Abstract Abstract
Species delimitation in the genus Populus is particularly challenging due to high levels of intraspecific polymorphism as well as frequent interspecific hybridisation and introgression. In this study, we aimed to examine the taxonomic status of Populusningshanica and P.wulianensis using an integrative taxonomy that considers multiple operational criteria. We carried out morphometric analyses of leaf traits and genetic examinations (including sequence variations at five barcoding DNAs and polymorphisms at 14 nuclear microsatellite SSR primers) at the population level between them and two closely related species P.adenopoda and P.davidiana. Results suggest that P.wulianensis belongs to the polymorphic species, P.adenopoda and should be considered as a synonym of the latter. P.ningshanica may have arisen as a result on the hybridisation between P.adenopoda and P.davidiana and therefore should be treated as P.×ningshanica. This study highlights the importance of the integrated evidence in taxonomic decisions of the disputed species.
Keywords: Geometric morphometrics, microsatellites, DNA barcodes, integrated species delimitation
Introduction
Species delimitation is essential to conserve and assess biodiversity (Agapow et al. 2004). Any incorrect species recognition may result in serious after-effects in related studies, for example, by an increase in species conservation (Wiens 2007) and under- or over-estimation of biodiversity (Douady 2007). Therefore, in addition to morphological traits, significant efforts have been made to delimit species based on DNA sequence variation (Wiens and Penkrot 2002; Sites and Marshall 2003; Kress et al. 2005; Bond and Stockman 2008; Fujita et al. 2012; Hendrixson et al. 2013) or other genetic polymorphisms that can assess gene flow and identify interspecific hybrids according to the biological species concept (Pérez-Losada et al. 2005). These molecular markers have been used to differentiate species, hybrids and even clones in the genus Populus (Salicaceae) (Hamzeh and Dayanandan 2004; Cervera et al. 2005; Hamzeh et al. 2006; Fladung and Buschbom 2009; Schroeder et al. 2012; Feng et al. 2013; Wan et al. 2013). Poplars are widely distributed in the Northern Hemisphere with an important ecological role in natural and artificial forests in both boreal and temperate regions (Dickmann et al. 2001). However, due to high levels of morphological variation and extensive inter-specific hybridisation, species delimitation within the genus is highly contentious (Eckenwalder 1996; Dickmann and Kuzovkina 2008). The number of the proposed species ranges from 22 to 85, plus hundreds of hybrids, varieties and cultivars (Dickmann and Stuart 1983; Fang et al. 1999). Numerous described species were doubted as being hybrids of the other independently evolving lineages (good species) or intra-specific variations of the polymorphic species. However, these ambiguous species have not been well examined.
In this study, we aimed to determine the taxonomic status of two species described from China: P.wulianensis S.B.Liang & X.W.Li and P.ningshanica C. Wang & Tung (Fang et al. 1999) based on morphometric analyses and genetic examinations at the population level as recently suggested for an integrated species delimitation (Liu 2016). P.wulianensis is restricted to eastern Shandong while P.ningshanica is distributed in southern Shaanxi and Northwest Hubei. Both are morphologically similar to P.davidiana Dode and P.adenopoda Maxim. of sect. Populus with widespread distributions in northern or middle to southern China. The key traits for their diagnosis are mainly based on leaf characters: blade and apex shape and margin incision (Fang et al. 1999). We firstly conducted morphometric analyses of leaf traits for representative populations of all four species. Then we examined genetic delimitations between them based on evidence from sequence variation of internal transcribed spacer (ITS) and four chloroplast DNA (cpDNA) and genetic polymorphisms from nuclear microsatellite loci (nSSR).
Materials and methods
Sample collection
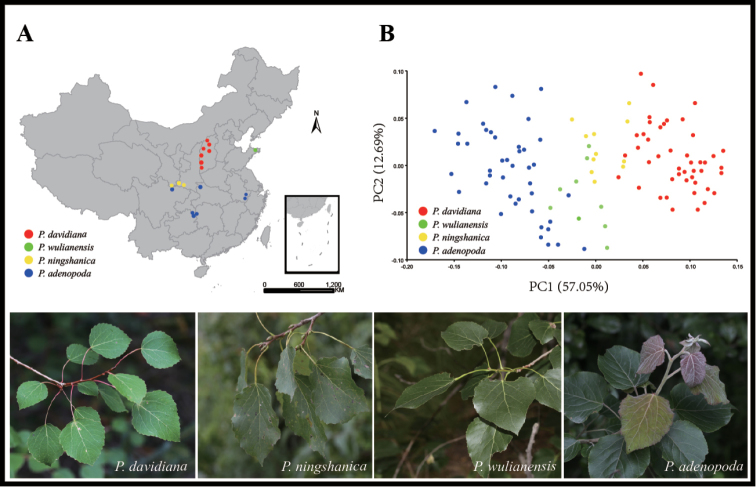
We sampled 163 individuals from 17 populations of four species (Table 1), including all recorded natural populations of both P.ningshanica and P.wulianensis. All individual trees were chosen with typical morphological leaf traits (Fang et al. 1999). Each tree was set apart by at least 50m in each population. Except for collecting specimens (SZ, herbarium of Sichuan University, Chengdu, China) for geometric morphometric analyses, we further selected healthy and fresh leaves from each tree and dried them immediately in silica gel for DNA extraction. We also used an Etrex GIS monitor (Garmin, Taiwan) to record latitude, longitude and altitude of each sampled population (Table 1; Fig. 1A).
