Abstract

Bioinspired designs of superhydrophobic and superhydrophilic materials have been an important and fascinating area of research in recent years for their extensive potential application prospects from industry to our daily life. Despite extensive progress, existing research achievements are far from real applications. From biomimetic performance to service life, the related research has faced serious problems at present. A timely outlook is therefore necessary to summarize the existing research, to discuss the challenges faced, and to propose constructive advice for the ongoing scientific trend. Here, we comb the process of development of bioinspired superhydrophobic and superhydrophilic materials at first. Then, we also describe how to design artificial superhydrophobic and superhydrophilic materials. Furthermore, current challenges faced by bioinspired designs of superhydrophobic and superhydrophilic materials are pointed out, separately, and the possible solutions are discussed. Emerging applications in this field are also briefly considered. Finally, the development trend within this field is highlighted to lead future research.
Short abstract
The development of bioinspired superhydrophobic and superhydrophilic materials is summarized with a time-line. Challenges and trends of bioinspired materials and interfacial sciences are explored.
1. Introduction
Nature is always the wise mentor of humans. Learning from nature makes for rapid development of our science and technology. Bionics is born out on the basis of this background, which is gradually becoming an interdisciplinary central science research field. Superhydrophobic and superhydrophilic materials1−7 are the most representative biomimetic examples in surface and interfacial research of physical chemistry. The discovery and development of superhydrophobic and superhydrophilic materials led to a series of innovations and opened the floodgates to new research.
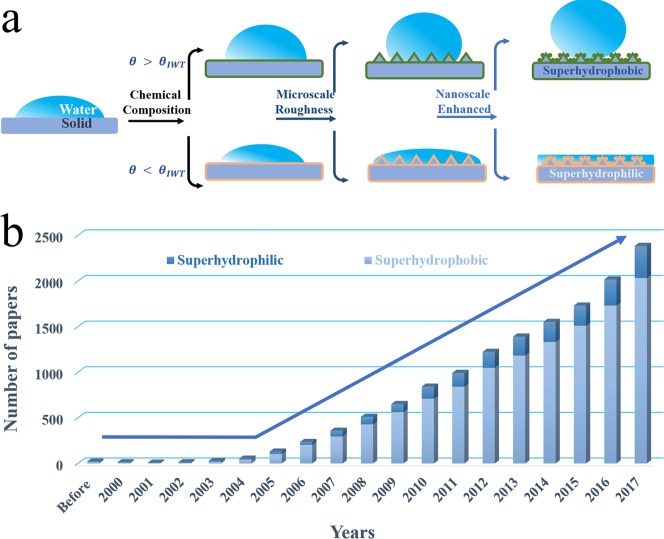
When a water drop is placed on a solid surface, it tends to spread toward a lower energy state. Then, there is a water contact angle (WCA) measured at the edge (solid–liquid–air three-phase contact line) of each droplet. Different WCAs can reflect the wettability of the solid surface. In 1805, Thomas Young,8 a pioneer scientist, proposed the concept of the contact angle of a liquid and established Young’s equation to define the notion of surface wettability. Some surfaces with extreme wettability have attracted much of the attention of later scientists,2,9−13 which are the above-mentioned superhydrophobic and superhydrophilic surfaces, defined with a WCA larger than 150° or smaller than 10°, respectively.14 The water drop can stand on the superhydrophobic surface like a ball but spread completely forming a water film on the superhydrophilic surface. A surface’s morphology (surface roughness) and intrinsic material properties (surface energy) cooperatively decide its wettability.1,9,15
As shown in Figure 1a, how surface roughness and surface energy
affect surface wettability
is determined by the intrinsic wetting threshold (IWT) of the water,
which is the boundary of the wettability between hydrophilic and hydrophobic
when the liquid is deposited.4,9 When the intrinsic WCA
(θ) on a flat solid surface is larger than θIWT, superhydrophobic surfaces can
be obtained by increasing the surface roughness. Conversely, when
the intrinsic WCA (θ) on a flat solid surface is smaller than θIWT, superhydrophilic
surfaces can be obtained. The relationship between surface roughness
and apparent contact angle (θa) can be described
by the Koch curve fractal formula:15,16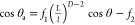 . f2 is the
fraction of the air surface under the water droplet, and f1 + f2 = 1. L and l are the upper- and lower-limit scales of
the surface, respectively, and D is the fractal dimension.
Surface roughness and apparent contact angle have close ties to the
value of L/l. Thus, the existence
of a nanoscale structure promotes superwettability. It is worth mentioning
that 90° is considered as the intrinsic wetting threshold of
water according to Young’s equation. However, by considering
the interphase water molecular interactions and structures, a lower
intrinsic wetting threshold of 65° is proposed. The WCA of 65°
has also been proven to be the limit between hydrophilicity and hydrophobicity
when increasing surface roughness to construct superhydrophilic or
superhydrophobic surfaces.9,17,18 It is the key criteria to construct artificial superhydrophilic
and superhydrophobic materials.
. f2 is the
fraction of the air surface under the water droplet, and f1 + f2 = 1. L and l are the upper- and lower-limit scales of
the surface, respectively, and D is the fractal dimension.
Surface roughness and apparent contact angle have close ties to the
value of L/l. Thus, the existence
of a nanoscale structure promotes superwettability. It is worth mentioning
that 90° is considered as the intrinsic wetting threshold of
water according to Young’s equation. However, by considering
the interphase water molecular interactions and structures, a lower
intrinsic wetting threshold of 65° is proposed. The WCA of 65°
has also been proven to be the limit between hydrophilicity and hydrophobicity
when increasing surface roughness to construct superhydrophilic or
superhydrophobic surfaces.9,17,18 It is the key criteria to construct artificial superhydrophilic
and superhydrophobic materials.
Figure 1.
(a) Intrinsic wetting threshold (θIWT) of water, which is regarded as the limit between hydrophilicity and hydrophobicity. When the intrinsic WCA (θ) on a flat solid surface is larger than θIWT, superhydrophobic surfaces can be obtained by increasing the surface roughness. Conversely, when the intrinsic WCA (θ) on a flat solid surface is smaller than θIWT, superhydrophilic surfaces can be obtained. (b) Rapid increase of research interest (number of papers) on the topic of “superhydrophobic” and “superhydrophilic” materials.
With the rapid development of nanotechnology at the beginning of the 21st century, basic scientific research started moving toward practical applications. Therefore, the research of superhydrophobic or superhydrophilic materials also enters a new period of rapid growth. By searching in the ISI (Institute for Scientific Information) Web of Science using the topic of “superhydrophobic” or “superhydrophilic”, we can observe an accelerating increase for the number of papers in 2004 (Figure 1b). Under amazing development momentum, some fundamental problems still hide. As for superhydrophobic materials, weak mechanical stability is the true crux of this topic.14,19,20 Scientists have taken notes of this point, and some research has also been carried out. So far, however, no practical solutions have been proposed. More seriously, no authoritative, standard, or effective evaluation system is established in this field. The challenges in superhydrophilic materials are more obvious. The ability of artificial superhydrophilic materials to imitate nature is limited.4,21 In addition, insufficient application development of superhydrophilic materials is another bottleneck. How to overcome these difficulties in the future is the main focus of this Outlook.
