Abstract
The purpose of this study was to determine the relationships among body condition score (BCS), radiography, and computed tomography (CT), and to establish a method for body fat assessment on CT in dogs. Thirty eight Beagles with 2 to 7 BCS were examined. Subcutaneous fat thickness (ST) on radiograph and body area (BA), total fat area (TA), subcutaneous fat area (SA), and visceral fat area (VA) on CT were measured at the level of L3 and L6 vertebra. Ratios of each value to the L6 length were obtained (rST, rTA, rSA, rVA) and the correlations with BCS were estimated. The value of VA/SA, VA/TA, TA/BA, VA/BA, and SA/BA were selected for measuring fat and the correlations with BCS were estimated. The rST, rTA, rSA, and rVA were significantly correlated with BCS, and the rTA and rSA were significantly correlated with rST. At the level of L3, rTA and rVA had stronger relationships with BCS than at L6 while rSA had a higher correlation with BCS at L6. The TA/BA, VA/BA, and SA/BA were significantly correlated with BCS, and the upper limits were 15.11, 6.31, and 8.92%, respectively. Our results showed that CT could be useful to assess body fat and TA/BA, VA/BA, and SA/BA are suitable criteria for measuring fat on CT. In addition, L3 was a more suitable location for evaluating total and visceral fat, and L6 was more suitable for evaluating subcutaneous fat.
Keywords: body condition score, body fat, computed tomography, dog, radiography
Obesity is an increasing problem in dogs, and studies suggest that 22 to 40% of dogs worldwide are obese [6]. Obesity is a risk factor for canine health and is closely linked to metabolic, endocrine, orthopedic, cardiorespiratory, urogenital, and dermatological disorders, as well as neoplasia [2, 6, 15]. It also reportedly increases anesthetic and surgical risks [3]. Therefore, clinical awareness and therapeutic management of canine obesity are needed. Especially, excess visceral fat is associated with cardiovascular disease or hyperadrenocorticism and quantitative assessments of subcutaneous and visceral fat separately are needed in these patients [2, 15].
Currently, deuterium oxide dilution and dual-energy x-ray absorptiometry are regarded as noninvasive and accurate methods for estimating body fat mass [11, 17]. However, these are not practical methods for quantifying body fat in veterinary medicine because of the variability of hydration status, the need for special equipment, and the impossibility of distinguishing subcutaneous and visceral fat from total fat. The most widely and easily used method for evaluating body fat composition in veterinary practice is body condition score (BCS) system. Although BCS shows good correlation with other body fat-measuring methods such as dual-energy x-ray absorptiometry [13] and serum leptin concentrations [8], it is a subjective method based on palpation and visual inspection, which has varying results among clinicians. BCS is also associated more with subcutaneous fat than visceral fat [12].
In human medical practice, computed tomography (CT) is a widely used and reliable tool for estimating abdominal fat accumulation and evaluating obesity [4]. It is possible to assess subcutaneous and visceral fat separately by CT, and parameters such as the visceral to subcutaneous fat ratio are generally considered important prognostic factors for obesity [4]. In veterinary medicine, fat assessment by CT has been studied [9, 10]. In addition, previous research has found that there is an association between BCS and fat thickness seen on thoracic radiographs [12]. However, in fat measuring methods, relationships among BCS, radiography, and CT have not been reported and there is no reliable reference range for body fat content seen on CT. The purpose of this study was to assess the relationships among BCS, radiography, and CT; to establish a method to assess body fat content on CT; and to quantify the body fat and establish reference ranges observed on CT in normal Beagle dogs.
MATERIALS AND METHODS
Animals and general procedures
The study was approved by the Institutional Animal Care and Use Committee of Kyungpook National University (Daegu, South Korea). The subjects were 38 adult Beagles (29 males and 9 females) with a BCS between 2 and 7; their body weights were recorded. All dogs showed normal findings on regular physical examination, complete blood count, serum biochemistry, and thoracic radiography. None of the dogs showed clinical signs and or a history of endocrine disease. Visual and palpation-based assessments were performed to determine the BCS of each dog by a single investigator (DN). A 9-point scale was used to assess BCS. The dogs were fasted for 12 hr.
