. The current literature states that the most common causes of abscess formation include Escherichia coli and Klebsiella pneumoniae, respectively, with K. pneumoniae being of particular importance in nosocomial infections.14 P. micra is an anaerobic Gram-positive bacteria and is indicated in periodontal disease.15 While no known cases of hepatic abscess from P. micra have been described, this may speak to the difficulty of culturing this bacterium. As recently as 2013, a strain of P. micra known as strain A293 was isolated from an abdominal abscess in United Kingdom.16
Regardless of the causative microbe, hepatic abscesses when untreated are often fatal. Prompt empiric treatment with antibiotics and surgical drainage of large abscesses is recommended. Success of treatment can be monitored by leukocyte count and trending the fever curve. Given the high incidence of hepatic abscess formation in the setting of malignancy, a thorough work-up for primary cancers should be considered. As identification techniques increase in specificity and more data are added in regard to this rare pathogen, more cases may be identified. Resistance has been noted in several cases, although reports seem to be varied and may be related to location of infection and penetrability of chosen therapy.17 Effective treatment of P. micra is usually achieved with metronidazole, clindamycin, or penicillin. Treatment should be guided by individual susceptibility results. Given the rare incidence of this infection, consensus of treatment duration does not yet exist and should be guided by clinical judgment.
Disclosures
Author contributions: All authors contributed equally in manuscript creation. M. Yadav is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Frat JP, Godet C, Robert R. Cervical spinal epidural abscess and meningitis due to Prevotella oris and Peptostreptococcus micros after retropharyngeal surgery. Intensive Care Med. 2004;30:1695. [DOI] [PubMed] [Google Scholar]
- 2.Uemura H, Hayakawa K, Shimada K, et al. Parvimonas micra as a causative organism of spondylodiscitis: A report of two cases and a literature review. Int J Infect Dis. 2014;23:53–5. [DOI] [PubMed] [Google Scholar]
- 3.Wenisch C, Wiesinger E, Werkgartner T, Makristathis A, Graninger W. Treatment of Peptostreptococcus micros endocarditis with teicoplanin. Clin Infect Dis. 1995;21:446–7. [DOI] [PubMed] [Google Scholar]
- 4.Riesbeck K, Sanzén L. Destructive knee joint infection caused by Peptostreptococcus micros: Importance of early microbiological diagnosis. J Clin Microbiol. 1999;37:2737–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poetter C, Pithois C, Caty S, Petit V, Combier JP, Mourtialon P, Mattner F. Hiding behind confusion: Pleural empyema caused by Parvimonas micra. Surg Infect (Larchmt). 2014;15:356–7. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Segade S, Velasco D, Marcos PJ. Empyema due to Aggregatibacter aphrophilus and Parvimonas micra coinfection. Arch Bronconeumol. 2015;51:254–5. [DOI] [PubMed] [Google Scholar]
- 7.García Carretero R, Luna-Heredia E, Olid-Velilla M, Vazquez-Gomez O. Bacteraemia due to Parvimonas micra, a commensal pathogen, in a patient with an oesophageal tumour. BMJ Case Rep. 2016;2016:bcr2016217740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George I, Pande A, Parsaei S. Delayed infection with Parvimonas micra following spinal instrumentation. Anaerobe. 2015;35:102–4. [DOI] [PubMed] [Google Scholar]
- 9.Gomez C, Gerber D, Zambrano E, Banaei N, Deresinski S, Blackburn B. First case of infectious endocarditis caused by Parvimonas micra. Anaerobe. 2015;36:53–5. [DOI] [PubMed] [Google Scholar]
- 10.Gorospe L, Bermudez-Coronel-Prats I, Gomez-Barbosa C, Olmedo-Garcia M, Ruedas-Lopez A, Gomez del Olmo V. Parvimonas micra chest wall abscess following transthoracic lung needle biopsy. Korean J Intern Med. 2014;29(6):834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones S, Riordan J, Glasgow A, Botes J, Boutlis C. Two cases of spondylodiscitis caused by Parvimonas micra. Intern Med J. 2015;45(10):1090–1. [DOI] [PubMed] [Google Scholar]
- 12.Ko J, Baek J, Kang C, et al. Bacteremic meningitis caused by Parvimonas micra in an immunocompetent host. Anaerobe. 2015;34:161–3. [DOI] [PubMed] [Google Scholar]
- 13.Marcus S, Walsh T, Pizzo P, Danforth D. Hepatic abscess in cancer patients: Characterization and management. Arch Surg. 1993;128(12):1358–64. [DOI] [PubMed] [Google Scholar]
- 14.Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. Virulent clones of Klebsiella pneumoniae: Identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One. 2009;4(3):e4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tindall BJ, Euzéby JP. Proposal of Parvimonas gen. nov. and Quatrionicoccus gen. nov. as replacements for the illegitimate, prokaryotic, generic names Micromonas Murdoch and Shah 2000 and Quadricoccus Maszenan et al. 2002, respectively. Int J Syetm Evolut Microbiol. 2006;56:2711–3. [DOI] [PubMed] [Google Scholar]
- 16.Ang M, Dymock D, Tan J, Thong M, Tan Q, Wong G, Paterson I, Choo S. Genome sequence of Parvimonas micra strain A293, isolated from an abdominal abscess from a patient in the United Kingdom. Genome Announc. 2013;1:e01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdoch DA. Gram-positive anaerobic cocci. Clin Microbiol Rev. 1998;11:81–120. [DOI] [PMC free article] [PubMed] [Google Scholar]