In this Outlook, we first briefly introduce the processes of the development of bioinspired superhydrophobic and superhydrophilic materials, mainly focused on research during the past two decades. On the basis of this understanding, in the following sections, the designs of bioinspired superhydrophobic and superhydrophilic materials have been summarized. Their respective challenges are pointed out, and the potential solutions are also put forward. In Section 4, recently emerging applications of bioinspired superhydrophobic and superhydrophilic materials are discussed as well. Finally, we briefly present our personal view of the open questions, remaining challenges, and development trend of this field.
2. Bioinspired Superhydrophobic and Superhydrophilic Processes of Development
2.1. Bioinspired Superhydrophobic Processes
Researchers found that duck feathers cannot be wetted by water a long time ago (Figure 2a).22 The modern study of superhydrophobic surfaces has enjoyed a resurgence in the past two decades with the rise of bionics. Scientists try to detect the mechanism behind the lotus effect with the aid of scanning electron microscopy. In 1997, the microscale papillae of lotus leaves were identified as the key to its water-repellency (Figure 2b).23 For further exploration, dual-scale micro-/nanostructures of lotus leaves allowing the achievement of both high apparent contact angles and low adhesion were demonstrated by Jiang et al. in 2002 (Figure 2c).15 This was the first work to reveal that the multiscale structure is crucial to superhydrophobicity. In the same year, Jiang et al. also discovered that the rice leaf displayed anisotropic wettability (Figure 2d).15 Rice leaf papillae are parallel to the leaf edge and randomly in the other direction. In 2004, Gao and Jiang24 found that the special hierarchical structure of superhydrophobic water strider legs (Figure 2e) is the main reason for water striders’ standing effortlessly and moving quickly on water. Researchers reported the second anisotropic creature surface in 2007, namely, butterfly wings (Figure 2f).25 A water droplet easily rolls off the wings along the outward direction of the body but pinned in an inward direction, which resulted from the direction-dependent arrangement of flexible nanotips on nanostripes and microscales overlapped on the wings.
Figure 2.
Time-line of bioinspired superhydrophobic and superhydrophilic process of development. (a) Duck feather.22 (b) Lotus effect.23 (c) Micro–nano cooperative lotus effect.15 (d) Rice leaf.15 (e) Legs of water striders.24 (f) Butterfly wings.25 (g) Rose effect.26 (h) Salvinia molesta.27 (i) Bacterial biofilm.28 (j) Collembola.29 (k) Precorneal tear film. (l) Fish scale.30 (m) Spider Silk.31 (n) Shark skin.32 (o) Cactus.33 (p) Tree frog.34 (q) Lizard skin.35 (r) Nepenthes alata.36 Copyright 2002, 2009, 2010, 2013 Wiley; 2004, 2010, 2012, 2016 Nature publishing group; 2007, 2012 Royal Society of Chemistry; 2008 American Chemical Society; 2015 Royal Society; 2011 PNAS; and 2012 Springer.
The adhesion force of superhydrophobic surfaces is also of great interest to scientists. In the following year, a superhydrophobic rose petal (Figure 2g) with high adhesion force26 was reported. A water droplet can stand on the surface of the petal with spherical shape, but it cannot roll off even when the petal is turned upside down. This phenomenon is also called the “petal effect”. Another unique superhydrophobic plant is discovered in 2010, called Salvinia. The Salvinia leaf (Figure 2h)27 offers a novel mechanism for long-term air retention. Miraculously, the terminal hair lacks the wax crystals and forms hydrophilic patches. Authors demonstrate that these hydrophilic patches can stabilize the air layer by pinning the air–water interface, which is also called the “Salvinia effect”. The discovery of these characteristic creature surfaces mentioned above has promoted a deep understanding of superhydrophobicity. The revelation of an intrinsic mechanism has also enriched the theory and guided the bioinspired designs of artificial superhydrophobic materials.
Aizenberg et al.28 reported a surprising observation that Bacillus subtilis biofilm colonies and pellicles (Figure 2i) were shown surpassing the repellency toward water and liquids with lower surface tension. This nonwetting property benefits from a synergistic effect of ECM (extracellular matrix, the slimelike “cement” of biofilm) composition, multiscale roughness, reentrant topography, and so on. Then, researchers (in 2012) found that springtails (Collembola)29 (Figure 2j) can live in water and oil environments. It is because they have robust surface plastrons consisting of hexagonal or rhombic comblike patterns to capture an air cushion formed around them.
2.2. Bioinspired Superhydrophilic Processes
The bioinspired superhydrophilic concept has not been popularized for a long time. The essential reason is the lack of practical applications. Long before, scientists found that tears can spread, forming a film to protect human eyes (Figure 2k). By 2009, the discovery of superhydrophilic fish scales (Figure 2l) opened a new door for bioinspired superhydrophilic research.30 Fish scales display good superoleophobicity under water, which originates from the water-phase hierarchical micro-/nanostructures structures. The oil drop shows low adhesion on the surface, so that fish can keep clean under water. This work makes underwater self-cleaning become one of the most important applications of superhydrophilicity. In the past decade, intriguing wetting phenomena on the superhydrophilic creature surfaces have been discovered continuously. Zheng and co-workers31 have demonstrated that spider silk (Figure 2m) can collect water efficiently from air in 2010. A unique silk structure with spindle-knots results in a surface energy gradient and a difference in Laplace pressure to achieve continuous directional water condensation around spindle-knots.
Shark skin (Figure 2n) has been detected with an underwater drag reduction ability a long time ago. In 2012, researchers also found its superhydrophilicity similar to that of fish scales.32 In the same year, anther water-collection creature, the cactus, was in scientists’ spotlight (Figure 2o). Opuntia microdasys (cactus) have an efficient fog collection system similar to spider silk.33 Three main reasons, gradient of the Laplace pressure, surface-free energy, and multifunction integration, endow the cactus with an efficient fog collection ability. Tree-frog toe secretion (Figure 2p) can wet all kinds of surfaces,34 and then adhere to the surfaces. The high-adhesion wetting state of a tree-frog toe profits from its microstructure and superhydrophilicity. In 2015, scientists found that special skin adaptations enable the Texas horned lizard35 (Figure 2q) to access water sources such as moist sand and dew. Their skin is capable of directionally transporting water without external energy input. Another classical creature surface with a continuous directional water transport ability found more recently is the peristome surface of Nepenthes alata(36) (Figure 2r). The multiscale structure of the peristome surface optimizes and enhances capillary rise in the transport direction, and prevents water backflow in the reverse direction. This discovery can be used to design bioinspired fluid-transport superhydrophilic systems for practical applications. In general, according to all this research, development of bioinspired superhydrophilic processes has entered a new historical stage.
3. Current Developments and Challenges of Designs of Superhydrophobic and Superhydrophilic Materials
The most critical part of bioinspired superwetting material design is synergistic action between hierarchical dual-scale structures and the intrinsic material properties.1,9,14 Basically, two biomimetic principles can be summarized: Hierarchical dual-scale structures have played a key role in determining the wetting property. The arrangement, orientation, and curvature of micro-/nanoscale structures can control the motion of the liquid.