Right lateral abdominal radiography using a digital radiographic system was performed. Under general anesthesia, the dogs were positioned in dorsal recumbency on the CT table. CT scanning was performed using a 32-row multi-detector CT scanner (Alexion, Toshiba, Tokyo, Japan) under the following conditions: 120 kV; 200 mA; Field of view, 210 to 240 mm (210 mm for 36 dogs, 230 mm for one dog, and 240 mm for one dog); and contiguous images with 3 mm slice thickness. The CT images included the diaphragm and continued caudally to the coxofemoral joint.
Image analysis
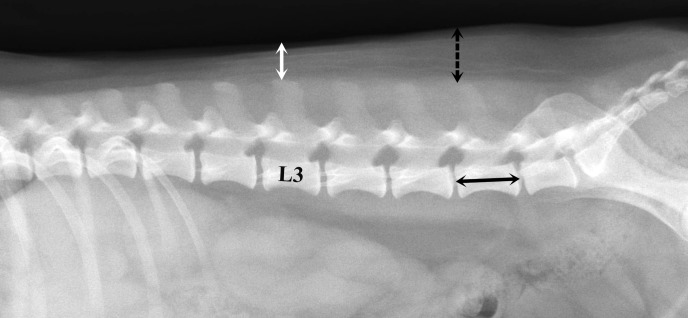
On a lateral abdominal radiograph, subcutaneous fat thickness (ST) was measured at the level of L3 and L6 vertebra, respectively. Straight lines extending from the highest level of the spinous processes to the skin-air interface were drawn at L3 and L6 vertebra, respectively (Fig. 1). To account for body size differences, the subcutaneous fat thickness ratios at L3 and L6 vertebra were calculated in all dogs (rST3, ST at the level of L3/length of the midbody of L6 vertebra; rST6, ST at the level of L6/length of the midbody of L6 vertebra).
Fig. 1.
Measurement of subcutaneous fat thickness and vertebral body length at L6 on a right lateral radiographic view. Subcutaneous fat thickness is measured from the highest level of the spinous process to the skin-air interface at L3 (white arrow) and L6 (dashed arrow) vertebra. The length of L6 vertebra is measured from the shortest part of the vertebral midbody (black arrow).
On CT transverse images at the level of L3 and L6 vertebra, regions of interest were drawn manually around the body to estimate body area (BA) and total fat area (TA) (Fig. 2, arrow). To assess visceral fat area (VA), regions of interest were drawn surrounding the peritoneal cavity (Fig. 2, arrowhead). The subcutaneous fat area (SA) was calculated by subtracting the VA from the TA. The fat areas were measured using a range of −135 HU to −105 HU. To account for body size differences, the ratios of TA, SA, and VA at the level of L3 and L6 vertebra to the length of the midbody of L6 were calculated (rTA, rSA, and rVA). For fat assessment, the VA/SA, VA/TA, TA/BA, VA/BA, and SA/BA were calculated at the level of L3 vertebra.
Fig. 2.
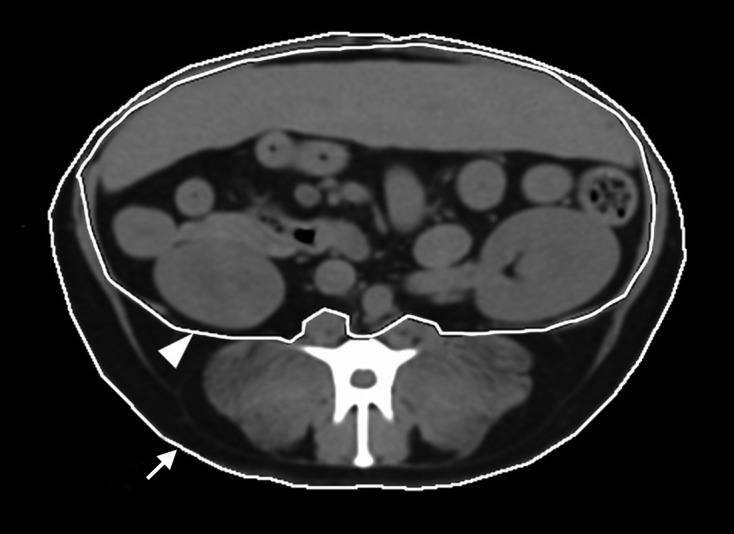
Estimation of body area and fat areas using CT. The region of interest is drawn on the body-air interface at the level of L3 and L6, and body area (BA) is acquired (arrow). In BA, total fat area (TA) was calculated using the range of −135 HU to −105 HU. An additional region of interest is drawn on the peritoneal line to estimate visceral fat area (VA) using the aforementioned HU (arrowhead). To calculate the subcutaneous fat area (SA), VA is subtracted from TA.