Table 1.
Detailed information for the 17 sampled populations of the sect. Populus species that were adopted for Data analysis using SSR and Geometric morphology.
| Species | Pop | Individuals | Lon (N) | Lat (E) | Alt (m) | CS | Vouchers |
|---|---|---|---|---|---|---|---|
| P. davidiana | 1 | 21 | 111.2848 | 38.21627 | 1467 | Lvliang, SX | LiuJQ-MZL-2013-117 |
| 2 | 8 | 111.3395 | 38.14662 | 1587 | Lvliang, SX | LiuJQ-MZL-2013-121 | |
| 3 | 6 | 112.3880 | 38.92512 | 1402 | Qizhou, SX | LiuJQ-MZL-2013-109 | |
| 4 | 10 | 112.0744 | 38.8556 | 1855 | Qizhou, SX | LiuJQ-MZL-2013-115 | |
| 5 | 8 | 111.4328 | 37.8976 | 1961 | Lvliang, SX | LiuJQ-MZL-2013-124 | |
| 6 | 9 | 111.2637 | 37.203483 | 1459 | Lvliang, SX | LiuJQ-MZL-2013-136 | |
| P. ningshanica | 7 | 8 | 105.249 | 32.74979 | 657 | Longnan, GS | LiuJQ-SHX-2015-20 |
| 8 | 1 | 107.1394 | 32.60744 | 865 | Hanzhong, SaX | LiuJQ-SHX-2015-14 | |
| 9 | 3 | 106.0741 | 33.55506 | 768 | Hanzhong, SaX | LiuJQ-SHX-2015-10 | |
| P. wulianensis | 10 | 10 | 121.7556 | 37.2983 | 188 | Yantai, SD | LiuJQ-ZL-2016-300 |
| P. adenopoda | 11 | 5 | 108.8565 | 28.1423 | 798 | Tongren, GZ | MaoKS-CX-2014-326 |
| 12 | 5 | 109.1866 | 28.2958 | 643 | Tongren, GZ | MaoKS-CX-2014-327 | |
| 13 | 5 | 108.7551 | 28.3148 | 707 | Tongren, GZ | MaoKS-CX-2014-328 | |
| 14 | 18 | 105.3035 | 32.5254 | 598 | Guangyuan, SC | LiuJQ-ZF-2016-01 | |
| 15 | 10 | 117.8054 | 30.4742 | 677 | Liuan, AH | LiuJQ-ZF-2016-02 | |
| 16 | 17 | 117.9531 | 30.5850 | 26 | Chizhou, AH | LiuJQ-ZF-2016-03 | |
| 17 | 19 | 110.3215 | 32.6738 | 683 | Shiyan, HB | LiuJQ-ZF-2016-04 |
Abbreviations: Pop, Population; Lon (N), Longitude; Lat (E), Latitude; Alt (m), Altitude; CS, Collection site. SX, Shanxi; GS, Gansu; SaX, Shaanxi; SD, Shandong; GZ, Guizhou; SC, Sichuan; AH, Anhui; HB, Hubei.
Figure 1.
A Geographical distribution of 17 populations of the four species (Populusadenopoda, Populusdavidiana, Populusningshanica, Populuswulianensis) B The Principal Component Analysis (PCA) plot for the morphological variations of 17 populations of 4 species.
Geometric morphometrics
Although we failed to find type specimens of P.ningshanica and P.wulianensis, we included the newly collected specimens from their type localities. A Canon 60D digital camera was used to photograph typical leaves of all specimens. We transformed every image into a vector diagram using TpsUtil version 1.64 (Rohlf 2013). Thirty-two homologous landmarks were assigned in order to quantify leaf blades shape in all specimens. Landmark positions of leaves included base, tip and margin. All landmarks were digitised for each individual using the software TpsDig version 2.22 (Rohlf 2015). We created a combined data file including all specimens. We implemented morphometrics analyses in MorphoJ version 1.01b (Klingenberg 2011), within which a principal component analysis of morphological variations was conducted and plotted.