3.1. Current Developments and Challenges of Designs of Superhydrophobic Materials
Scientists have detected microscale papillae (Figure 3a) on the lotus leaf as early as 199723 and deduced that this structure plays an important role in superhydrophobicity. In 2002, the micro-/nanostructure (Figure 3b) on lotus leaves was uncovered further.15 The synergistic action of the micro-/nanostructure makes the water droplet tend to stand on the surface in the Cassie–Baxter state (Figure 3c). The water drop cannot penetrate into small cavities between rough dual-scale structures. The air is trapped in these cavities,22 resulting in a solid–air–liquid heterogeneous interface. Academics believe that the main contribution of the microscale structure is to enhance the mechanical stability of the superhydrophobic surface, and the aim of the nanoscale structure is to retain the Cassie–Baxter state on the superhydrophobic surface.
Figure 3.
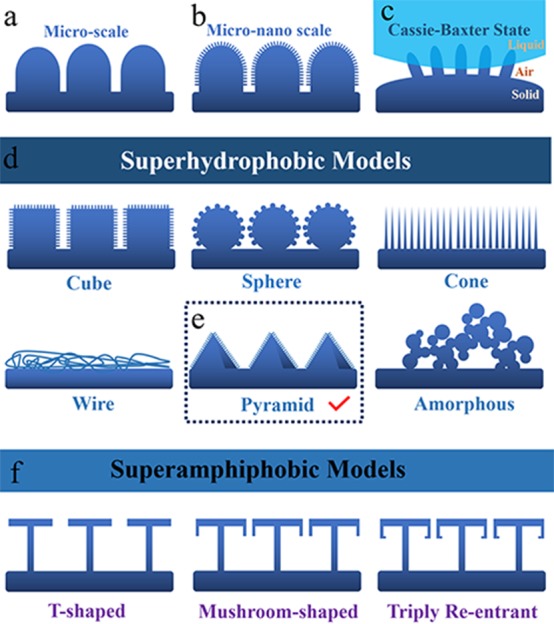
Models of the evolution from superhydrophobicity to superamphiphobicity. (a) Microscale synapse model. (b) Micro-/nanoscale synapse model. (c) Cassie–Baxter state model. (d) Various developed artificial micro-/nanoscale models. (e) Pyramid micro-/nanoscale model shows optimal comprehensive performance. (f) Classical superamphiphobic models from T-shaped to mushroom-shaped to triply reentrant structure.
On the basis of this understanding, scientists have designed a series of different micro–nano dual-scale superhydrophobic materials (Figure 3d). Regular structures (cube, sphere, pyramid, and so on) can be fabricated through physical methods, such as laser processing or lithography.37,38 After chemical modification, various regular micro–nano dual-scale superhydrophobic materials can be obtained. In addition, irregular micro–nano dual-scale superhydrophobic materials can be prepared by chemical methods including electrodeposition, sol–gel process, hydrothermal method, and so on.39,40 As to micro-/nanowires or micro-/nanofibers, the electrospinning technique is one of the best choices.41 Dual-scale amorphous superhydrophobic (carbon) materials can be achieved by incomplete combustion of long-chain alkane.42
We have summarized some typical works around the development of bioinspired superhydrophobic and superhydrophilic materials. In 2004, Jiang et al.43 reported one kind of lotus-leaf-like superhydrophobic polystyrene film with a novel composite structure consisting of porous microspheres and nanofibers by the versatile electro-hydrodynamic method. Later scientists also tried to construct a lotus-leaf-like artificial surface to obtain a similar superhydrophobicity.44,45 One work made new progress recently by Lee et al.46 They prepared artificial rice leaf surfaces in which nanoporous multilayers were of a wavy microstructure. An increase in nanostructure roughness led to a transition in the dynamic water droplet anisotropic wetting states. Research about water strider legs is mostly about the mechanism. Bioinspired designs of water striders can achieve microrobots who can walk on the water surface.47,48 Herein, Tan and co-workers49 presented a versatile route via selective surface functionalization and subsequent electroless deposition to duplicate the intact 3D organic butterfly wing scales on metals such as cobalt, copper, nickel, and so on. Malvadkar et al.50 reported an array of poly(p-xylylene) nanorod film with anisotropic wetting behavior by means of a pin-release droplet ratchet mechanism. The nanofilm achieves directional transport of microliter droplets on a smooth surface. In 2010, lotus/petal effect surfaces were successfully fabricated. With the control of UV irradiation and heating profiles of the photochromic compound, surface wettability can be controlled.51 As for the Salvinia leaf with a fine eggbeater shape, it is very difficult to construct this unique structure through common physical or chemical methods. However, with the aid of advanced 3D printing technology, Yang et al.52 have reported a superhydrophobic eggbeater structure with controllable surface morphology. Through the research on superoleophobicity creature surfaces, scientists revealed that only one certain type of structure with reentrant geometries is appropriate for constructing superoleophobic surfaces (Figure 3f). Tuteja et al.53 first came up with the T-shaped reentrant structure, which will not only strengthen the stability of the superamphiphobic surface but also facilitate the choice of corresponding oleophilic materials for preparing superamphiphobicity. Furthermore, Kim et al.54 designed one kind of mushroom-shaped doubly reentrant structure. This surface with doubly reentrant structure can repel completely wettable liquid, where even perfluorohexane can bounce off this surface. Most recently, using two-photon polymerization 3D printing technology, Gu et al.55 fabricated triply reentrant arrays with super-repellence from water to various organic liquids successfully. Even after treatment by oxygen plasma, the superamphiphobicity of the triply reentrant array surface can be kept well. This work proposed an advanced method to construct a complex bioinspired reentrant structure. Further, except for superhydrophobicity and superoleophobicity, in different media, 64 unique wetting states are achieved totally through different combinations of water, oil, and air.2,9
The tremendous application potential of bioinspired superhydrophobic materials in areas such as water repellence, self-cleaning, anti-icing, drag reduction, and corrosion resistance1,10,14,56 will make significant changes in our daily life and industry. Despite significant progress,57−60 bioinspired superhydrophobic materials were never as widespread in fact as we expected. It is because two core issues have not been addressed yet, which are chemical stability and, especially, mechanical durability.19 Micro-/nanostructures on the superhydrophobic surface are highly susceptible to mechanical wear, while surface compositions may also be altered by UV or acid–base conditions.20,61 Both processes can lead to the loss of superhydrophobicity. Lots of scientists have committed to resolve this problem. From the structural stability aspect, the micro–nano dual-scale pyramid (Figure 3e) is considered the best choice.62−64 The pyramid structure has a large contact area with the substrate to resist a greater degree of wear, while the acuminate top can reduce contact area with liquid to increase the WCA. However, it is not realistic to promote superhydrophobic materials with micro–nano dual-scale pyramids in extensive areas because of the complex preparation and high cost. Thus, the most mainstream approaches are a self-healing superhydrophobic material40,65−67 and a superhydrophobic surface with bonding layer.60,61 These two approaches bring the research into a new stage but not the end.
