Abstract
Angiogenesis is required for tumor growth. Dihydroartemisinin (DHA), a the effective anti-malarial derivative of artemisinin, demonstrated potent anti-angiogenic activities that closely related to the regulation of vascular endothelial growth factor (VEGF) signaling cascade. VEGF receptor 1 (VEGFR1), a receptor in endothelial cells (ECs), coordinately regulate angiogenic activity triggered by ligand-receptor binding. Here we aimed to explore the effects of DHA on VEGFR1 expression in ECs. We found that DHA significantly increases VEGFR1 expression in human umbilical vein endothelial cells (HUVECs). In addition, DHA significantly upregulates the level of V-Ets Avian Erythroblastosis Virus E26 Oncogene Homolog 1 (ETS-1), a transcriptional factor which binds to the human VEGFR1 promoter. ChIP assay showed that DHA increases ETS-1 binding to the -52 ETS motif on the VEGFR1 promoter. Knockdown of ETS-1 by RNA interference abolished DHA-induced increase of VEGFR1 expression. Taken together, we demonstrated that DHA elevates VEGFR1 expression via up-regulation of ETS-1 transcription in HUVECs.
Keywords: dihydroartemisinin, angiogenesis, endothelial cells, VEGFR1, ETS-1
Introduction
Angiogenesis refers to the form of new vascular network by the proliferation and migration from the original vascular endothelial cells 1. Angiogenesis is essential both in the adult new blood vessel formation and in embryogenesis, while abnormal angiogenesis is related to certain pathological states such as cancer 2, 3. During cancer growth, newly formed blood vessels are required to provide nutrients and oxygen, and remove the waste products 3, 4.
Among the regulators of angiogenesis, VEGF is the most potent stimulator in physiological and pathological situations 5, 6. To promote angiogenesis, VEGF acts through its receptor VEGFR2, which is highly expressed in ECs 7-9. VEGFR1, another receptor of VEGF, is considered to be a 'decoy' receptor which isolates VEGF and reduces its incorporation to VEGFR2 10-12. Previous studies suggested that VEGFR1 suppresses the pro-angiogenic signals induced by VEGFR2 in ECs 13. In addition, the soluble VEGFR1 which carries only the extracellular domain, is considered to be a natural inhibitor of VEGF-A 7.
Dihydroartemisinin (DHA) is a semi-synthetic derivative of artemisinin, which is extracted from Chinese herb Artemisia annua 14, 15. Like other artemisinin derivatives, DHA displayed strong anti-inflammatory and anti-angiogenic activities 16-18. Although DHA have a positive role in angiogenesis in zebrafish 19, it suppressed the growth, proliferation, migration, and tube formation of mammalian ECs, which are essential processes in angiogenesis 20-22. In addition, DHA inhibited VEGF expression in cancer cells and reduced VEGF binding to its receptors in HUVECs 18, 23. However, the role and mechanisms by which DHA affects VEGFR1 expression in ECs have not been studied.
ETS-1 is a member of the ETS transcriptional factor family which contains about 30 related proteins and a basic 80-90 aa DNA-binding domain 24. As a transcription factor, ETS-1 is highly expressed in vascular system 25, 26. In ECs, ETS-1 regulates downstream target genes including Tie1, Tie2, MMPs, p53 and VEGFR1 27-30. On the VEGFR1 promoter, ETS-1 binds to a conserved ETS responsive element between -49 and -52 region to promote VEGFR1 expression 31.
In this study, we reported that DHA significantly upregulates the expression of VEGFR1 and ETS-1 in HUVECs. ChIP assays validated that the binding of ETS-1 on the VEGFR1 promoter was significantly increased by DHA treatment. In addition, knockdown of ETS-1 eliminates DHA-induced expression of VEGFR1. These results indicated that DHA elevates VEGFR1 expression via up-regulation of ETS-1 in HUVECs.
Materials and Methods
Cell culture
HUVECs were obtained from Lonza (Basel, Switzerland) and cultured in DMEM (Corning, NY, USA) with 10% FBS (Lonza), 100 IU/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA). HUVECs were treated with DHA (Sigma-Aldrich) for different time points (0, 1, 6, 12, 24 h) or concentrations (0, 5, 10, 25, 50, 100 μM).