Statistical analysis
Statistical analysis was performed using a statistical software program (SPSS, version 22.0, SPSS Inc., Chicago, IL, U.S.A.). The normality of BCS, subcutaneous fat thickness, BA, TA, SA, and VA was evaluated using the Shapiro-Wilk test. The Pearson correlation method was used to test whether the ST at L3 and L6 vertebra measured on radiography, the rTA, rSA, and rVA at the level of L3 and L6 vertebra measured on CT, and the VA/SA, VA/TA, TA/BA, VA/BA, and SA/BA measured on CT were correlated with the BCS. In addition, the Pearson correlation method was used to test whether rST3 was correlated with rTA, rSA, and rVA at L3 and L6 vertebra on CT. The Pearson correlation method was used to test whether rST3 and rST6 were correlated with SA/BA. The Mann-Whitney U test was used to identify differences of VA/SA, VA/TA, TA/BA, VA/BA, and SA/BA at L3 vertebra between the male and female groups. Upper limits of 95% confidence intervals for TA/BA, VA/BA, and SA/BA were calculated.
RESULTS
The median BCS of the dogs was 5 (range, 2 to 7; mean, 5.05), with a dog assigned a BCS of 2, 3 dogs assigned a BCS of 3, 8 dogs assigned a BCS of 4, 12 dogs assigned a BCS of 5, 9 dogs assigned a BCS of 6, and 5 dogs assigned a BCS of 7. The mean body weight was 10.5 kg (range, 6.5 to 22.0 kg).
Relationships among BCS, radiography, and CT
Relationships among BCS, radiography, and CT were summarized in Table 1. On radiography, BCS was significantly correlated with rST3 and rST6. On CT, all fat areas (rTA, rSA, and rVA at L3 and L6 vertebra) showed good correlations with BCS. The rTA showed a better correlation with BCS at L3 than at L6 vertebra in the total and female groups, while the male group showed a better correlation at L6 than at L3 vertebra. The rSA had a better correlation with BCS at L6 than at L3 vertebra in all dogs. All dogs showed better correlations between rVA and BCS at L3 than at L6 vertebra. All dogs also showed significant correlations between rST3 and rTA at L3 vertebra, and between rST3 and rTA at L6 vertebra. The rSA at both L3 and L6 vertebra showed a high correlation with rST3. Both rST3 and rST6 were correlated with SA/BA (P<0.01; r=0.823, 0.887, respectively). The rVA at both L3 and L6 vertebra was correlated with rST3 in the total and male groups. There was no correlation between rST3 and rVA at L3 vertebra, or between rST3 and rVA at L6 vertebra in females.
Table 1. Correlation coefficients among BCS, and fat measurement on radiography and CT.
| Variable | Modality | BCS |
rST3 |
||||
|---|---|---|---|---|---|---|---|
| All | Female | Male | All | Female | Male | ||
| (n=38) | (n=9) | (n=29) | (n=38) | (n=9) | (n=29) | ||
| rST3 | Radiography | 0.851 a) | 0.755 b) | 0.880 a) | – | – | – |
| rST6 | 0.847 a) | 0.709 b) | 0.901 a) | – | – | – | |
| rTA at L3 | CT | 0.809 a) | 0.985 a) | 0.791 a) | 0.864 a) | 0.684 b) | 0.914 a) |
| rTA at L6 | 0.795 a) | 0.933 a) | 0.793 a) | 0.875 a) | 0.756 b) | 0.917 a) | |
| rSA at L3 | 0.771 a) | 0.831 a) | 0.795 a) | 0.859 a) | 0.722 b) | 0.918 a) | |
| rSA at L6 | 0.804 a) | 0.880 a) | 0.814 a) | 0.887 a) | 0.845 a) | 0.917 a) | |
| rVA at L3 | 0.779 a) | 0.936 a) | 0.755 a) | 0.811 a) | 0.542 | 0.875 a) | |
| rVA at L6 | 0.661 a) | 0.810 a) | 0.635 a) | 0.718 a) | 0.332 | 0.803 a) | |
a) P<0.01, b) P<0.05.
Fat accumulation differences between male and female groups
Median ranks and P values of VA/SA, VA/TA, TA/BA, VA/BA, and SA/BA at the level of L3 vertebra were summarized in Table 2. There were significant differences between males and females in the median ranks of VA/SA, VA/TA, TA/BA, and SA/BA. There was no significant difference in the median rank of VA/BA between males and females. The median ranks of VA/SA and VA/TA for males were higher than those for females. The median ranks of TA/BA and SA/BA for females were higher than for males.
Table 2. Correlation coefficients between BCS and fat measuring criteria, and median rank of the male and female groups.
| Variable | BCS |
Median rank |
||||
|---|---|---|---|---|---|---|
| All | Female | Male | Female | Male | P value | |
| (n=38) | (n=9) | (n=29) | (n=9) | (n=29) | ||
| VA/SA | −0.268 | 0.354 | −0.309 | 9.89 | 22.48 | 0.003 |
| VA/TA | −0.234 | 0.397 | −0.334 | 9.89 | 22.48 | 0.003 |
| TA/BA | 0.821 a) | 0.926 a) | 0.833 a) | 27.11 | 17.74 | 0.019 |
| VA/BA | 0.812 a) | 0.911 a) | 0.797 a) | 23.89 | 18.14 | 0.175 |
| SA/BA | 0.781 a) | 0.856 a) | 0.820 a) | 28.00 | 16.86 | 0.009 |
a) P<0.01.
Relationship between BCS and fat assessment criteria
The relationships between BCS and 5 criteria (VA/SA, VA/TA, TA/BA, VA/BA, and SA/BA) were summarized in Table 2. BCS was not significantly correlated to VA/SA, or VA/TA in all dogs. However, BCS showed significant positive correlations with TA/BA, VA/BA, and SA/BA in all dogs. The upper limits of the 95% confidence interval for the mean predicted values of TA/BA, VA/BA, and SA/BA were 15.11, 6.31, and 8.92%, respectively (Table 3).
Table 3. Mean values, ranges, and 95% confidence interval upper limits of fat measuring criteria using CT.
| Variable | Mean (%) | Range (%) | Upper limit of 95% confidence interval (%) |
|---|---|---|---|
| TA/BA | 11.84 | 0.93–37.02 | 15.11 |
| VA/BA | 4.95 | 0.40–17.24 | 6.31 |
| SA/BA | 6.89 | 0.43–20.54 | 8.92 |
DISCUSSION
In the present study, there was a significant correlation between BCS and rST3 as well as between BCS and rST6 using lateral abdominal radiography. In addition, rST3 on radiograph and rTA and rSA on CT showed good correlation. Therefore abdominal radiography may be a useful tool for measuring subcutaneous fat. However, we were unable to estimate subcutaneous and visceral fat separately in a radiographic image. In addition, subcutaneous fat thickness measurements on radiograph, from the spinous process to the skin-air interface at L3 and L6 vertebra, have included some muscles, which may have reduced the accuracy of measurements. There were no interactions between rVA at L3 vertebra and rST3 or between rVA at L6 vertebra and rST3 in females. This may be due to a difference in fat accumulation tendency based on sex.
On CT, all fat areas had a good correlation with BCS. When comparing the correlation coefficient of fat areas at L3 and L6 vertebra, rTA and rVA had a better correlation at L3 than at L6 vertebra, which indicated that the cross-section of L3 vertebra is a better location for total fat and visceral fat measurements. Conversely, rSA had a better correlation at L6 than at L3 vertebra. In addition, SA/BA had a better correlation with rST6 than with rST3. These results proved that the cross-section of L6 vertebra was a more suitable location for subcutaneous fat measurements. CT was confirmed to be better than radiography because it can estimate subcutaneous fat and visceral fat accumulation tendencies separately.