Scientists who professionally engaged in studying the durability of superhydrophobic materials all face a common confusion, which is the lack of a unified, authoritative, and comprehensive evaluation system. At present, several strategies have been used to test the durability of superhydrophobicity, such as the abrasion test, tape peeling, blade scratching, sand abrasion, water jet tests, ultraviolet radiation, high/low temperature, and acid–base marinating.19,61,68 However, there is no consistency about the test conditions such as types of abradant, applied pressure, velocity, irradiation intensity, and so on. As a result, the lack of standardization always makes comparison of different research results impossible. In addition, how to evaluate the durability of superhydrophobic materials is also an issue. By only using WCA, it is insufficient and unconvincing. If these problems cannot be solved urgently, all current research is blind and unavailing in this field. Therefore, it is necessary and urgent to develop a unified, authoritative, and comprehensive characterization and evaluation system. In addition to developing new test methods, we need to introduce tribology knowledge to analyze, solve and guide the design of bioinspired superhydrophobic materials. The related research is still basically in the blank condition. Then, WCA, SA, advancing/receding contact angle, adhesive force, and integrity of microtopography should all be taken into evaluation systems.69 The best solution is to develop a highly integrated testing system which can test all-around surface wettability. With the aid of an external humidity/temperature control system, gas circuit system, and super-high-speed camera, liquid volatilization and icing processes can be studied via this integrated system in different atmospheres or in vacuum. Ultimately, professional evaluation institutions have to be instituted.
3.2. Current Developments and Challenges of Designs of Superhydrophilic Materials
The research of bioinspired superhydrophilic materials can be traced back to the research of the cornea of the human eye. Water (tears) can spread completely forming a water film to eliminate light scattering. Scientists have established some relatively mature fabrication methods, which can be divided into two categories: physical methods and chemical methods (Figure 4a).
Figure 4.
(a) Common superhydrophilic surface fabrication physical and chemical methods. (b) Water spreading process on superhydrophilic interfaces with the help of 2D capillary forces.75 (c) Fish-scale-inspired artificial superhydrophilic/underwater superoleophobic surfaces.30 (d) Conical spine structure of cactus spine,33 spindle-knot fiber structure of spider silk,31 and in situ observation of water transport in carbon nanotubes.80 (e) Through continual liquid deposition, dyed water could unidirectionally spread in one single direction and pin in all the others on superhydrophilic peristome-mimetic surfaces. Deposited liquid also could flow downward along the spiral.81 Copyright 2016, 2017 Wiley; 2010, 2012 Nature publishing group; and 2004 American Chemical Society.
Physical methods can be applied to create regular patterned microstructures, such as laser treatment,70 and to obtain a new relatively homogeneous layer by physical vapor deposition or spray coating.71 For instance, Vorobyev and Guo70 created a novel surface pattern that transformed regular silicon to being superhydrophilic using high-intensity femtosecond laser pulses. Because of the superhydrophilicity, water can spread vertically uphill against gravity. The driving force of water motion is the supercapillary effect benefited from the surface structures. Zheng and co-workers72 have reported superhydrophilic colloidal TiO2/SiO2 nanoparticle coatings in a spray-and-dry way. Li et al.71 fabricated one kind of long-time stable superhydrophilic hierarchical TiO2 ordered by pulsed laser deposition and subsequent annealing. This TiO2 hierarchical particle array exhibited superhydrophilicity with a water contact angle of nearly 0° without further UV irradiation.
As for chemical methods, it is more beneficial to obtain complex precise structures in micro-/nanoscale. The chemical method can also create hydrophobic functional groups on the surface through external field stimulation, such as UV illumination. TiO2 has become one of the most famous “photoresponsive” wetting materials since the discovery of its photoinduced superhydrophilic property was reported in Nature.73 Recently, superhydrophilic/-hydrophobic fabric with self-propelled directional wetting patterns controlled by UV illumination and temperature has been successfully created by a one-step coating process involving nano-TiO2.74 Random frameworks can also be generated by chemical methods such as etching/oxidation, layer-by-layer, sol–gel methods, and so on. Zhu et al.75 reported a simple three-step treatment method to generate superamphiphilic silicon wafer surfaces with contact angles near 0° for both water and typical organic liquids. Taking advantage of lateral force mode (LFM) atomic force microscopy images, the alternating hydrophilic and hydrophobic nanodomains can be detected. The mechanism has also been revealed to explain its superamphilicity. Liquids infiltrate the hydrophilic (or hydrophobic) nanodomains giving rise to a 2D capillary effect (Figure 4b), which pulls liquids across the nanodomains continuously. By making use of these superamphiphilic silicon wafer surfaces, uniform organic thin films were generated in both water and organic solvent. Most recently, Zheng and co-workers76 have reported a superhydrophilic cellulose membrane through an electrospinning cellulose acetate method followed by a process of hydrolysis inspired from Chinese Xuan papers. Water can spread out rapidly along the X–Y 2D plane but seldom permeates through the Xuan papers. This is because of the multilayered spreading mechanism resulting from the layered anisotropic micro-/nanofibrous structures.
After being proposed, the concept of superhydrophilicity has not been widely dispersed and studied. This is because the potential applications have not been discovered for a long time. The mystical nature gives us answers again, which brings a new revolution for bioinspired superhydrophilic materials research. In 2009, Liu et al. found that fish scales showed natural superhydrophilicity.30 Superhydrophilic fish scales displayed a unique underwater superoleophobicity. The reason is that the water layer can be trapped within the micro-/nanostructures of fish scale surfaces. By virtue of this property, fish can keep clean under water showing a great antifouling property. Bioinspired superhydrophilic/underwater superoleophobic materials are also achieved in this work. Superhydrophilic/underwater superoleophobic artificial polyacrylamide film with fish-scale structures and silicon surface with microstructure or micro-/nanostructure (Figure 4c) has been fabricated successfully. These studies extended superwettability research to oil/water/solid triphasic systems. Thus, the underwater antifouling property of the superhydrophilic surface has drawn increasing attention. On the basis of this understanding, researchers further explore the oil/water separation property of superhydrophilic/underwater superoleophobic materials. Xue et al.77 prepared a novel superhydrophilic and underwater superoleophobic polyacrylamide hydrogel-coated mesh which can achieve selective separation of water from oil/water mixtures with high separation efficiency.
From the perspective of bioinspired designs, the relevant superhydrophilic materials for antifouling and oil/water separation are concentrated in 2D films or membranes. One-dimensional superhydrophilic materials seem to have not much room for development. A turning point came in 2010 and 2012; the discovery of the unique liquid transport ability of spider silk31 and cactus33 opens the new door for the research of 1D superhydrophilic fibers and channels (Figure 4d). The combination of Laplace pressure and surface energy resulted in controllable transport of liquid by designing 1D superhydrophilic interfaces. It is also the core guiding ideology of bioinspired designs of 1D superhydrophilic materials. Bai et al.78 designed a series of artificial spider silks with spindle-knots to drive tiny water drops (tens of picoliters) with controllable transport by optimizing the cooperation of curvature, chemical composition, and roughness gradients on fiber surfaces. The key for artificial spider silk bioinspired designs for fog or water collection is to construct a spindle-knot structure, while the main aim of artificial cactus bioinspired designs is to obtain microscale cone structures. Ju and co-workers79 bioinspired designed a dual-gradient one-dimensional fog collector, namely, conical copper wire with gradient wettability. With the help of field-emission scanning electron microscopy, scientists detected that water can transport in carbon nanotubes.80 The WCAs in carbon nanotubes range from 5° to 15°. On the basis of this pioneering research, superhydrophilic nanochannel materials have attracted more and more attention in recent years. These great works have opened up a favorable new situation of bioinspired designs of superhydrophilic materials.