Western blotting
After treatment with DHA, HUVECs were lysed in RIPA lysis buffer with 0.1% SDS, 1 mg/ml leupeptin and 1 mM PMSF on ice. Proteins were quantified by BCA assay (Bio‑Rad, Hercules, CA, USA). The protein samples were loaded and separated on a 8% SDS‑polyacrylamide gel, then electroblotted onto the PVDF membranes. The membranes were blocked 1 h in 5% skim milk in TBS-T (TBS containing 0.05% Tween-20), and then incubated with the primary antibody at 4˚C overnight. The primary antibodies include anti-VEGFR1 antibody (Abcam, Cambridge, MA, USA), anti-ETS-1 antibody (Abcam) and anti-β-actin antibody (Sigma-Aldrich). The membranes were washed in TBS-T, and incubated with a HRP-linked goat anti-rabbit secondary antibody (Proteintech, Chicago, IL, USA) for 2 h at room temperature. The protein bands were visualized with an ECL kit (Millipore, Billerica, MA, USA).
RNA extraction and quantitative real-time PCR(qRT-PCR)
Total RNA of HUVECs was isolated using Trizol (Invitrogen, Carlsbad, CA, USA), and the cDNAs was generated from the reverse transcription by the RevertAid First Strand cDNA Synthesis Kit (ThermoFisher, Grand Island, NY, USA). Then the mRNAs levels were evaluated by qRT-PCR executed by a Applied Biosystems PCR System (Waltham, MA, USA) with SYBR supermix (TaKaRa Biotechnology, Shiga, Japan) and following the thermocycling conditions: 94°C 1 min; 95°C 1 min; 95°C 12 s, 62 °C 1 min; 40 cycles from step 3 to 4. The primers are as follows: VEGFR1: sense, 5ˊ-TGGCCATCACTAAGGAGCACTCC-3ˊ; anti-sense, 5ˊ-GGAACTGCTGATGGCCACTGTG-3ˊ; ETS-1: sense, 5ˊ-TTCACTAAAGAACAGCAAC-3ˊ; anti-sense, 5ˊ-TGTCCCCAACAAAGTCTG-3ˊ; β‑actin: sense, 5ˊ-TTGCCGACAGGATGCAGAA-3ˊ; anti-sense, 5ˊ- GCCGATCCACACGGAGTACT-3ˊ. Results was normalized against β‑actin.
Immunofluorescence (IF)
HUVECs were grown fluency on cover glass. After DHA treatment, 4% paraformaldehyde was added to fix the cells. Then the cells were penetrated with Triton X-100 (0.1%) for 10 min and blocked with 5% BSA. Proteins were labeled with primary antibody against VEGFR1 (Abcam) at 4˚C overnight and an Alex-546 labelled anti-rabbit IgG secondary antibody (Molecular Probes, Eugene, OR, USA) for 1 h at room temperature. The cell nucleus was stained with DAPI (ThermoFisher). Positive staining were detected using a fluorescence microscopy (Olympus, Tokyo, Japan).
Chromatin immunoprecipitation (ChIP)
A ChIP assay Kit (Millipore) was used as previously described 32. HUVECs were exposed to 1% paraformaldehyde for 10 min to achieve in vivo crosslinking, then the crosslinked DNA were sheared to 200-1000 bp fragments by sonication. The chromatin fragments were immunoprecipitated with antibodies against ETS-1 (Abcam) and IgG (Millipore) using protein A/G agrose beads. The immunoprecipitated gDNA was enriched by centrifuging and purified by Phenolic chloroform isoamyl alcohol (25:24:1). The immunoprecipitated fragments of the VEGFR1 promoter were amplified by PCR. The primers are as follows: forward, 5ˊ-CCCTCGGCTGCTCTTCATC-3ˊ; reverse, 5ˊ-TTCCTCCCAGGCTCGCTTCC-3ˊ.
siRNA transfection
Transfection of siRNAs was performed when HUVECs were reached 60% confluent using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Briefly, transfection reagent was mixed with siRNAs (Genepharma, Shanghai, China) with Opti-MEM (Invitrogen) separately. The mixture were incubated for 20 min and added into the cell culture. The cells were collected 48 h later. The following siRNAs were used: ETS-1 siRNA: sense, 5ˊ-AUAGAGAGCUACGAUAGUUdTT-3ˊ; anti-sense, 5ˊ-AACUAUCGUAGCUCUCUAUdTT-3ˊ; control siRNA: sense, 5ˊ-UUCUCCGAACGUGUCACGUTT-3ˊ; anti-sense, 5ˊ-ACGUGACACGUUCGGAGAATT-3ˊ.
Statistical analysis
Statistical significance was evaluated with paired-sample t-test by using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). p<0.05 was considered significant.