In the present study, we used abdominal radiography to measure subcutaneous thickness because the abdominal area is the main site used to assess BCS, deposition of fat over the lumbar and base of the tail, presentation of the waist, abdominal tuck, and abdominal distention. On CT, L3 and L6 vertebra were used to measure subcutaneous fat thickness. In human medicine, cross-sectional images of the abdominal umbilical region on CT are used to estimate obesity [16]. In this study, L3 vertebra was chosen for measuring subcutaneous fat thickness based on a previous study. The previous study suggested L3 vertebra as an ideal location for measuring total fat on CT [9], but whether L3 vertebra is also an ideal location for measuring visceral and subcutaneous fat separately was not proven. L6 vertebra was selected because subcutaneous fat is the thickest at L6 to L7 vertebra on lateral abdominal radiograph, which makes it easy to estimate fat thickness. The subcutaneous fat thickness of thin dogs, which do not have a large amount of fat, can also be measured without error at L6 vertebra. At L7 vertebra, accurate measurements were difficult because the spinous process of L7 vertebra overlaps with the ilium. Therefore, we concluded that L6 vertebra is an easy and accurate location for measuring subcutaneous fat thickness.
In veterinary medicine, subcutaneous and visceral fat accumulation tendency according to sex has not yet been reported, to our knowledge. In this experiment, 5 fat measuring criteria were compared between males and females to determine whether there is a difference in fat accumulation tendency based on sex. The significant differences between males and females, the higher median rank VA/SA and VA/TA values in males, and the higher median rank SA/BA values in females suggests that males have a tendency to accumulate more visceral fat than subcutaneous fat, whereas females have a tendency to accumulate more subcutaneous fat than visceral fat. A similar tendency of fat accumulation was found in human studies, where men had more visceral fat and women had more subcutaneous fat [1, 4, 14]. It should be noted that our study had an imbalanced number of males and females, so further gender-balanced studies are needed.
In human medicine, central obesity is determined by calculating the visceral to subcutaneous fat ratio [5]. The TA/BA, VA/BA, and SA/BA have also been proposed to determine whether a patient is obese [7]. It was unknown whether these criteria are applicable to dogs so, similar to human studies, the fat measuring criteria (VA/SA, VA/TA, TA/BA, VA/BA, and SA/BA) were selected to determine whether they are associated with the degree of obesity measured by BCS. The results showed the VA/SA and VA/TA were not correlated with BCS, while TA/BA, VA/BA, and SA/BA were well correlated with BCS. Thus, TA/BA, VA/BA, and SA/BA can be regarded as predictors of the degree of obesity. Furthermore, the upper limits of the 95% confidence interval of the mean predicted values for the TA/BA, VA/BA, and SA/BA were 15.11, 6.31, and 8.92%, respectively. The 9-point canine BCS was used to define dogs with a score of 8 or 9 as obese. Our study used dogs with a BCS of 2 to 7, so dogs with score of 8 or 9 were assumed to be obese. Therefore, these upper limits may provide an index for determining obesity. Further studies with other breeds would be needed to validate these upper limits.
In conclusion, abdominal radiography and CT were valuable techniques for estimating body fat levels in dogs. CT was especially useful because it may provide more useful information about visceral and subcutaneous fat separately. When using CT, the L3 vertebra level may be more suitable for measuring total and visceral fat, whereas the level of L6 vertebra may be more suitable for measuring subcutaneous fat. Males had a tendency to accumulate more visceral fat, whereas females tended to accumulate more subcutaneous fat. Results of the present study suggested that TA/BA, VA/BA, and SA/BA measured on a CT transverse image could be useful to estimate the degree of obesity in dogs. We suggest the following upper limits of TA/BA, VA/BA, and SA/BA to determine whether dogs are obese: 15.11, 6.31 and 8.92%, respectively.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2017RIC1B5075777).
REFERENCES
- 1.Busetto L., Baggio M. B., Zurlo F., Carraro R., Digito M., Enzi G.1992. Assessment of abdominal fat distribution in obese patients: anthropometry versus computerized tomography. Int. J. Obes. Relat. Metab. Disord. 16: 731–736. [PubMed] [Google Scholar]
- 2.Cho K. D., Paek J., Kang J. H., Chang D., Na K. J., Yang M. P.2014. Serum adipokine concentrations in dogs with naturally occurring pituitary-dependent hyperadrenocorticism. J. Vet. Intern. Med. 28: 429–436. doi: 10.1111/jvim.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clutton R. E.1988. The medical implications of canine obesity and their relevance to anaesthesia. Br. Vet. J. 144: 21–28. doi: 10.1016/0007-1935(88)90149-2 [DOI] [PubMed] [Google Scholar]
- 4.Enzi G., Gasparo M., Biondetti P. R., Fiore D., Semisa M., Zurlo F.1986. Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am. J. Clin. Nutr. 44: 739–746. doi: 10.1093/ajcn/44.6.739 [DOI] [PubMed] [Google Scholar]
- 5.Fujioka S., Matsuzawa Y., Tokunaga K., Tarui S.1987. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 36: 54–59. doi: 10.1016/0026-0495(87)90063-1 [DOI] [PubMed] [Google Scholar]
- 6.German A. J.2006. The growing problem of obesity in dogs and cats. J. Nutr. 136 Suppl: 1940S–1946S. doi: 10.1093/jn/136.7.1940S [DOI] [PubMed] [Google Scholar]
- 7.Grauer W. O., Moss A. A., Cann C. E., Goldberg H. I.1984. Quantification of body fat distribution in the abdomen using computed tomography. Am. J. Clin. Nutr. 39: 631–637. doi: 10.1093/ajcn/39.4.631 [DOI] [PubMed] [Google Scholar]
- 8.Ishioka K., Hosoya K., Kitagawa H., Shibata H., Honjoh T., Kimura K., Saito M.2007. Plasma leptin concentration in dogs: effects of body condition score, age, gender and breeds. Res. Vet. Sci. 82: 11–15. doi: 10.1016/j.rvsc.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 9.Ishioka K., Okumura M., Sagawa M., Nakadomo F., Kimura K., Saito M.2005. Computed tomographic assessment of body fat in beagles. Vet. Radiol. Ultrasound 46: 49–53. doi: 10.1111/j.1740-8261.2005.00009.x [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T., Koie H., Kusumi A., Kitagawa M., Kanayama K., Otsuji K.2014. Comparative investigation of body composition in male dogs using CT and body fat analysis software. J. Vet. Med. Sci. 76: 439–446. doi: 10.1292/jvms.13-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauten S. D., Cox N. R., Brawner W. R., Jr., Baker H. J.2001. Use of dual energy x-ray absorptiometry for noninvasive body composition measurements in clinically normal dogs. Am. J. Vet. Res. 62: 1295–1301. doi: 10.2460/ajvr.2001.62.1295 [DOI] [PubMed] [Google Scholar]
- 12.Linder D. E., Freeman L. M., Sutherland-Smith J.2013. Association between subcutaneous fat thickness measured on thoracic radiographs and body condition score in dogs. Am. J. Vet. Res. 74: 1400–1403. doi: 10.2460/ajvr.74.11.1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawby D. I., Bartges J. W., d’Avignon A., Laflamme D. P., Moyers T. D., Cottrell T.2004. Comparison of various methods for estimating body fat in dogs. J. Am. Anim. Hosp. Assoc. 40: 109–114. doi: 10.5326/0400109 [DOI] [PubMed] [Google Scholar]
- 14.Seidell J. C., Oosterlee A., Thijssen M. A., Burema J., Deurenberg P., Hautvast J. G., Ruijs J. H.1987. Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am. J. Clin. Nutr. 45: 7–13. doi: 10.1093/ajcn/45.1.7 [DOI] [PubMed] [Google Scholar]
- 15.Thengchaisri N., Theerapun W., Kaewmokul S., Sastravaha A.2014. Abdominal obesity is associated with heart disease in dogs. BMC Vet. Res. 10: 131. doi: 10.1186/1746-6148-10-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshizumi T., Nakamura T., Yamane M., Islam A. H., Menju M., Yamasaki K., Arai T., Kotani K., Funahashi T., Yamashita S., Matsuzawa Y.1999. Abdominal fat: standardized technique for measurement at CT. Radiology 211: 283–286. doi: 10.1148/radiology.211.1.r99ap15283 [DOI] [PubMed] [Google Scholar]
- 17.Zanghi B. M., Cupp C. J., Pan Y., Tissot-Favre D. G., Milgram N. W., Nagy T. R., Dobson H.2013. Noninvasive measurements of body composition and body water via quantitative magnetic resonance, deuterium water, and dual-energy x-ray absorptiometry in awake and sedated dogs. Am. J. Vet. Res. 74: 733–743. doi: 10.2460/ajvr.74.5.733 [DOI] [PubMed] [Google Scholar]