Now, the main cutting-edge focus for bioinspired designs of superhydrophilic materials has become liquid’s continuously unidirectional transport inspired by lizard skin35 and Nepenthes alata.36 Most recently, Li et al.81 fabricated mimicking biological surfaces with sophisticated structures through the stereolithography fabrication method (Figure 4e). Liquids with varied surface tensions and viscosities can spontaneously propagate in a preferred single direction. The overflow-controlled64,82 unidirectional transport mechanism has been revealed by microcomputerized tomography scanning observations. Furthermore, this group found that the impact of a water drop can achieve unidirectional transport on the Nepenthes alata inspired superhydrophilic surface.83
The research of bioinspired designs of superhydrophilic materials has good momentum of development. However, the problems beneath the prosperous surface are pretty obvious. The performance of artificial superhydrophilic materials cannot reach natural superhydrophilic materials. For example, the water collection test of artificial 1D superhydrophilic materials is under high-humidity atmosphere. This condition can hardly exist in nature. Moreover, it is hard to simulate the fine structure on the creature surface of artificial superhydrophilic materials. Advanced 3D printing technology could resolve, to a certain extent, this challenge.84 However, the scale of printed structures is always much larger than the scale of the creature surface structure. Therefore, where is the future of bioinspired superhydrophilic materials? At first, scientists should make an earnest effort to find out new materials or methods to reach the properties of natural creatures. Developing high-precision 3D printer and laser writing technology is another good solution. Second, we must never cease to work for new applications of superhydrophilic materials from a static to dynamic fluid and from 1D to 3D. Combining superhydrophilicity and other material properties, such as humidity-response, is a good approach.85 Finally, the reversible rapid wettability transition between superhydrophobicity and superhydrophilicity is a promising research field.
4. Emerging Applications of Bioinspired Superhydrophobic and Superhydrophilic Materials
Scientists and engineers have investigated the utility of superhydrophobic and superhydrophilic materials in different fields. Nowadays, a great number of applications have been commercialized. Here, a systematic summary has been concluded in this section. We try to focus on both applications that take advantage of a single wetting state or two extreme wetting states.
Oil–water separation is of great importance for industrial water purification and oil spill collection. Both superhydrophobic and superhydrophilic materials have proven to be highly efficient for this purpose.86−88 After the development of recent years, the research of oil–water separation has entered the liquid–liquid separation stage.89,90 On the basis of the θIWT for different liquids, the membranes with different wettabilities can be further applied to separate two different organic liquids. Furthermore, Li and co-workers91 achieved tiny water-in-oil droplet separation into pure water and oil droplets via a “go-in-opposite-ways” mechanism on curved bioinspired peristome-mimetic surfaces without energy input (Figure 5a). The separations of tiny water-in-oil droplets, emulsions, and liquid/air will continue to be the focus of the future research.89,92−95 Superhydrophilic materials show dramatic antifogging behavior, where the water droplets will instantaneously spread to form a thin liquid film. It is interesting that, inspired by mosquito compound eyes (Figure 5b), superhydrophobic materials also display great antifogging behaviors, which can rapidly expel condensed droplets from their surfaces.96 Densely packed micro-ommatidia structures covered by hexagonal nanonipples resulted in this ability of mosquito eyes.97 The synergistic effect of wettability and surface topography can regulate the surface bioadhesion ability by cytophilic interactions and a size-matching mechanism (Figure 5c).98 The surfaces with great bioadhesion ability are applicable for cancer detection. Superhydrophilic nanowire arrays can selectively capture circulating tumor cells while inhibiting the adhesion of normal blood cells.98 Bioinspired superhydrophobic surfaces can be used to deliver analytes to Raman-sensitive sites in highly diluted fluids (Figure 5d). The essential reason is that the solution becomes increasingly concentrated during the no-pinning evaporation process.9,99 Improving biocompatibility of superwetting sensors and extending the range of usable fluids will be the major concerns. Another well-known commercial application of bioinspired superhydrophobic and superhydrophilic materials is self-cleaning (Figure 5e). Superhydrophobic materials are mainly aimed at solid pollutants.23 However, superhydrophilic materials are mainly aimed at liquid pollutants.100 Combining advantages of the two together to make omnipotent self-cleaning superwetting materials is significant. As mentioned in earlier sections, bioinspired superhydrophobic and superhydrophilic materials display a unique water collection property (Figure 5f). How to improve water collection efficiency under a low-humidity environment and how to prevent water evaporation are open questions. The novel green printing based on superoleophilic nanomaterials on superhydrophilic plate substrates has solved high-cost and environmental pollution problems (Figure 5g). Regulating the morphology and wettability of printed materials can achieve the contact line of the aqueous protecting liquid. Then, the printed ink microdroplet pinned on the nonprinted and printed regions results in high-quality images with fine resolution.101 Liquid unidirectional transport on a solid surface with special wettability is a very unique and intriguing phenomenon (Figure 5h). The driving mechanism of liquid unidirectional transport can be divided into four parts: Laplace pressure, gradient wettability, ratchet mechanism, and capillary rise.18,33,36,50,81,83,102,103 In general, asymmetric surface morphology and/or surface energy make the force or resistance at the three-phase contact line of a liquid uneven in different directions, thus driving the liquid in a particular direction. However, a further application based on the liquid unidirectional transport is insufficient. How to develop its value behind the phenomenon in a smart microfluidic system, laboratory-on-a-chip devices, and advanced biochemistry microreactor is the prime concern of many scientists.18,81,83,104,105
Figure 5.
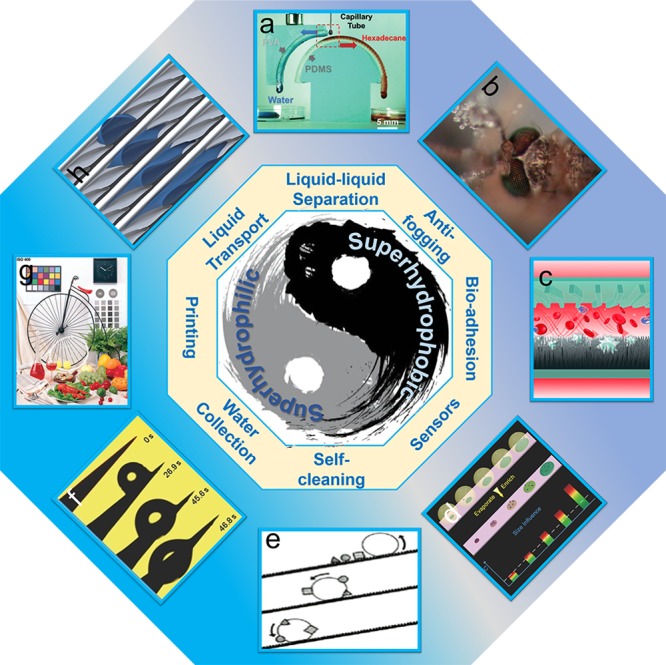
Emerging applications of superhydrophobic and superhydrophilic surfaces in various fields. (a) Liquid–liquid separation.91 (b) Antifogging.97 (c) Bioadhesion.98 (d) Sensors.99 (e) Self-cleaning.23 (f) Water collection.79 (g) Printing.101 (h) Liquid transport.36 Copyright 2007, 2011, 2013, 2015, 2017 Wiley; 2016 Nature publishing group; 1997 Springer; and 2013 Royal Society of Chemistry.
5. Outlook
In this Outlook, we have concluded the development of bioinspired superhydrophobic and superhydrophilic progress. The renaissance in this field, to a large extent, has been brought about by recent advances in nanotechnology. The designs of bioinspired superhydrophobic and superhydrophilic materials have also been summarized. The challenges, opportunities, and solutions are mentioned in this section. It is also worth it for us to pay attention to emerging applications of superhydrophobic and superhydrophilic materials. As we have highlighted in this outlook, superwetting materials with unprecedented properties and functionalities can be generated and integrated into devices for emerging applications.
Although great progress has been made, there are still some difficult challenges to overcome. How can further impulses in the development in this field be created? It is a tough question. Advanced 3D printing technology could be a good solution. Three-dimensional printing technology can rapidly, precisely prepare a bioinspired surface structure and morphology similar to that of creature surfaces.18,55,81,84 Compared with traditional chemical or physical methods, 3D printing technology displays great controllability, integration, and repeatability with low cost. More importantly, 3D printing technology brings light to efficiently construct superamphiphobic surfaces/substrates with specific delicate structures with reentrant geometries, which display high theoretical research and practical application values.42,55,105−107 Although it is still in its infancy, there are still some limitations, such as the size of products or the choice of raw materials, but predictably it will give play to enormous value in this field in the future.
Throughout the development of the superwetting field, we find that the current research focus is moving from static droplets to dynamic liquid flow.11,18,64,108−112 Because liquids in nature tend to have a certain velocity, the hydrodynamic properties or behavior of liquid have gradually become a major concern in the design of superhydrophilic and superhydrophobic materials, for example, the rapid, continuous, spontaneous flow or transport of a large amount of liquid on the surface or bulk phase of bioinspired superhydrophilic materials. The wetting/dewetting/impact process of fluids on the edge of the superhydrophobic surface is another hydrodynamic research topic in the future. The rapid transition between superhydrophilic and superhydrophobic by regulating liquid is also a new research hot-spot. Research on these problems is at an early stage, and it is possible to greatly expand the application of bioinspired superhydrophobic and superhydrophilic materials.
In terms of fundamental research, scientists should try to reveal the deeper essence of nature. Direct imaging and recording have attracted broad scientific attention for both theoretical research and practical applications. Nano-X-ray CT, confocal laser scanning microscopy (CLSM), environmental scanning electron microscopy (ESEM), and the ultra-high-speed camera are pretty useful for the future research. Moreover, tremendous effort should be concentrated on developing the highly integrated 3D imaging approaches with high resolution.113 New theories and concepts should be explored from microscale to nanoscale or molecular scale to understand delicate wetting phenomena. Setting up a perfect theoretical system can provide guidance for us to design required bioinspired superhydrophobic and superhydrophilic materials. Finally, there are still a great deal of secrets to find out from nature. With the joint efforts of researchers, the research of bioinspired designs of superhydrophobic and superhydrophilic materials will open a new page tomorrow.
Acknowledgments
We acknowledge project funding provided by the National Natural Science Foundation (21703270, 21431009, 91127025), the National Key R&D Program of China (2018YFA0208500, 2017YFA0206900), and the Key Research Program of the Chinese Academy of Sciences (KJZD-EW-M01).
The authors declare no competing financial interest.
References
- Su B.; Tian Y.; Jiang L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. 10.1021/jacs.5b12728. [DOI] [PubMed] [Google Scholar]
- Wang S.; Liu K.; Yao X.; Jiang L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. 10.1021/cr400083y. [DOI] [PubMed] [Google Scholar]
- Barthlott W.; Mail M.; Neinhuis C. Superhydrophobic Hierarchically Structured Surfaces in Biology: Evolution, Structural Principles and Biomimetic Applications. Philos. Trans. R. Soc., A 2016, 374, 20160191–20160231. 10.1098/rsta.2016.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.; Zheng S.; Peng S.; Zhao Y.; Tian Y. Superlyophilic Interfaces and Their Applications. Adv. Mater. 2017, 29, 1703120–1703132. 10.1002/adma.201703120. [DOI] [PubMed] [Google Scholar]
- Yao X.; Song Y.; Jiang L. Applications of Bio-Inspired Special Wettable Surfaces. Adv. Mater. 2011, 23, 719–734. 10.1002/adma.201002689. [DOI] [PubMed] [Google Scholar]
- Darmanin T.; Guittard F. Superhydrophobic and Superoleophobic Properties in Nature. Mater. Today 2015, 18, 273–285. 10.1016/j.mattod.2015.01.001. [DOI] [Google Scholar]
- Koch K.; Barthlott W. Superhydrophobic and Superhydrophilic Plant Surfaces: An Inspiration for Biomimetic Materials. Philos. Trans. R. Soc., A 2009, 367, 1487–1509. 10.1098/rsta.2009.0022. [DOI] [PubMed] [Google Scholar]
- Young T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. London 1805, 95, 65–87. 10.1098/rstl.1805.0005. [DOI] [Google Scholar]
- Liu M.; Wang S.; Jiang L. Nature-Inspired Superwettability Systems. Nat. Rev. Mater. 2017, 2, 17036–17052. 10.1038/natrevmats.2017.36. [DOI] [Google Scholar]
- Xia F.; Jiang L. Bio-Inspired, Smart, Multiscale Interfacial Materials. Adv. Mater. 2008, 20, 2842–2858. 10.1002/adma.200800836. [DOI] [Google Scholar]
- Si Y.; Yu C.; Dong Z.; Jiang L. Wetting and Spreading: Fundamental Theories to Cutting-Edge Applications. Curr. Opin. Colloid Interface Sci. 2018, 36, 10–19. 10.1016/j.cocis.2017.12.006. [DOI] [Google Scholar]
- Wen L.; Tian Y.; Jiang L. Bioinspired Super-Wettability from Fundamental Research to Practical Applications. Angew. Chem., Int. Ed. 2015, 54, 3387–3399. 10.1002/anie.201409911. [DOI] [PubMed] [Google Scholar]
- Zhang C.; McAdams D. A. 2nd; Grunlan J. C. Nano/Micro-Manufacturing of Bioinspired Materials: A Review of Methods to Mimic Natural Structures. Adv. Mater. 2016, 28, 6292–6321. 10.1002/adma.201505555. [DOI] [PubMed] [Google Scholar]
- Si Y.; Guo Z. Superhydrophobic Nanocoatings: From Materials to Fabrications and to Applications. Nanoscale 2015, 7, 5922–5946. 10.1039/C4NR07554D. [DOI] [PubMed] [Google Scholar]
- Feng L.; Li S. H.; Li Y. S.; Li H. J.; Zhang L. J.; Zhai J.; Song Y. L.; Liu B. Q.; Jiang L.; Zhu D. B. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. 10.1002/adma.200290020. [DOI] [Google Scholar]
- Koch K.; Bhushan B.; Jung Y. C.; Barthlott W. Fabrication of Artificial Lotus Leaves and Significance of Hierarchical Structure for Superhydrophobicity and Low Adhesion. Soft Matter 2009, 5, 1386–1393. 10.1039/b818940d. [DOI] [Google Scholar]
- Tian Y.; Jiang L. Wetting: Intrinsically Robust Hydrophobicity. Nat. Mater. 2013, 12, 291–292. 10.1038/nmat3610. [DOI] [PubMed] [Google Scholar]
- Si Y.; Wang T.; Li C.; Yu C.; Li N.; Gao C.; Dong Z.; Jiang L.. Liquids Unidirectional Transport on Dual-Scale Arrays. ACS Nano 2018, in press. 10.1021/acsnano.8b03924. [DOI] [PubMed] [Google Scholar]
- Tian X.; Verho T.; Ras R. H. Surface Wear. Moving Superhydrophobic Surfaces toward Real-World Applications. Science 2016, 352, 142–143. 10.1126/science.aaf2073. [DOI] [PubMed] [Google Scholar]
- Cohen N.; Dotan A.; Dodiuk H.; Kenig S. Superhydrophobic Coatings and Their Durability. Mater. Manuf. Processes 2016, 31, 1143–1155. 10.1080/10426914.2015.1090600. [DOI] [Google Scholar]
- Drelich J.; Chibowski E.; Meng D. D.; Terpilowski K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804–9828. 10.1039/c1sm05849e. [DOI] [Google Scholar]
- Cassie A. B. D.; Baxter S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–550. 10.1039/tf9444000546. [DOI] [Google Scholar]
- Barthlott W.; Neinhuis C. Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202, 1–8. 10.1007/s004250050096. [DOI] [Google Scholar]
- Gao X.; Jiang L. Biophysics: Water-Repellent Legs of Water Striders. Nature 2004, 432, 36. 10.1038/432036a. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Gao X.; Jiang L. Directional Adhesion of Superhydrophobic Butterfly Wings. Soft Matter 2007, 3, 178–182. 10.1039/B612667G. [DOI] [PubMed] [Google Scholar]
- Feng L.; Zhang Y. A.; Xi J. M.; Zhu Y.; Wang N.; Xia F.; Jiang L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. 10.1021/la703821h. [DOI] [PubMed] [Google Scholar]
- Barthlott W.; Schimmel T.; Wiersch S.; Koch K.; Brede M.; Barczewski M.; Walheim S.; Weis A.; Kaltenmaier A.; Leder A.; Bohn H. F. The Salvinia Paradox: Superhydrophobic Surfaces with Hydrophilic Pins for Air Retention under Water. Adv. Mater. 2010, 22, 2325–2328. 10.1002/adma.200904411. [DOI] [PubMed] [Google Scholar]
- Epstein A. K.; Pokroy B.; Seminara A.; Aizenberg J. Bacterial Biofilm Shows Persistent Resistance to Liquid Wetting and Gas Penetration. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 995–1000. 10.1073/pnas.1011033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerl J.; Helbig R.; Schulz H.-J.; Werner C.; Neinhuis C. Diversity and Potential Correlations to the Function of Collembola Cuticle Structures. Zoomorphology 2013, 132, 183–195. 10.1007/s00435-012-0181-0. [DOI] [Google Scholar]
- Liu M. J.; Wang S. T.; Wei Z. X.; Song Y. L.; Jiang L. Bioinspired Design of a Superoleophobic and Low Adhesive Water/Solid Interface. Adv. Mater. 2009, 21, 665–669. 10.1002/adma.200801782. [DOI] [Google Scholar]
- Zheng Y.; Bai H.; Huang Z.; Tian X.; Nie F. Q.; Zhao Y.; Zhai J.; Jiang L. Directional Water Collection on Wetted Spider Silk. Nature 2010, 463, 640–643. 10.1038/nature08729. [DOI] [PubMed] [Google Scholar]
- Bixler G. D.; Bhushan B. Bioinspired Rice Leaf and Butterfly Wing Surface Structures Combining Shark Skin and Lotus Effects. Soft Matter 2012, 8, 11271–11284. 10.1039/c2sm26655e. [DOI] [Google Scholar]
- Ju J.; Bai H.; Zheng Y.; Zhao T.; Fang R.; Jiang L. A Multi-Structural and Multi-Functional Integrated Fog Collection System in Cactus. Nat. Commun. 2012, 3, 1247–1252. 10.1038/ncomms2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drotlef D.-M.; Stepien L.; Kappl M.; Barnes W. J. P.; Butt H.-J.; del Campo A. Insights into the Adhesive Mechanisms of Tree Frogs Using Artificial Mimics. Adv. Funct. Mater. 2013, 23, 1137–1146. 10.1002/adfm.201202024. [DOI] [Google Scholar]
- Comanns P.; Buchberger G.; Buchsbaum A.; Baumgartner R.; Kogler A.; Bauer S.; Baumgartner W. Directional, Passive Liquid Transport: The Texas Horned Lizard as a Model for a Biomimetic ‘Liquid Diode’. J. R. Soc., Interface 2015, 12, 20150415–20150422. 10.1098/rsif.2015.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.; Zhang P.; Zhang L.; Liu H.; Jiang Y.; Zhang D.; Han Z.; Jiang L. Continuous Directional Water Transport on the Peristome Surface of Nepenthes Alata. Nature 2016, 532, 85–89. 10.1038/nature17189. [DOI] [PubMed] [Google Scholar]
- Li Y.; Luong D. X.; Zhang J.; Tarkunde Y. R.; Kittrell C.; Sargunaraj F.; Ji Y.; Arnusch C. J.; Tour J. M. Laser-Induced Graphene in Controlled Atmospheres: From Superhydrophilic to Superhydrophobic Surfaces. Adv. Mater. 2017, 29, 1700496–1700503. 10.1002/adma.201700496. [DOI] [PubMed] [Google Scholar]
- Kang H.; Heo Y. J.; Kim D. J.; Kim J. H.; Jeon T. Y.; Cho S.; So H. M.; Chang W. S.; Kim S. H. Droplet-Guiding Superhydrophobic Arrays of Plasmonic Microposts for Molecular Concentration and Detection. ACS Appl. Mater. Interfaces 2017, 9, 37201–37209. 10.1021/acsami.7b11506. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Chen D.; Kang Z. One-Step Electrodeposition Process to Fabricate Corrosion-Resistant Superhydrophobic Surface on Magnesium Alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. 10.1021/am507586u. [DOI] [PubMed] [Google Scholar]
- Si Y.; Zhu H.; Chen L.; Jiang T.; Guo Z. A Multifunctional Transparent Superhydrophobic Gel Nanocoating with Self-Healing Properties. Chem. Commun. 2015, 51, 16794–16797. 10.1039/C5CC06977G. [DOI] [PubMed] [Google Scholar]
- Hou L.; Wang N.; Wu J.; Cui Z.; Jiang L.; Zhao Y. Bioinspired Superwettability Electrospun Micro/Nanofibers and Their Applications. Adv. Funct. Mater. 2018, 1801114. 10.1002/adfm.201801114. [DOI] [Google Scholar]
- Deng X.; Mammen L.; Butt H. J.; Vollmer D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. 10.1126/science.1207115. [DOI] [PubMed] [Google Scholar]
- Jiang L.; Zhao Y.; Zhai J. A Lotus-Leaf-Like Superhydrophobic Surface: A Porous Microsphere/Nanofiber Composite Film Prepared by Electrohydrodynamics. Angew. Chem., Int. Ed. 2004, 43, 4338–4341. 10.1002/anie.200460333. [DOI] [PubMed] [Google Scholar]
- Guo Z.; Zhou F.; Hao J.; Liu W. Stable Biomimetic Super-Hydrophobic Engineering Materials. J. Am. Chem. Soc. 2005, 127, 15670–15671. 10.1021/ja0547836. [DOI] [PubMed] [Google Scholar]
- Sun M.; Luo C.; Xu L.; Ji H.; Ouyang Q.; Yu D.; Chen Y. Artificial Lotus Leaf by Nanocasting. Langmuir 2005, 21, 8978–8981. 10.1021/la050316q. [DOI] [PubMed] [Google Scholar]
- Lee S. G.; Lim H. S.; Lee D. Y.; Kwak D.; Cho K. Tunable Anisotropic Wettability of Rice Leaf-Like Wavy Surfaces. Adv. Funct. Mater. 2013, 23, 547–553. 10.1002/adfm.201201541. [DOI] [Google Scholar]
- Hu D. L.; Chan B.; Bush J. W. The Hydrodynamics of Water Strider Locomotion. Nature 2003, 424, 663–666. 10.1038/nature01793. [DOI] [PubMed] [Google Scholar]
- Yang E.; Son J. H.; Lee S. I.; Jablonski P. G.; Kim H. Y. Water Striders Adjust Leg Movement Speed to Optimize Takeoff Velocity for Their Morphology. Nat. Commun. 2016, 7, 13698–13706. 10.1038/ncomms13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.; Gu J.; Zang X.; Xu W.; Shi K.; Xu L.; Zhang D. Versatile Fabrication of Intact Three-Dimensional Metallic Butterfly Wing Scales with Hierarchical Sub-Micrometer Structures. Angew. Chem., Int. Ed. 2011, 50, 8307–8311. 10.1002/anie.201103505. [DOI] [PubMed] [Google Scholar]
- Malvadkar N. A.; Hancock M. J.; Sekeroglu K.; Dressick W. J.; Demirel M. C. An Engineered Anisotropic Nanofilm with Unidirectional Wetting Properties. Nat. Mater. 2010, 9, 1023–1028. 10.1038/nmat2864. [DOI] [PubMed] [Google Scholar]
- Uchida K.; Nishikawa N.; Izumi N.; Yamazoe S.; Mayama H.; Kojima Y.; Yokojima S.; Nakamura S.; Tsujii K.; Irie M. Phototunable Diarylethene Microcrystalline Surfaces: Lotus and Petal Effects Upon Wetting. Angew. Chem., Int. Ed. 2010, 49, 5942–5944. 10.1002/anie.201000793. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Li X.; Zheng X.; Chen Z.; Zhou Q.; Chen Y. 3d-Printed Biomimetic Super-Hydrophobic Structure for Microdroplet Manipulation and Oil/Water Separation. Adv. Mater. 2018, 30, 1704912–1704922. 10.1002/adma.201704912. [DOI] [PubMed] [Google Scholar]
- Tuteja A.; Choi W.; Ma M.; Mabry J. M.; Mazzella S. A.; Rutledge G. C.; McKinley G. H.; Cohen R. E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. 10.1126/science.1148326. [DOI] [PubMed] [Google Scholar]
- Liu T. L.; Kim C. J. Repellent Surfaces. Turning a Surface Superrepellent Even to Completely Wetting Liquids. Science 2014, 346, 1096–1100. 10.1126/science.1254787. [DOI] [PubMed] [Google Scholar]
- Liu X.; Gu H.; Wang M.; Du X.; Gao B.; Elbaz A.; Sun L.; Liao J.; Xiao P.; Gu Z. 3D Printing of Bioinspired Liquid Superrepellent Structures. Adv. Mater. 2018, 30, 1800103–1800110. 10.1002/adma.201800103. [DOI] [PubMed] [Google Scholar]
- Li N.; Wu L.; Yu C.; Dai H.; Wang T.; Dong Z.; Jiang L. Ballistic Jumping Drops on Superhydrophobic Surfaces Via Electrostatic Manipulation. Adv. Mater. 2018, 30, 1703838–1703845. 10.1002/adma.201703838. [DOI] [PubMed] [Google Scholar]
- Das A.; Deka J.; Raidongia K.; Manna U. Robust and Self-Healable Bulk-Superhydrophobic Polymeric Coating. Chem. Mater. 2017, 29, 8720–8728. 10.1021/acs.chemmater.7b02880. [DOI] [Google Scholar]
- Das S.; Kumar S.; Samal S. K.; Mohanty S.; Nayak S. K. A Review on Superhydrophobic Polymer Nanocoatings: Recent Development and Applications. Ind. Eng. Chem. Res. 2018, 57, 2727–2745. 10.1021/acs.iecr.7b04887. [DOI] [Google Scholar]
- Peng C.; Chen Z.; Tiwari M. K. All-Organic Superhydrophobic Coatings with Mechanochemical Robustness and Liquid Impalement Resistance. Nat. Mater. 2018, 17, 355–360. 10.1038/s41563-018-0044-2. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Sathasivam S.; Song J.; Crick C.; Carmalt C.; Parkin I. Robust Self-Cleaning Surfaces That Function When Exposed to Either Air or Oil. Science 2015, 347, 1132–1135. 10.1126/science.aaa0946. [DOI] [PubMed] [Google Scholar]
- Si Y.; Guo Z.; Liu W. A Robust Epoxy Resins @ Stearic Acid-Mg(OH)2 Micronanosheet Superhydrophobic Omnipotent Protective Coating for Real-Life Applications. ACS Appl. Mater. Interfaces 2016, 8, 16511–16520. 10.1021/acsami.6b04668. [DOI] [PubMed] [Google Scholar]
- Xiu Y.; Zhu L.; Hess D. W.; Wong C. P. Hierarchical Silicon Etched Structures for Controlled Hydrophobicity/Superhydrophobicity. Nano Lett. 2007, 7, 3388–3393. 10.1021/nl0717457. [DOI] [PubMed] [Google Scholar]
- Xiu Y.; Liu Y.; Hess D. W.; Wong C. P. Mechanically Robust Superhydrophobicity on Hierarchically Structured Si Surfaces. Nanotechnology 2010, 21, 155705–155709. 10.1088/0957-4484/21/15/155705. [DOI] [PubMed] [Google Scholar]
- Dong Z.; Wu L.; Wang J.; Ma J.; Jiang L. Superwettability Controlled Overflow. Adv. Mater. 2015, 27, 1745–1750. 10.1002/adma.201405387. [DOI] [PubMed] [Google Scholar]
- Li Y.; Chen S.; Wu M.; Sun J. All Spraying Processes for the Fabrication of Robust, Self-Healing, Superhydrophobic Coatings. Adv. Mater. 2014, 26, 3344–3348. 10.1002/adma.201306136. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Wang H.; Niu H.; Zhao Y.; Xu Z.; Lin T. A Waterborne Coating System for Preparing Robust, Self-Healing, Superamphiphobic Surfaces. Adv. Funct. Mater. 2017, 27, 1604261–1604268. 10.1002/adfm.201604261. [DOI] [Google Scholar]
- Tian X.; Shaw S.; Lind K. R.; Cademartiri L. Thermal Processing of Silicones for Green, Scalable, and Healable Superhydrophobic Coatings. Adv. Mater. 2016, 28, 3677–3682. 10.1002/adma.201506446. [DOI] [PubMed] [Google Scholar]
- