Abstract
The effect of riboflavin supplement in Eimeria tenella-infected chickens was evaluated. Mortality, fecal consistency, and oocysts per gram of feces were monitored for groups of E. tenella-infected chickens administered a basal diet supplemented with either riboflavin, the anti-coccidial drug amprolium, or with both compounds. The number of oocysts shed per chicken in the riboflavin-treated group was significantly higher than the positive non-treated control group. No significant difference in oocyst number between the amprolium-treated group and riboflavin plus amprolium-treated group was observed. Thus, the addition of 0.8 g/kg of riboflavin to basal diet can increase oocyst number in E. tenella-infected chicken, but has no effect on the efficacy of amprolium.
Keywords: amprolium, chicken, Eimeria tenella, oocyst production, riboflavin
Chicken coccidiosis is an intestinal protozoan disease caused by nine different species of Eimeria, which occurs worldwide [1, 15, 16]. This parasite causes a major impact on poultry production. E. tenella, E. acervulina, E. maxima, and E. necatrix are the four major pathogenic species [1]. E. tenella infection causes a severe disease characterized by bleeding, hemorrhagic lesion development, mortality, and reduced weight gain [7]. Coccidia damage epithelial cells of the intestine and hinder the absorption of nutrients or production of the vitamins indispensable for the chicken’s metabolism [13]. Apart from its general deleterious effects, the resulting hypovitaminosis also plays a role in increasing the severity of the clinical course of the coccidiosis [13].
Riboflavin, also known as vitamin B2, is an essential water-soluble vitamin that is required for the proper functioning of the nervous system, growth, metabolism, and cellular antioxidant protection in chickens [2, 6]. It is generally added to poultry feeds to prevent paralytic nervous symptoms and to preserve normal growth and health [6]. The active forms of riboflavin are flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), both of which act as cofactors for a variety of flavoprotein enzymatic reactions [2]. The normal riboflavin requirement for young, growing broiler chickens is 3.67 to 6.00 mg/kg of feed [6]. Riboflavin has a role not just in the host, but it is also necessary for the development of the parasite within the host [5]. The objectives of this study were to investigate the effects of riboflavin supplement on oocyst output in the chickens experimentally infected with E. tenella, and also to investigate the influence of riboflavin supplement on the efficacy of amprolium, an anticoccidial drug.
Parasite: The E. tenella Taiwanese strain that had been passaged and maintained in our laboratory for several years was used. Species identification and purification of the strain were confirmed by the manifestation of gross lesions and a multiplex PCR assay based on the regions of ITS-1 [18].
Experimental design: Seven-day-old Boris Brown chicks were divided into five groups of 12–22 chicks per group with the following treatments: negative control (no parasite, no drug or vitamin treatment fed with basal diet), positive control (infected with the parasite, no drug or vitamin treatment fed with basal diet), riboflavin-treated group (infected with the parasite and fed with the basal diet supplemented with riboflavin), amprolium-treated group (infected with the parasite and fed with the basal diet supplemented with amprolium), and riboflavin plus amprolium-treated group (infected with the parasite and fed with the basal diet supplemented with riboflavin and amprolium). Riboflavin (Wako Pure Chemical Industries Ltd., Tokyo, Japan) and amprolium (Tokyo chemical industry Co., Ltd., Tokyo, Japan) were mixed in the feed and given to the chicks beginning at the challenge day until necropsy. The amprolium dose used in our study was 0.125 g/kg of feed following the dose for prophylactic treatment for coccidiosis in chickens recommended by Kant et al. (2013) [8] as well as Peek and Landman (2011) [12]. The dose of riboflavin used was 0.8 g/kg of feed; we selected this dose because we would like to assess the effect of the riboflavin supplement at a higher concentration than the normal daily requirement on oocyst production. Moreover, there have been no reports of the riboflavin toxicity studies in poultry as most data are conducted on rats, and the results suggested that dietary levels between 10 and 20 times, or even 100 times higher than the requirement levels can be tolerated safety [4, 11]. The chicks, with ad libitum access to water and feed, were kept and handled according to the rules and regulations laid down by the Institutional Animal Care and Use Committee (IACUC) of Azabu University. Inoculation of oocysts for each chick was carried out by administering a 0.3 ml suspension of 1 × 103 sporulated oocysts of E. tenella in distilled water directly into the crop via oral gavage. From 1 to 7 days post-inoculation (DPI), clinical signs such as mortality and fecal consistency were recorded. The chicks were kept in plastic cages, and feces contaminated with oocysts were removed every day from 1 DPI, and then the oocysts per gram of feces (OPG) were estimated using the O-ring technique [14]. Chicks were necropsied at 7.5 DPI and oocyst counts were determined in cecal content samples individually from each chick using a McMaster slide. Oocyst numbers were transformed to the cube-root scale prior to one-way ANOVA. Statistical comparisons among the groups were performed with Tukey’s HSD test and P<0.05 was considered as significant.
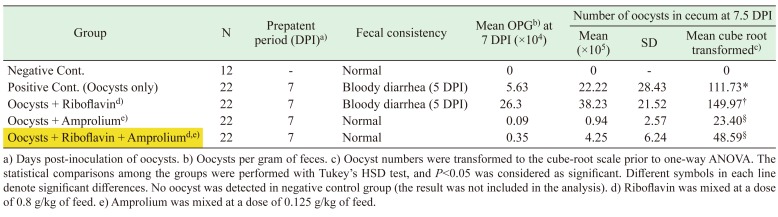
Prepatent period, fecal consistency, OPG, and mean number of oocysts in the cecum of chickens experimentally inoculated with 1 × 103 sporulated oocysts of E. tenella are shown in Table 1. Bloody diarrhea was observed at 5 DPI in all infected groups except for the amprolium-treated group and riboflavin plus amprolium-treated group, but the oocysts were recovered from the feces for the first time at 7 DPI from all experimentally infected groups. This might be because after the ingestion of the sporulated oocysts, the sporozoites are released and penetrate into the intestinal epithelial cells. Then, the sporozoites transform into merozoites, which multiply asexually [7, 9]. During asexual stage, bloody diarrhea occurred due to the destruction of epithelial cells by the merozoites. This is followed by the sexual stage and continuous oocysts excretion [7]. The OPG of the riboflavin-treated group was higher than the positive control group; the former being 2.63 × 105 and the latter 5.63 × 104. The mortality in the negative control, riboflavin-treated, amprolium-treated, and riboflavin plus amprolium-treated groups were zero, and only one chick from the positive control group died at 2 DPI. On the day of the necropsy (7.5 DPI), the mean number of oocysts in the cecum of the riboflavin-treated group was significantly higher than the positive control group (P<0.05). However, there was no significant difference in the mean oocyst number between the amprolium-treated and riboflavin plus amprolium-treated groups (P>0.05) (Table 1).
Table 1. Prepatent period, fecal consistency, OPG, and mean number of oocysts in the cecum of the chickens experimentally inoculated with 1 × 103 sporulated oocysts of Eimeria tenella.
A number of vitamins such as biotin, thiamine, nicotinic acid, folic acid, and riboflavin are known to be necessary for the complete development of coccidia within the host [3, 17]. For normal development of parasites, E. acervulina and E. tenella in the chicken, dietary thiamine, riboflavin, biotin, nicotinic acid, and folic acid are required. E. tenella required thiamine and riboflavin for gametogony, biotin for first schizogony, and nicotinic acid for second schizogony [3, 13, 17].
Warren (1968) [17] fed E. acervulina or E. tenella-infected chickens with various single vitamin-deficient diets and monitored the oocyst production. The author observed that thiamine, biotin, and nicotinic acid are the three most needed vitamins, and their deficiency led to over 90% suppression in oocyst production. The author also found that riboflavin deficiency caused 75–89% inhibition in oocyst production. In our study, chickens supplemented with riboflavin and then infected with E. tenella showed significant increased shedding in the number of oocysts per chicken because riboflavin is a precursor of FAD, which is an essential cofactor for carbohydrate metabolism in the parasite [10]. Riboflavin, pyridoxine, biotin, and vitamin B12 are required by both the host and the parasite, the former for prevention of paralytic nervous symptoms, and the latter for multiplication. However, vitamin A, D, E, and K are essential for only the host and not for endogenous development by coccidia [3, 5].
Amprolium, which is a thiamine antagonist, completely blocks the absorption of thiamine and also has an antagonistic effect on the biotin supply [8, 12]. Because of the high demand of thiamine during schizogony, amprolium is especially efficacious during this stage. Amprolium also affects the first generation of schizonts, and to a lesser extent, the gametogony allows the immune response to develop [12]. In our study, there was no significant difference between the amprolium-treated group and riboflavin plus amprolium-treated group for the number of oocysts per chicken. This might be because riboflavin enhances the development of the coccidia only during gametogony, but amprolium has an effect on schizogony, the first generation of schizonts, and gametogony [3, 12, 13, 17].
This study demonstrated that the addition of riboflavin at a dose of 0.8 g/kg to the basal diet could increase oocyst numbers in E. tenella-infected chickens, but has no effect on the efficacy of the anticoccidial drug.
REFERENCES
- 1.Aziz A. E., Orma O. A., Awadin W. F., Seady Y. E. L.2016. Effects of supplementation of broiler diets with fish oil and linseed oil on growth performance, cytokine, and cecal histopathological changes in broiler chicken infected by Eimeria tenella. Int. J. Agric. Sc. & Vet. Med. 4: 12–27. [Google Scholar]
- 2.Belinda T. J.2014. Significance of riboflavin (vitamin-B2) for health. J. Pharm. Sci. & Res. 6: 285–287. [Google Scholar]
- 3.Doran D. J., Augustine P. C.1978. Eimeria tenella: vitamin requirements for development in primary cultures of chicken kidney cells. J. Protozool. 25: 544–546. doi: 10.1111/j.1550-7408.1978.tb04183.x [DOI] [PubMed] [Google Scholar]
- 4.EFSA FEEDAP Panel (European Food Safety Authority Panel on Additives and Products or Substances used in Animal Feed). 2016. Scientific Opinion on the safety and efficacy of vitamin B2 (riboflavin and riboflavin 5′-phosphate ester monosodium salt) produced by Bacillus subtilis for all animal species, based on a dossier submitted by DSM. EFSA 14: 1–26.
- 5.Hammond D. M.1973. The coccidia (Eimeria, Isospora, Toxoplasma, and Related Genera), University Park Press, Baltimore and Butterworth & Co., Ltd., London. [Google Scholar]
- 6.Johnson W. D., Storts R. W.1988. Peripheral neuropathy associated with dietary riboflavin deficiency in the chicken. I. Light microscopic study. Vet. Pathol. 25: 9–16. doi: 10.1177/030098588802500102 [DOI] [PubMed] [Google Scholar]
- 7.Jordan A., Caldwell D. J., Klein J., Coppedge J., Pohl S., Fitz-Coy S., Lee J. T.2011. Eimeria tenella oocyst shedding and output in cecal or fecal contents following experimental challenge in broilers. Poult. Sci. 90: 990–995. doi: 10.3382/ps.2010-01228 [DOI] [PubMed] [Google Scholar]
- 8.Kant V., Singh P., Verma P. K., Bais I., Parmar M. S., Gopal A., Gupta V.2013. Anticoccidial drugs used in the poultry: an overview. Sci. Int 1: 261–265. doi: 10.17311/sciintl.2013.261.265 [DOI] [Google Scholar]
- 9.Khaier M. A. M., Abdelhalim A. I., Abukashawa S. M. A.2015. Isolation and morphological identification of Eimeria tenella (Family : Eimeriidae) from Khartoum State (Sudan). J. Appl. Ind. Sci. 3: 177–181. [Google Scholar]
- 10.Long P. L.1982. The biology of the coccidia, University Park Press, Baltimore. [Google Scholar]
- 11.NRC (National Research Council). 1987. Riboflavin (Vitamin B2). pp. 53–57. In: Vitamin Tolerance of Animals. The National Academies Press, Washington, D.C. [Google Scholar]
- 12.Peek H. W., Landman W. J. M.2011. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 31: 143–161. doi: 10.1080/01652176.2011.605247 [DOI] [PubMed] [Google Scholar]
- 13.Pellerdy L. P.1974. Coccidia and coccidiosis, 2nd ed., Verlag Paul Parey, Berlin und Hamburg. [Google Scholar]
- 14.Taira N., Ando Y., Williams J. C.2003. A Color Atlas of Clinical Helminthology of Domestic Animal, Revised ed., Elsevier, Amsterdam. [Google Scholar]
- 15.Tsuji N., Kawazu S., Ohta M., Kamio T., Isobe T., Shimura K., Fujisaki K.1997. Discrimination of eight chicken Eimeria species using the two-step polymerase chain reaction. J. Parasitol. 83: 966–970. doi: 10.2307/3284302 [DOI] [PubMed] [Google Scholar]
- 16.Vrba V., Poplstein M., Pakandl M.2011. The discovery of the two types of small subunit ribosomal RNA gene in Eimeria mitis contests the existence of E. mivati as an independent species. Vet. Parasitol. 183: 47–53. doi: 10.1016/j.vetpar.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 17.Warren E. W.1968. Vitamin requirements of the Coccidia of the chicken. Parasitology 58: 137–148. doi: 10.1017/S0031182000073492 [DOI] [PubMed] [Google Scholar]
- 18.You M. J.2014. Detection of four important Eimeria species by multiplex PCR in a single assay. Parasitol. Int. 63: 527–532. doi: 10.1016/j.parint.2014.01.006 [DOI] [PubMed] [Google Scholar]