Results
The effects of DHA on VEGFR1 expression in ECs
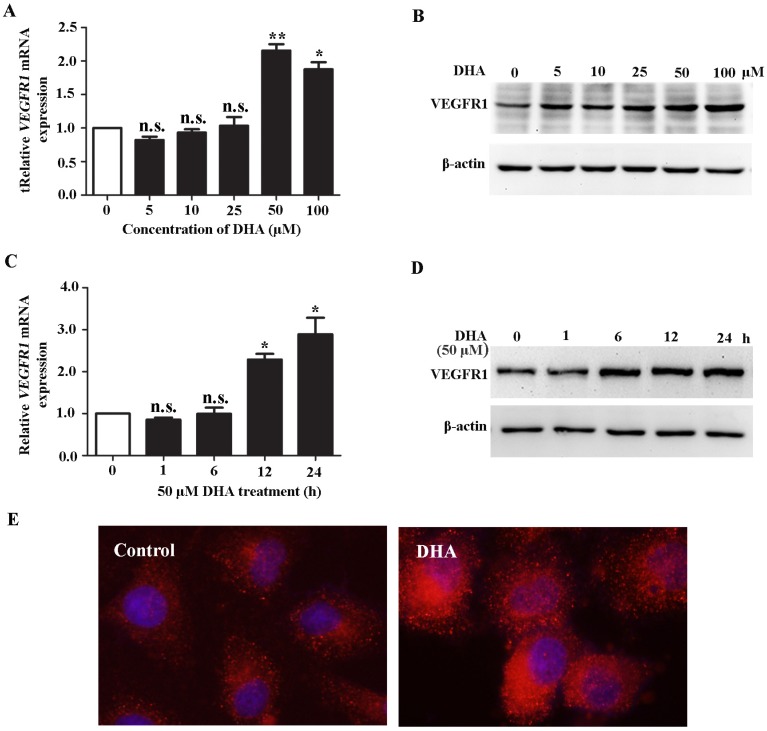
HUVECs were treated with DHA at different dose and time course. We found that DHA remarkably increased the mRNA (Fig. 1A) and protein level (Fig. 1B) of VEGFR1 at a concentration of 50 µM and 100 µM. By treatment with 50 µM DHA, the mRNA and protein level of VEGFR1 was significantly increased in a time-dependent manner (Fig. 1C, D). Immunofluorescence staining with VEGFR1 antibody showed that the VEGFR1 expression on the EC membrane was remarkably increased by DHA treatment (Fig. 1E). Together, DHA increases VEGFR1 expression in HUVECs.
Figure 1.
DHA up-regulates VEGFR1 expression in ECs. (A) Relative VEGFR1 mRNA expression in HUVECs treated with increasing concentrations of DHA for 24 h. n = 6; n.s., non-significant; *, P < 0.05; **, P < 0.01. (B) Representative immunoblots of VEGFR1 from HUVECs treated with increasing concentrations of DHA for 24 h. (C) Relative VEGFR1 mRNA expression in HUVECs treated with 50 μM DHA at different time points. n = 5; n.s., non-significant; *, P < 0.05. (D) Representative immunoblots of VEGFR1 from HUVECs treated with 50 μM DHA at different time points. (E) Immunofluorescence staining of VEGFR1 in HUVECs treated with 50 μM DHA for 24 h.
The effects of DHA on ETS-1 expression in ECs
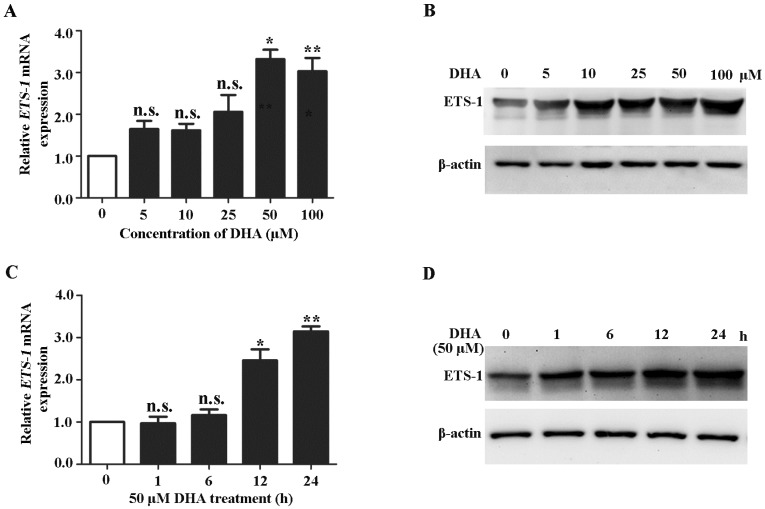
Because ETS-1 is a major regulator of VEGFR1 33, 34, we next investigated the effects of DHA on expression of ETS-1. qRT-PCR analysis showed that the ETS-1 mRNA expression levels were significantly increased by DHA treatment at 50 µM and 100 µM for 24 h (Fig. 2A). Western blot analysis showed a similarly pattern of increase of ETS-1 protein levels by 50 µM and 100 µM DHA treatment (Fig. 2 B). At a concentration of 50 µM, DHA significantly increase the mRNA and protein levels of ETS-1 after 12 h and 24 h treatment (Fig 2C, D). These results indicated ETS-1 expression is enhanced during DHA treatment in HUVECs.
Figure 2.
DHA up-regulates ETS-1 expression in ECs. (A) Relative ETS-1 mRNA expression in HUVECs treated with increasing concentrations of DHA for 24 h. n = 6; n.s., non-significant; *, P < 0.05; **, P < 0.01. (B) Representative immunoblots of ETS-1 from HUVECs treated with increasing concentrations of DHA for 24 h. (C) Relative ETS-1 mRNA expression HUVECs treated with 50 μM DHA at different time points. n = 6; n.s., non- significant; *, P < 0.05; **, P < 0.01. (D) Representative immunoblot of ETS-1 from HUVECs treated with 50 μM DHA at different time points.
DHA enhances ETS-1 binding to the VEGFR1 promoter
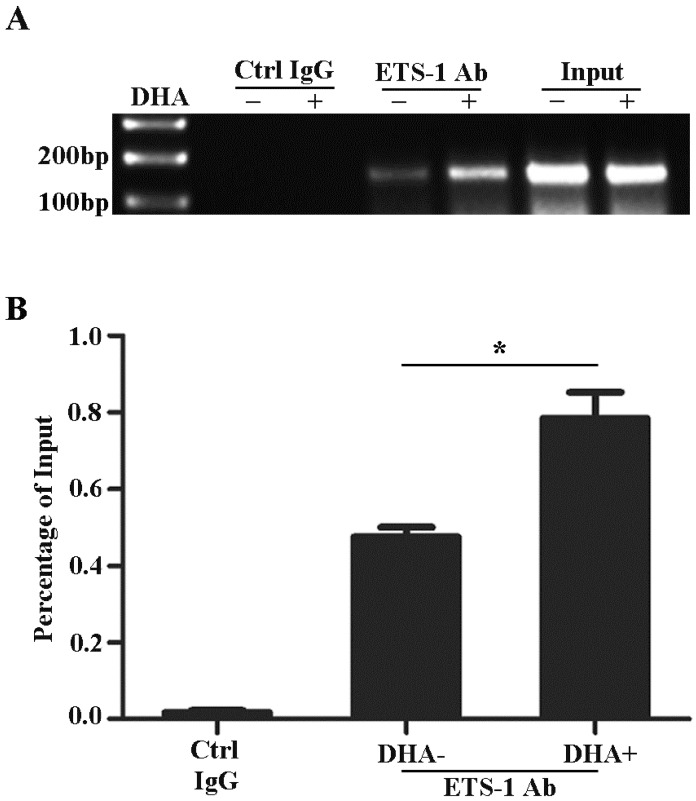
Previously studies reported that the promoter of VEGFR1 gene contains ETS binding motifs 29, 31, 34. To examine the effects of DHA on the interactions between ETS-1 and VEGFR1 promoter, HUVECs were treated with 50 µM DHA for 24 h and collected for CHIP assay. As shown on Fig. 3, ETS-1 binds to -52 ETS motif on the VEGFR1 promoter (Fig. 3A) and DHA further enhanced the binding of ETS-1 to the motif (p<0.05)(Fig. 3B).
Figure 3.
DHA enhances ETS-1 binding to the VEGFR1 promoter. (A) ChIP assay for ETS-1 binding to VEGFR1 promoter in HUVEC with 50 µM DHA treatment for 24 h. (B) Binding ratio relative to total input chromatin in the ChIP reaction. n = 6; n.s., non-significant; *, P < 0.05.
Knockdown of ETS-1 eliminates DHA-induced expression of VEGFR1 in ECs
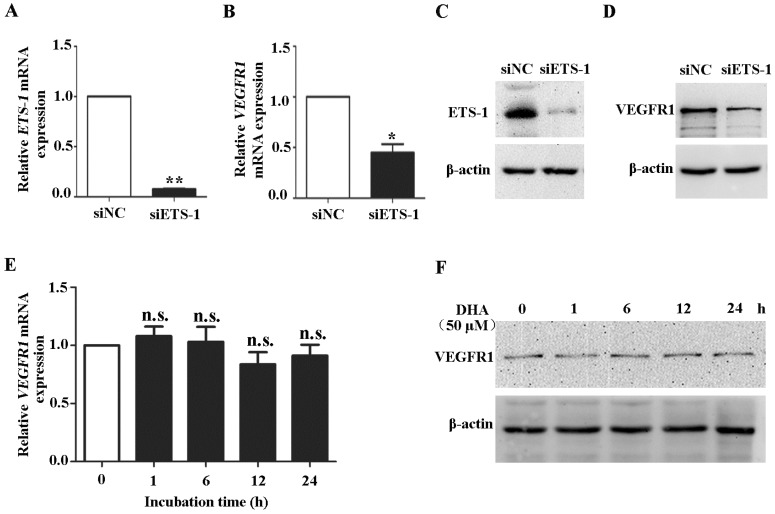
To confirm the role of ETS-1 in DHA induced VEGFR1 expression, ETS-1 were knocked down in HUVECs by siRNA interference. Transfection of ETS-1 siRNA in HUVECs reduced both ETS-1 (P<0.01) (Fig. 4A) and VEGFR1 transcription (P<0.05)(Fig. 4B), and induced measureable decrease of ETS-1 (Fig. 4C) and VEGFR1 protein (Fig. 4D). Next, HUVECs with ETS-1 siRNA transfection were treated with 50 µM DHA for 24 h. In the ETS-1-silenced HUVECs, no increase in VEGFR1 mRNA (Fig. 4E) and protein (Fig. 4F) was observed after DHA treatment. Therefore, DHA-induced elevation of VEGFR1expression is mediated by ETS-1.
Figure 4.
Knockdown of ETS-1 eliminates DHA-induced expression of VEGFR1 in ECs. HUVECs were transfected with control siRNA or siRNA against ETS-1. The expression of ETS-1 were assessed by qRT-PCR (A) and by Western blot (B). n = 4; *, P < 0.05; **, P < 0.01. After transfection, the expression of VEGFR1 were assessed by qRT-PCR (C) or by Western blot (D). HUVECs with ETS-1 siRNA interference were treated with 50 µM DHA. VEGFR1 expression was assessed by qRT-PCR (E) or by Western blot (F) at different time points. n = 6; n.s., non-significant.
Discussion
DHA is an effective anti-malarial agent with few side effects 15, 20. Recent studies revealed a potent anti-tumor and anti-angiogenic activity of DHA 35, 36. On cellular level, DHA exerted a significant inhibitory effect on apoptosis, migration and tube-like formation of ECs 37. Several signaling cascades including NF-κB, PKC, ERK, JNK, and p38 MAPK pathway have been reported to mediate the effects of DHA 38-42. For example, DHA activates JNK signaling pathway and then increases the expression of cyclooxygenase-2 and matrix metalloproteinase-13 (MMP-13) 43. Moreover, DHA plays a negative role in the expression of hypoxia inducible factor (HIF)-1α, angiogenic mediators VEGF, MMP9, MMP11, and collagens 30, 44. Structurally, DHA may bind to VEGF and its receptors 45. Although dozens of papers have been published regarding the role of DHA on endothelial cell function, to date the effects of DHA on VEGFR1 expression in ECs have not been reported. In this study, we found that transcriptional factor ETS-1 mediates dihydroartemisinin-induced VEGFR-1 expression. This novel finding was validated by mRNA and protein expression analyses, siRNA interference and examination of protein-DNA interactions.
VEGFR1 is a one of the major regulators in vascular development and angiogenesis 7, 46. The responses of VEGFR1 are influenced by the binding of ligands and the indirectly interaction with VEGFR2 7, 29. Loss of VEGFR1 increases VEGFR2 phosphorylation and activity, resulting in vessel overgrowth 47, 48. Overexpression of VEGFR1 suppresses VEGFR2-mediated EC proliferation 13, 49. In addition, VEGFR1 directly mediates a series of signal responses during angiogenesis and might prevent tumor growth 48. In this study, we demonstrated that DHA treatment significantly enhances the expression of VEGFR1, which may suppresses VEGFR2-mediated pro-angiogenic responses. Therefore, our study provided a novel mechanism of the anti-angiogenic effect of DHA.
ETS-1 is a master regulator of endothelial gene transcription 50. The transcriptional regulation of VEGFR1 by ETS-1 during embryonic or tumorous angiogenesis has been systematically studied 33, 34. Binding of ETS-1 on -52 site activates VEGFR1 expression 51. Our results showed that DHA significantly upregulates the expression of ETS-1 in HUVECs. ChIP assay also showed that DHA increases ETS-1 binding to the -52 ETS motif on the VEGFR1 promoter. In addition, knockdown of ETS-1 abolished DHA-induced VEGFR1 expression. To our knowledge, this is the first study which validated the functional relevance between DHA and ETS-1. This suggest that ETS-1 as a novel mediator for the cellular functions of DHA and other artemisinin derivatives.
Although VEGFR-1 is highly expressed in ECs, it has also been detected in monocyte/macrophages, hematopoietic stem cells, and a subset of epithelial cancer cells 52, 53. In other types of cells, VEGFR-1 also involves in regulation of cellular functions, e.g., it supports the growth and survival of human breast carcinoma 53. ETS-1 is widely expressed in most cell types, and facilitates malignant transformation and tumour progression 54. Therefore, ETS-1-regulated VEGFR-1 expression might exist in non-endothelial cells, which could also be effected by DHA treatment. Further studies are needed to explore the role of DHA on VEGFR-1 expression in non-endothelial cells, particularly in tumor cells.
In this study, we demonstrated that DHA induces VEGFR1 expression by up-regulating ETS-1 transcription factor. This is a novel mechanism contributing towards the effect of DHA on endothelium, and helps explore its clinical applications in chemotherapy.
Acknowledgments
This study was supported by the grants from Traditional Chinese Medicine Research Projects of Shandong Province (2015-285), The Key Science and Technology Research Plan of Shandong Province (2017GSF218037), Natural Science Foundation of Shandong Province (ZR2014HQ058, ZR2014HM043) and Shandong Taishan Scholarship (Ju Liu).
Abbreviations
- BMP1
Bone Morphogenetic Protein1
- ChIP
Chromatin immunoprecipitation
- DHA
dihydroartemisinin
- ECs
endothelial cells
- ETS-1
V-Ets Avian Erythroblastosis Virus E26 Oncogene Homolog 1
- HIF-1α
hypoxia-inducible factor-1α
- HUVECs
human umbilical vein endothelial cells
- MMPs
matrix metalloproteinases
- NF-κB
nuclear Factor-κB
- PKC
protein kinase C
- PVDF
polyvinylidene fluoride
- p38 MAPK
p38 mitogen-activated protein kinase
- siRNA
small interference RNA
- VEGF
vascular endothelial growth factor
- VEGFR1
vascular endothelial growth factor receptor 1
- VEGFR2
vascular endothelial growth factor receptor 2.
References
- 1.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 2.Costa C, Soares R, Schmitt F. Angiogenesis: now and then. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2004;112:402–12. doi: 10.1111/j.1600-0463.2004.apm11207-0802.x. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 4.Gao P, Niu N, Wei T, Tozawa H, Chen X, Zhang C. et al. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget. 2017;8:69139–61. doi: 10.18632/oncotarget.19932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D. et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–8. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 6.Takuwa N, Du W, Kaneko E, Okamoto Y, Yoshioka K, Takuwa Y. Tumor-suppressive sphingosine-1-phosphate receptor-2 counteracting tumor-promoting sphingosine-1-phosphate receptor-1 and sphingosine kinase 1 - Jekyll Hidden behind Hyde. Am J Cancer Res. 2011;1:460–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H. et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–24. [PubMed] [Google Scholar]
- 9.Shen BQ, Lee DY, Zioncheck TF. Vascular endothelial growth factor governs endothelial nitric-oxide synthase expression via a KDR/Flk-1 receptor and a protein kinase C signaling pathway. The Journal of biological chemistry. 1999;274:33057–63. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 11.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. The Journal of biological chemistry. 1994;269:25646–54. [PubMed] [Google Scholar]
- 12.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9349–54. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. J Biol Chem. 2000;275:16986–92. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- 14.Longo M, Zanoncelli S, Torre PD, Riflettuto M, Cocco F, Pesenti M. et al. In vivo and in vitro investigations of the effects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos. Reprod Toxicol. 2006;22:797–810. doi: 10.1016/j.reprotox.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang SJ, Sun B, Cheng ZX, Zhou HX, Gao Y, Kong R. et al. Dihydroartemisinin inhibits angiogenesis in pancreatic cancer by targeting the NF-kappaB pathway. Cancer Chemother Pharmacol. 2011;68:1421–30. doi: 10.1007/s00280-011-1643-7. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Z, Qi R, Li L, Liu Q, Zhang W, Zhou X. et al. Dihydroartemisinin ameliorates sepsis-induced hyperpermeability of glomerular endothelium via up-regulation of occludin expression. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;99:313–8. doi: 10.1016/j.biopha.2018.01.078. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Chen X, Dong F, Liu Q, Zhang C, Xu D. et al. Dihydroartemisinin up-regulates VE-cadherin expression in human renal glomerular endothelial cells. Journal of cellular and molecular medicine. 2017;22(3):2028–2032. doi: 10.1111/jcmm.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HH, Zhou HJ, Wang WQ, Wu GD. Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemother Pharmacol. 2004;53:423–32. doi: 10.1007/s00280-003-0751-4. [DOI] [PubMed] [Google Scholar]
- 19.Ba Q, Duan J, Tian JQ, Wang ZL, Chen T, Li XG. et al. Dihydroartemisinin promotes angiogenesis during the early embryonic development of zebrafish. Acta pharmacologica Sinica. 2013;34:1101–7. doi: 10.1038/aps.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacological Research. 2003;48:231–6. doi: 10.1016/s1043-6618(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 21.Wu GD, Zhou HJ, Wu XH. Apoptosis of human umbilical vein endothelial cells induced by artesunate. Vascul Pharmacol. 2004;41:205–12. doi: 10.1016/j.vph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 22.D'Alessandro S, Basilico N, Corbett Y, Scaccabarozzi D, Omodeo-Sale F, Saresella M. et al. Hypoxia modulates the effect of dihydroartemisinin on endothelial cells. Biochem Pharmacol. 2011;82:476–84. doi: 10.1016/j.bcp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhou HJ, Wang WQ, Wu GD, Lee J, Li A. Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul Pharmacol. 2007;47:131–8. doi: 10.1016/j.vph.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Wasylyk B, Hahn SL, Giovane A. The Ets family of transcription factors. European journal of biochemistry. 1993;211:7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto J, Aoki I, Toyoki H, Khatun S, Sato E, Tamaya T. Expression of ETS-1 Related to Angiogenesis in Uterine Endometrium during the Menstrual Cycle. Journal of Biomedical Science. 2003;10:320–7. doi: 10.1007/BF02256451. [DOI] [PubMed] [Google Scholar]
- 26.Hahne JC, Okuducu AF, Fuchs T, Florin A, Wernert N. Identification of ETS-1 target genes in human fibroblasts. Int J Oncol. 2011;38:1645–52. doi: 10.3892/ijo.2011.981. [DOI] [PubMed] [Google Scholar]
- 27.Dube A, Thai S, Gaspar J, Rudders S, Libermann TA, Iruela-Arispe L. et al. ELF-1 Is a Transcriptional Regulator of the Tie2 Gene During Vascular Development. Circulation Research. 2001;88:237–44. doi: 10.1161/01.res.88.2.237. [DOI] [PubMed] [Google Scholar]
- 28.Baillat D, Laitem C, Leprivier G, Margerin C, Aumercier M. Ets-1 binds cooperatively to the palindromic Ets-binding sites in the p53 promoter. Biochem Biophys Res Commun. 2009;378:213–7. doi: 10.1016/j.bbrc.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Dutta D, Ray S, Vivian JL, Paul S. Activation of the VEGFR1 chromatin domain: an angiogenic signal-ETS1/HIF-2alpha regulatory axis. J Biol Chem. 2008;283:25404–13. doi: 10.1074/jbc.M804349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto J, Aoki I, Toyoki H, Khatun S, Sato E, Sakaguchi H. et al. Clinical implications of expression of ETS-1 related to angiogenesis in metastatic lesions of ovarian cancers. Oncology. 2004;66:420–8. doi: 10.1159/000079491. [DOI] [PubMed] [Google Scholar]
- 31.Morishita K, Johnson DE, Williams LT. A novel promoter for vascular endothelial growth factor receptor (flt-1) that confers endothelial-specific gene expression. The Journal of biological chemistry. 1995;270:27948–53. doi: 10.1074/jbc.270.46.27948. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Pan S, Liu J, Dong F, Cheng Z, Zhang J. et al. GATA3-induced vWF upregulation in the lung adenocarcinoma vasculature. Oncotarget. 2017;8:110517–29. doi: 10.18632/oncotarget.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K. et al. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Annals of the New York Academy of Sciences. 2000;902:201–5. doi: 10.1111/j.1749-6632.2000.tb06314.x. discussion 5-7. [DOI] [PubMed] [Google Scholar]
- 34.Valter MM, Hugel A, Huang HJ, Cavenee WK, Wiestler OD, Pietsch T. et al. Expression of the Ets-1 transcription factor in human astrocytomas is associated with Fms-like tyrosine kinase-1 (Flt-1)/vascular endothelial growth factor receptor-1 synthesis and neoangiogenesis. Cancer Res. 1999;59:5608–14. [PubMed] [Google Scholar]
- 35.Dong F, Zhou X, Li C, Yan S, Deng X, Cao Z. et al. Dihydroartemisinin targets VEGFR2 via the NF-kappaB pathway in endothelial cells to inhibit angiogenesis. Cancer biology & therapy. 2014;15:1479–88. doi: 10.4161/15384047.2014.955728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei T, Liu J. Anti-angiogenic properties of artemisinin derivatives (Review) Int J Mol Med. 2017;40:972–8. doi: 10.3892/ijmm.2017.3085. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Guo Y, Zhang BC, Chen ZT, Gao JF. Induction of apoptosis and inhibition of cell migration and tube-like formation by dihydroartemisinin in murine lymphatic endothelial cells. Pharmacology. 2007;80:207–18. doi: 10.1159/000104418. [DOI] [PubMed] [Google Scholar]
- 38.Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW, Jeong HG. Suppression of PMA-induced tumor cell invasion by dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol. 2010;79:1714–26. doi: 10.1016/j.bcp.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Cheng C, Ho WE, Goh FY, Guan SP, Kong LR, Lai WQ. et al. Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS One. 2011;6:e20932. doi: 10.1371/journal.pone.0020932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo L, Dong F, Hou Y, Cai W, Zhou X, Huang AL. et al. Dihydroartemisinin inhibits vascular endothelial growth factor-induced endothelial cell migration by a p38 mitogen-activated protein kinase-independent pathway. Exp Ther Med. 2014;8:1707–12. doi: 10.3892/etm.2014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong F, Tian H, Yan S, Li L, Dong X, Wang F. et al. Dihydroartemisinin inhibits endothelial cell proliferation through the suppression of the ERK signaling pathway. International journal of molecular medicine. 2015;35:1381–7. doi: 10.3892/ijmm.2015.2140. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Guo L, Zhou X, Dong F, Li L, Cheng Z. et al. Dihydroartemisinin induces endothelial cell anoikis through the activation of the JNK signaling pathway. Oncology letters. 2016;12:1896–900. doi: 10.3892/ol.2016.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong F, Han J, Jing G, Chen X, Yan S, Yue L. et al. Dihydroartemisinin transiently activates the JNK/SAPK signaling pathway in endothelial cells. Oncology letters. 2016;12:4699–704. doi: 10.3892/ol.2016.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anfosso L, Efferth T, Albini A, Pfeffer U. Microarray expression profiles of angiogenesis-related genes predict tumor cell response to artemisinins. Pharmacogenomics J. 2006;6:269–78. doi: 10.1038/sj.tpj.6500371. [DOI] [PubMed] [Google Scholar]
- 45.Saeed ME, Kadioglu O, Seo EJ, Greten HJ, Brenk R, Efferth T. Quantitative structure-activity relationship and molecular docking of artemisinin derivatives to vascular endothelial growth factor receptor 1. Anticancer research. 2015;35:1929–34. [PubMed] [Google Scholar]
- 46.Wei T, Jia J, Wada Y, Kapron CM, Liu J. Dose dependent effects of cadmium on tumor angiogenesis. Oncotarget. 2017;8:44944–59. doi: 10.18632/oncotarget.16572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL. The Vascular Endothelial Growth Factor (VEGF) Receptor Flt-1 (VEGFR-1) Modulates Flk-1 (VEGFR-2) Signaling During Blood Vessel Formation. The American Journal of Pathology. 2004;164:1531–5. doi: 10.1016/S0002-9440(10)63711-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–56. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 49.Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276:26969–79. doi: 10.1074/jbc.M103213200. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Fu Y, Day DS, Sun Y, Wang S, Liang X. et al. VEGF amplifies transcription through ETS1 acetylation to enable angiogenesis. Nat Commun. 2017;8:383. doi: 10.1038/s41467-017-00405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin E, Liu J, Suehiro J, Yuan L, Okada Y, Nikolova-Krstevski V. et al. Differential roles for ETS, CREB, and EGR binding sites in mediating VEGF receptor 1 expression in vivo. Blood. 2009;114:5557–66. doi: 10.1182/blood-2009-05-220434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–79. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S. et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–29. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 54.Hahne JC, Okuducu AF, Sahin A, Fafeur V, Kiriakidis S, Wernert N. The transcription factor ETS-1: its role in tumour development and strategies for its inhibition. Mini Rev Med Chem. 2008;8:1095–105. doi: 10.2174/138955708785909934. [DOI] [PubMed] [Google Scholar]