Genetic analyses
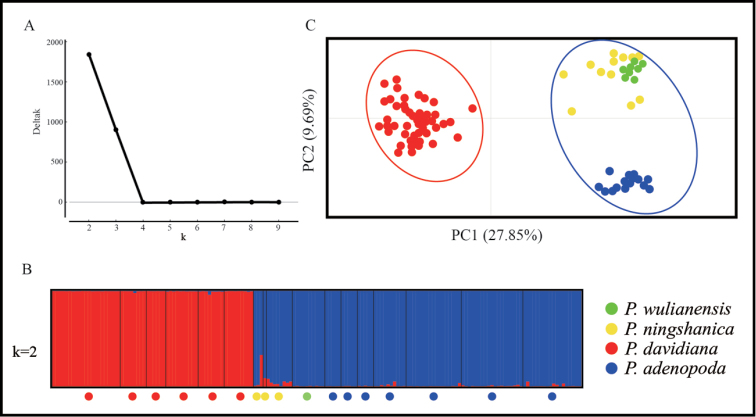
We isolated the total genomic DNA from leaves of each individual, based on the hexadecyltrimethyl ammonium bromide (CTAB) method (Doyle 1987). We used a total of 14 SSRs primers (Suppl. material 1: Table S1) developed previously, based on the genome sequences of Populuseuphratica and P.trichocarpa (Ma et al. 2013; Jiang et al. 2016) to genotype our samples. The PCRs were performed in a volume of 25 ml, which contained: 50–100 ng diluted genomic DNA, 0.5 mM of each dNTP, 0.5 µl of each primer, 2.5 µl 10 × Taq buffer and 0.5 units of Taq polymerase (Vazyme Biotech, Nanjing, China). The PCR programme used was: initially a single cycle at 95 °C for 5 min, followed by 36 cycles at 95 °C for 45s, 55 °C for 40 s and 72 °C for 80 s, with a final extension at 72 °C for 10 min. The PCR products at each locus were analysed on an ABI 3830xl DNA analyser (Applied Biosystems, Inc., Foster City CA) at Tsingke Biological Technology (Beijing, China). We used STRUCTURE version 2.3.4 (Falush et al. 2003) that allows a Bayesian hybrid mixture computation to identify genetic compositions of all sampled trees. We pre-assigned a number of genetic clusters (K) ranging from 1 to 10. All runs involved 1,000,000 Markov chain Monte Carlo repetitions after a burn-in period of 500,000 iterations. We used the long burn-in and run lengths as well as 10 replicates to ensure the reproducibility of STRUCTURE results (Gilbert et al. 2012). We estimated the posterior probability of K and Delta K (ΔK), the rate of change of Ln P (K) between successive K values (Evanno et al. 2005). We determined the most likely number of clusters.
We also sequenced internal transcribed spacer (ITS) and four chloroplast DNA (cpDNA) fragments: matK, trnH-psbA, trnG-psbK and psbK-psbI for three to five individuals from each sampled population of four species used for nSSR genotyping. In addition, one individual of P.euphratica was sequenced as the outgroup. Primers, PCRs and sequencing followed Feng et al. (2013) (Suppl. material S1: Table S2). Sequences for each fragment were aligned and sequences from four cpDNAs were connected using MEGA 7.0 (Kumar et al. 2016). We constructed unrooted neighbour-joining (NJ) trees for both ITS and cpDNAs datasets by MEGA 7.0 (Kumar et al. 2016) respectively, using pairwise deletion and the P-distance model. Bootstrap values were estimated with 1000 random addition sequence replicates.
Results
PCA analyses of geometric morphometric data
Geometric morphometric analyses of leaf traits yielded 30 principal components (PC), which accounted for all leaf variations. PC1 to PC3 were the only PCs that individually represented >5% of the variance (PC1=57.05%; PC2=12.69%; PC3=7.68%) and they together represented 77.43% of the variance. All other PCs accounted for <5% of the variance individually. The greatest amount of shape variance is observed across PC1 and PC2 (Fig. 1B). Across these two axes, individuals of P.davidiana and P.adenopoda were treated as a clear division, whereas individuals of P.wulianensis and P.ningshanica are clustered into one subgroup of the P.adenopoda group. All other PCs showed similar relationships.
Clustering analyses based on the SSR polymorphisms
We genotyped 14 nuclear SSR loci for 163 sampled individuals of four species. Using the method originally described by Pritchard et al. (Pritchard et al. 2000) and also the ΔK approach described by Evanno et al. (Evanno et al. 2005), we found the most likely number of Bayesian clusters was two (K = 2) (Fig. 2A). When K = 2, individuals from P.davidiana clustered into one group and those from P.adenopoda into the other. Within each group, some samples indicated the weak genetic introgression from the other. All sampled individuals of both P.wulianensis and P.ningshanica were assigned to the group represented by P.adenopoda (Fig. 2B). However, approximately 10% of the genetic composition of P.ningshanica derived from the cluster represented by P.davidiana, while more than 90% was from P.adenopoda. Similar results were obtained based on PCA analyses of genetic polymorphisms and that two groups were identified to be, respectively, represented by P.davidiana and the other three (Fig. 2C).
Figure 2.
Principal Coordinates Analysis (PCA) of the 17 populations of 4 species based on genetic distance using SSR data (A); the optimal K value was estimated using (B) the distribution of delta K (K=2) and Bayesian clustering plots for 17 populations of 4 species based on variation at 14 nSSR loci (C).
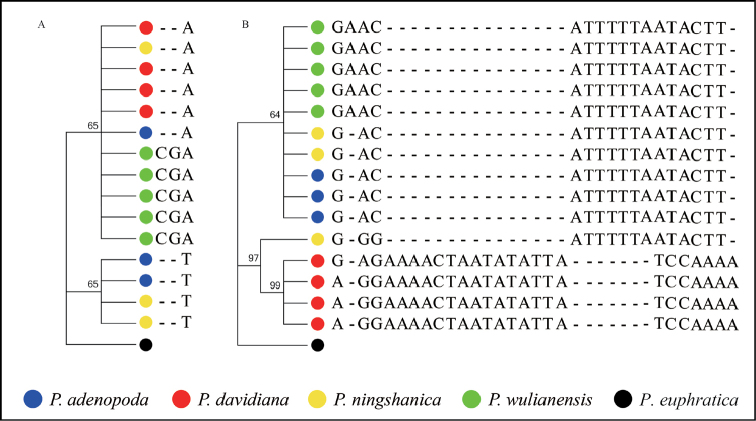
We have combined sequences of four cpDNAs for each individual into one cpDNA sequence. We aligned the cpDNA sequences of all individuals and identified 2, 1, 1 and 2 sequences for P.davidiana, P.adenopoda, P.wulianensis and P.ningshanica, respectively. The total length of the aligned cpDNA sequence was 1866 bp with 9 variable sites amongst different sequences from four species (Fig. 3B). NJ clustering of all different cpDNA sequences from four species similarly identified two tentative groups: one comprised of P.davidiana and P.ningshanica, while the other included those from P.adenopoda, P.wulianensis and P.ningshanica. We identified 1, 2, 1 and 2 different ITS sequences for the sampled individuals for P.davidiana, P.adenopoda, P.wulianensis and P.ningshanica. We aligned these ITS sequences from four species, which were 552 bps long with 1 variable site amongst all the different sequences from four species (Suppl. material S1: Table S3; Fig. 3A). NJ analyses of the ITS dataset identified two tentative groups: one comprised 4 sequences from P.adenopoda and P.ningshanica while the other, all four species.
Figure 3.
The neighbour-joining (NJ) tree of ITS variable sites (A); The neighbour-joining (NJ) tree of four cpDNA variable sites (B).
Discussion
Statistical analyses based on geometric morphometric measurements are highly successful at separating similar species (Villemant et al. 2007; Francuski et al. 2009), even when the individual character shows the overlapped variations between them (Lumley and Sperling 2010; Buck et al. 2012). Especially, geometric morphometrics could differentiate the overall changes in the gross morphology (Rohlf and Marcus 1993). Poplar leaves are ideal for geometric morphometric analyses, as they are two-dimensional, easily imaged and the venation provides many points that are clearly homologous and straightforward to landmark accurately. In addition, flower traits are highly static across the genus without variations and leaf characters are therefore used to classify different species (Dickmann and Stuart 1983; Eckenwalder 1996; Fang et al. 1999; Dickmann and Kuzovkina 2008). We tried to classify four popular species based on geometric morphometric analyses of leaf traits. Our results obviously suggested that P.davidiana and P.adenopoda differed distinctly from each other. P.wulianensis and P.ningshanica could not be distinguished from each other and they together clustered into one subgroup, which obviously belonged to the P.adenopoda group (Fig. 1B). Therefore, this statistical clustering indicated that both P.wulianensis and P.ningshanica may belong to the polymorphic P.adenopoda.
Genetic evidence, based on nuclear SSR loci, similarly recognised the distinct species boundary between P.davidiana and P.adenopoda (Fig. 2A, B). However, all sampled individuals of P.wulianensis belong to the P.adenopoda group without distinct introgression from P.davidiana. All sampled individuals of P.ningshanica shared similar genetic compositions, together belonging to the P.adenopoda group but with obvious genetic introgressions from the P.davidiana group. These individuals comprise the obvious backcrosses from P.adenopoda. Similarly, sequence variations from five DNAs (ITS, matK, trnH-psbA, trnG-psbK and psbK-psbI) seem to support these inferences. The connected sequences of four cpDNAs distinguished P.davidiana and P.adenopoda while all P.wulianensis individuals shared the same cpDNA sequences with P.adenopoda. We found two types of cpDNA sequences in P.ningshanica (Fig. 3B), clustering respectively with those from P.davidiana and P.adenopoda, which further suggested the hybrid origin of P.ningshanica. However, the initial hybrids must have repeatedly backcrossed with P.adenopoda, which resulted in the high genetic similarity of the sampled individuals of P.ningshanica to P.adenopoda but with introgression with P.davidiana (Fig. 3B). The interspecific hybrids in the genus Populus could be F1, F2 to multiple generation backcrossing hybrids (Braatne et al. 1992; Bradshaw et al. 2000; Feng et al. 2013; Jiang et al. 2016). We failed to find stable ITS differences between P.davidiana and P.adenopoda. It is highly probably that the gene flow, mediated by interspecific hybrids, had caused the concerted evolutions and indistinct differences in the ITS sequence variations (Feng et al. 2013; Jiang et al. 2016).
Overall, multiple lines (Figs 1B, 2B, C;) of evidence suggested that P.wulianensis was described based on the intraspecific variations of the polymorphic P.adenopoda and individuals ascribed to P.ningshanica are, in fact, hybrids between P.adenopoda and P.davidiana with the repeated backcrosses to the former. Both taxa should be treated accordingly in the taxonomic revision of the genus Populus.
Additional specimens examined. China. Anhui: Jiuhuashan mountain, on slope, 500 m elev., 18 Aug 1934 C. S. Fan & Y. Y. Li 262 (NAS!). Huoshan county, on slope, 17 Apr 1959, M. B. Deng & J. Q. Pan 0208 (NAS!). She county, in woods, 300 m elev., 04 May 1959, S. She 1218 (NAS!). Jinzhai county, on slope, 12 Jul 1959, Z. Jin 6044 (PE!). Jin county, in woods, 300 m elev., 10 Oct 1959, Anonymous 793 (NAS!). Xiuning county, in roadside, 450 m elev., 29 Jun 1959, R. H. Shan et al. 2661 (NAS!). Xuancheng city, in woods, 130 m elev., 02 Nov 1959 Anonymous 262 (NAS!). ChongQing: Fengjie county, 860 m elev., 29 Apr 1959, J. C. Zhang, 174 (SM!) Nanchuan county, 970 m elev., 13 Apr 1957, G. F. Li 60474 (PE!). Nanchuan county, jin fo mountain, in forest edge, 1070 m elev., 20 Apr 1957, J. H. Xiong & Z. L. Zhou 90383 (PE!). Qianjiang county, on slope, 980 m elev., 14 Aug 1988, Z. C. Zhao, 88-1502 (PE!). Pengshui county, on slope, 800 m elev., 26 May 1959, J. Z. Chuan, 03125 (PE!). Wushan county, 1080 m elev., 31 Mar 1958, G. H. Yang, 57592 (PE!). Wushan county, huangniba mountain, 1100 m elev., 14 Apr 1958, G. H. Yang 57715 (PE!). Wushan county, on slope, 1500 m elev., 17 May 1939, T.P.Wang 10653 (PE!). Gansu: Wen county, 16 Oct 1958, Z. P. Wei, 3047 (HIMC!). Wen county, 04 Apr 1964, Z. B. Wang, 18862 (HNWP!). Guangxi: Longlin county, in woods, 1600 m elev., 09 Apr 1991, H. Q. Wen 00375 (IBK!). Rongshui county, on slope, 1280 m elev., 20 Aug 1958, S. Q. Chen 16359 (PE!). Tianyang county, 29 Nov 1978, Z. Y. Chen 54101 (IBK!). Yangshuo county, 19 Apr 1956, H. F Qin 700139 (IBK!). Guizhou: Dushan county, in grassland, 900 m elev., 24 Jul 1959, Team of Libo 1198 (PE!). Guiding county, 400 m elev., 29 Jun 1930, Y. Tsiang 5435 (IBSC!). Guiding county, in woods, 16 Jun 2014, K. S. Mao & L. Zhang 2014-313 (SZ!). Guiyang city, Baiyun county, on slope, 1320 m elev., 22 Mar 2003, M. T. An 5014 (PE!). Huangping county, in bushwoods, 1505 m elev., 04 May 1987, J. M. Li 14 (GZTM!). Huishui county, on slope, 20 Jun 2014 K. S. Mao & L. Zhang 2014-306 (SZ!). Luodian county, 300 m elev., 20 Mar 1960, Z. S. Zhang & Y. T. Zhang 634 (IBSC!). Luodian county, in woods, 400 m elev., 22 Mar 1960, Z. S. Zhang & Y. T. Zhang 133 (PE!). Pingtang county, in woods, 15 Jun 2014, K. S. Mao & L. Zhang 2014-310 (SZ!). Qinglong county, in woods, 1600 m elev., 25 May 1987, F. J. Li 403 (GZTM!). Suiyang county, in woods, 17 Jun 2014, K. S. Mao & L. Zhang 2014-315 (SZ!). Wangmo county, in woods, 850 m elev., 01 Apr 2005, G. F. Wang 1-1048 (PE!). Tongzi county, 23 May 1987, K. M. Lan 870314 (GFS!). Yuqing county, in woods, 16 Jun 2014, K. S. Mao & L. Zhang 2014-315 (SZ!). Zunyi county, in woods, 17 Jun 2014, K. S. Mao & L. Zhang 2014-320 (SZ!). Henan: Luanchuan county, in woods, 28 Jun 2013, K. S. Mao & L. Zhang 2013-078A (SZ!). Nanyang city, funiu mountain, in woods, 1000 m elev., Jun 1959, Anonymous 063 (HENU!). Tongbai county, tongbai mountain, on slope, 1000 m elev., 01 Apr 1960, S. S. Kuang 468 (HENU!). Tongbai county, in woods, 27 Jun 2013, K. S. Mao & L. Zhang 2013-063 (SZ!). Xixia county, 04 Agu 1956, forestry department of Henan 27 (PE!). Hubei: Enshi city, in woods, 18 Jun 2013, K. S. Mao & L. Zhang 2013-050 (SZ!). Hefeng county, 1250 m elev., 27 Aug 1958, H. J. Li 5862 (PE!). Jianshi county, in woods, 23 Jun 2013, K. S. Mao & L. Zhang 2013-048 (SZ!). Luotian county, in woods, 700 m elev., 10 Jul 1979, Q. G. He 75-3 (PE!). Shennongjia, in woods, 06 Apr 1977, Team of shennongjia 20635 (PE!). Xianfeng county, in woods, 25 Sep 1958, H. J. Li 9252 (PE!). Xianfeng county, in woods, 22 Jun 2013, K. S. Mao & L. Zhang 2013-053 (SZ!). Xinshan county, in woodlands, 1993 m elev., 27 March 2012, D. G. Zhang 4383 (JIU!). Xinshan county, on slope, 1300 m elev., 14 May 1975, Z. F. Fang et al 2005 (NAS!). Xinshan county, in woods, 20 Jun 2013, K. S. Mao & L. Zhang 2013-046 (SZ!). Xuanen county, in woods, 22 Jun 2013, K. S. Mao & L. Zhang 2013-052 (SZ!). Yun county, in woods, 20 Jun 2013, K. S. Mao & L. Zhang 2013-038A (SZ!). Hunan: Cili county, on slope, 840 m elev., 07 May 1986, C. L. Peng 86040 (CSFI!). Dao county, on slope, 550 m elev., 04 May 1978, Q. Z. Lin 0262 (CSFI!). Longshan county, 31 May 1958, L. H. Liu 1885 (IBK!). Longshan county, in woods, 25 Jun 2013, K. S. Mao & L. Zhang 2013-055 (SZ!). Luxi county, on slope, 400 m elev., 09 Apr 1982, K. W. Liu 30045 (CSFI!). Sangzhi county, in woods, 25 Jun 2013, K. S. Mao & L. Zhang 2013-061 (SZ!). Shimen county, 09 Jul 1979, P. C. Cai 20198 (CSFI!). Shimen county, in woods, 420 m elev., 01 May 1980, D. C. Xiao 80311 (CSFI!). Zhangjiajie city, zhangjiajie mountain, in woods, 870 m elev., 15 Apr 2015, H. Zhou & D. S. Zhou 15041503 (CSFI!). Yizhang county, in woods, 09 Aug 1942, S. Q. Chen 2107 (PE!). Yuanling county, in woods, 600 m elev., 22 Apr 1976, Z. H. Shen 058 (CSFI!). Yuanling county, 600 m elev., 22 Apr 1976, Anonymous 58 (IBSC!). Jiangxi: Lushan mountain, 15 May 1977, C. F. Liang 34455 (IBK!). Tonggu county, 400 m elev., 06 Jun 1959, J. Xiong 04268 (LBG!). Yushan county, 500 m elev., 14 Sep 1977, S. K. Lai & H. R. Shan & D. F. Huang 039 (LBG!). Zhejiang: Chunan county, in broad-leaved forest, 700 m elev., 31 May 1959, M. L. She 26991 (NAS!). Linan city, tianmu mountains in woods, 1 Oct 1934, J. Shen 264 (NAS!). Linan city, tianmu mountain, on roadside, 430 m elev., 20 Jun 1983, Q. X. Zheng S815-16 (PE!). Linan city, tianmu mountains in woods, 400 m elev., 22 Aug 1959, Anonymous 28877 (NAS!). Taishun county, 25 May 2007, Anonymous 24100 (HHBG!). Shaanxi: Foping county, in woods, 15 Jun 2013 K. S. Mao & L. Zhang 2013-027A (SZ!). Lueyang county, in valley, 600 m elev., 11 Nov 1989 T. Y. Ding 2159 (IFP!). Mian county, 23 May 1942, K.T.Fu 3508 (PE!). Nanzhen county, in woods, 15 Jun 2015 L. Zhang 2015-19 (SZ!). Pingli county, on slope, 550 m elev., Apr 1959,Y. L. Qiao, 1114 (PE!). Shiquan county, in woods, 15 Jun 2013, K. S. Mao & L. Zhang 2013-033B (SZ!). Xixiang county, on slope, 650 m elev., 08 Apr 1958, J. Q. Xing 18 (NAS!). Xixiang county, in woods, 16 Jun 2013, K. S. Mao & L. Zhang 2013-032 (SZ!). Shandong: Kunyushan mountain, 12 Jul 1957, Anonymous 3095 (IBSC!, PE!). Kunyushan mountain, in woods, 188 m elev., 12 May 2016, L. Zhang 2016300 (SZ!). Sichuan: Cangxi county, 1070m elev., 08 May 1959, Z. S. Qin 02663 (CDBI!). Da county, 800 m elev., 23 Feb 1979, Team of Bazhong 830 (SM!). Da county, 1000 m elev., 20 Aug 1978, Team of Kaijiang 706 (SM!). Dujiangyan city, in valley, 1200 m elev., 11 May 1930, F. T. Wang 20749 (PE!). Emeishan mountain, on slope, 400 m elev., 03 Apr 1940, W. P. Fang 13968 (WUK!). Jiangjin county, 1100 m elev., 26 Jul 1978, Team of Dazu 584 (SM!). Jiulong county, on slope, 1000 m elev., 03 May 1959, M. X. Wang 7680 (PE!). Leibo county, 1100 m elev., Jun 1963, Z. T. Guan 373 (IBSC!). Mabian county, 1000 m elev., 31 May 1962, Q. L. Zhang 10123 (IBSC!). Qingchuan county, in woods, 10 May 2015, L. Zhang 201501 (SZ!). Tianquan county, on slope, 950 m elev., 14 Sep 1963, K. J. Guan & W. C. Wang 3470 (PE!). Tongjiang county, on roadside, 1900 m elev., 19 Sep 1978, Team of Tongjiang 1385 (SM!). Guangyuan city, on roadside, 1720 m elev., 08 Jul 1978, Team of Guangyuan 0880 (SM!).
Populus×ningshanica C. Wang & Tung in Journal of Beijing Forestry University 4: 19. 1979. TYPE: China (holotype, WUK not seen).
Additional specimens examined. China. Gansu: Wen county, 660 m elev., 15 Jun 2015 L. Zhang & Z. Q. Wang, 01014868 (SZ!). Shaanxi: Lueyang county, 770 m elev., 10 Jun 2015, L. Zhang & Z. Q. Wang, 01014866 (SZ!).
Acknowledgments
We thank the National Natural Science Foundation of China (31561123001, 31590821, 31500502) for financial support, Youth Science and Technology Innovation Team of Province (2014TD003), National Key Project for Basic Research (2012CB114504) and International Collaboration 111 Projects of China and 985 and 211 Projects of Sichuan University. The anonymous reviewers and editors are sincerely acknowledged.
Citation
Zhang L, Wang M, Ma T, Liu J (2018) Taxonomic status of Populuswulianensis and P.ningshanica (Salicaceae). PhytoKeys 108: 117–129. https://doi.org/10.3897/phytokeys.108.25600
Supplementary materials
Tables S1–S3
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Lei Zhang, Mingcheng Wang, Tao Ma, Jianquan Liu
Data type: molecular data
Explanation note: Table S1. Details for the 14 microsatellite loci adopted in genetic survey. Table S2. Details for the four chloroplast DNA fragments adopted in genetic survey. Table S3. Length and variations for each DNA region and for the combination of the four plastid regions
References
- Agapow PM, Binindaemonds OR, Crandall KA, Gittleman JL, Mace GM, Marshall JC, Purvis A. (2004) The impact of species concept on biodiversity studies. The Quarterly Review of Biology 79(2): 161–179. 10.1086/383542 [DOI] [PubMed] [Google Scholar]
- Bond JE, Stockman AK. (2008) An integrative method for delimiting cohesion species: Finding the population-species interface in a group of Californian trapdoor spiders with extreme genetic divergence and geographic structuring. Systematic Biology 57(4): 628–646. 10.1080/10635150802302443 [DOI] [PubMed] [Google Scholar]
- Braatne JH, Hinckley TM, Stettler RF. (1992) Influence of soil water on the physiological and morphological components of plant water balance in Populustrichocarpa, Populusdeltoides and their F1 hybrids. Tree Physiology 11(4): 325–339. 10.1093/treephys/11.4.325 [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Ceulemans R, Davis J, Stettler R. (2000) Emerging model systems in plant biology: Poplar (Populus) as a model forest tree. Journal of Plant Growth Regulation 19(3): 306–313. 10.1007/s003440000030 [DOI] [Google Scholar]
- Buck M, Cobb TP, Stahlhut JK, Hanner RH. (2012) Unravelling cryptic species diversity in eastern Nearctic paper wasps, Polistes (Fuscopolistes), using male genitalia, morphometrics and DNA barcoding, with descriptions of two new species (Hymenoptera: Vespidae). Zootaxa 3502(3502): 1–48. [Google Scholar]
- Cervera MT, Storme V, Soto A, Ivens B, Van MM, Rajora OP, Boerjan W. (2005) Intraspecific and interspecific genetic and phylogenetic relationships in the genus Populus based on AFLP markers. Theoretical and Applied Genetics 111(7): 1440–1456. [DOI] [PubMed] [Google Scholar]
- Dickmann DI, Isebrands JG, Eckenwalder JE, Richardson J. (Eds) (2001) Poplar Culture in North America. NRC Research Press, Ottawa, Canada.
- Dickmann DI, Kuzovkina YA. (2008) Poplars and willows of the world. Forest Management, Rome, Italy.
- Dickmann DI, Stuart KW. (1983) The culture of Poplars in Eastern North America. Department of Forestry, Michigan State University.
- Doyle J. (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19(1): 11–15. [Google Scholar]
- Douady CJ. (2007) Contribution of molecular techniques to the species delimitation problem. Bulletin de la Société Zoologique de France 132(4): 281–291. [Google Scholar]
- Eckenwalder JE. (1996) Taxonomic signal and noise in multivariate interpopulational relationships in Populusmexicana (Salicaceae). Systematic Botany 21(3): 261–271. 10.2307/2419658 [DOI] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. (2005) Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Molecular Ecology 14(8): 2611–2620. 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. (2003) Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 164(4): 1567–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Zhao S, Skvortsov A. (1999) Salicaceae In: Wu Z, Raven P (Eds) Flora of China. Volume 4. Science Press, Beijing, China.
- Feng J, Jiang DC, Shang HY, Dong M, Wang GN, He XY, Zhao CM, Mao KS. (2013) Barcoding Poplars (Populus L.) from Western China. PLoS One 8(8): e71710. 10.1371/journal.pone.0071710 [DOI] [PMC free article] [PubMed]
- Fladung M, Buschbom J. (2009) Identification of single nucleotide polymorphisms in different Populus species. Trees (Berlin) 23(6): 1199–1212. 10.1007/s00468-009-0359-3 [DOI] [Google Scholar]
- Francuski L, Ludoski J, Vujic A, Milankov V. (2009) Wing geometric morphometric inferences on species delimitation and intraspecific divergent units in the Merodonruficornis group (Diptera, Syrphidae) from the Balkan Peninsula. Zoological Science 26(4): 301–308. 10.2108/zsj.26.301 [DOI] [PubMed] [Google Scholar]
- Fujita MK, Leaché AD, Burbrink FT, McGuire JA, Moritz C. (2012) Coalescent-based species delimitation in an integrative taxonomy. Trends in Ecology & Evolution 27(9): 480–488. 10.1016/j.tree.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Gilbert KJ, Andrew RL, Bock DG, Franklin MT, Kane NC, Moore JS, Moyers BT, Renaut B, Rennison DJ, Veen T, Vines TH. (2012) Recommendations for utilizing and reporting population genetic analyses: The reproducibility of genetic clustering using the program structure. Molecular Ecology 21(20): 4925–4930. 10.1111/j.1365-294X.2012.05754.x [DOI] [PubMed] [Google Scholar]
- Hamzeh M, Périnet P, Dayanandan S. (2006) Genetic relationships among species of Populus (Salicaceae) based on nuclear genomic data. The Journal of the Torrey Botanical Society 133(4): 519–527. 10.3159/1095-5674(2006)133[519:GRASOP]2.0.CO;2 [DOI]
- Hamzeh M, Dayanandan S. (2004) Phylogeny of Populus (Salicaceae) based on nucleotide sequences of chloroplast trnt-trnf region and nuclear rDNA. American Journal of Botany 91(9): 1398–1408. 10.3732/ajb.91.9.1398 [DOI] [PubMed] [Google Scholar]
- Hendrixson BE, Derussy BM, Hamilton CA, Bond JE. (2013) An exploration of species boundaries in turret-building tarantulas of the Mojave Desert (Araneae, Mygalomorphae, Theraphosidae, Aphonopelma). Molecular Phylogenetics and Evolution 66(1): 327–340. 10.1016/j.ympev.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Jiang D, Feng JJ, Dong M, Wu GL, Mao KS, Liu JJ. (2016) Genetic origin and composition of a natural hybrid poplar Populus×jrtyschensis from two distantly related species. BMC Plant Biology 16(1): 89. 10.1186/s12870-016-0776-6 [DOI] [PMC free article] [PubMed]
- Klingenberg CP. (2011) MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources 11(2): 353–357. 10.1111/j.1755-0998.2010.02924.x [DOI] [PubMed] [Google Scholar]
- Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. (2005) Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences of the United States of America 102(23): 8369–8374. 10.1073/pnas.0503123102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7): 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. (2016) The integrative species concept and species on the speciation way. Shengwu Duoyangxing 24(9): 1004–1008. 10.17520/biods.2016222 [DOI] [Google Scholar]
- Lumley LM, Sperling FAH. (2010) Integrating morphology and mitochondrial DNA for species delimitation within the spruce budworm (Choristoneurafumiferana) cryptic species complex (Lepidoptera: Tortricidae). Systematic Entomology 35(3): 416–428. 10.1111/j.1365-3113.2009.00514.x [DOI] [Google Scholar]
- Ma T, Junyi Wang JY, Zhou GK, Yue Z, Hu QJ, Chen Y, Liu BB, Qiu Q, Wang Z, Zhang J, Wang K, Jiang D, Gou CY, Yu LL, Zhan DL, Zhou R, Luo WC, Ma H, Yang YZ, Pan SK, Fang DM, Luo YD, Wang X, Wang GN, Wang J, Wang Q, Lu X, Chen Z, Liu JC, Lu Y, Yin Y, Yang HM, Abbott RJ, Wu YX, Wan DS, Li J, Yin TM, Lascoux M, DiFazio SP, Tuskan GA, Wang J, Liu JQ. (2013) Genomic insights into salt adaptation in a desert poplar. Nature Communications 4(1): 2797. 10.1038/ncomms3797 [DOI] [PubMed]
- Pérez-Losada M, Eiroa J, Mato S, Domínguez J. (2005) Phylogenetic species delimitation of the earthworms Eiseniafetida (Savigny, 1826) and Eiseniaandrei Bouché, 1972 (Oligochaeta, Lumbricidae) based on mitochondrial and nuclear DNA sequences. Pedobiologia 49(4): 317–324. 10.1016/j.pedobi.2005.02.004 [DOI] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. (2000) Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf FJ. (2015) TpsDig. Version 2.22. Ecology and Evolution, SUNY at Stony Brook.
- Rohlf FJ. (2013) TpsUtility. Version 1.64. Ecology and Evolution, SUNY at Stony Brook.
- Rohlf FJ, Marcus LF. (1993) A revolution in morphometrics. Trends in Ecology & Evolution 8(4): 129–132. 10.1016/0169-5347(93)90024-J [DOI] [PubMed] [Google Scholar]
- Sites Jr JW, Marshall JC. (2003) Delimiting species: A Renaissance issue in systematic biology. Trends in Ecology & Evolution 18(9): 462–470. 10.1016/S0169-5347(03)00184-8 [DOI] [Google Scholar]
- Schroeder H, Hoeltken AM, Fladung M. (2012) Differentiation of Populus species using chloroplast single nucleotide polymorphism (SNP) markers-essential for comprehensible and reliable poplar breeding. Plant Biology 14(2): 374–381. 10.1111/j.1438-8677.2011.00502.x [DOI] [PubMed] [Google Scholar]
- Villemant C, Simbolotti G, Kenis M. (2007) Discrimination of Eubazus (Hymenoptera, Braconidae) sibling species using geometric morphometrics analysis of wing venation. Systematic Entomology 32(4): 625–634. 10.1111/j.1365-3113.2007.00389.x [DOI] [Google Scholar]
- Wan XQ, Zhang F, Zhong Y, Ding YH, Wang CL, Hu TX. (2013) Study of genetic relationships and phylogeny of the native Populus in Southwest China based on nucleotide sequences of chloroplast trnt-trnf and nuclear DNA. Plant Systematics and Evolution 299(1): 57–65. 10.1007/s00606-012-0702-9 [DOI] [Google Scholar]
- Wiens JJ. (2007) Species delimitation: New approaches for discovering diversity. Systematic Biology 56(6): 875–878. 10.1080/10635150701748506 [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Penkrot TA. (2002) Delimiting species using DNA and morphological variation and discordant species limits in spiny lizards (Sceloporus). Systematic Biology 51(1): 69–91. 10.1080/106351502753475880 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Lei Zhang, Mingcheng Wang, Tao Ma, Jianquan Liu
Data type: molecular data
Explanation note: Table S1. Details for the 14 microsatellite loci adopted in genetic survey. Table S2. Details for the four chloroplast DNA fragments adopted in genetic survey. Table S3. Length and variations for each DNA region and for the combination of the four plastid regions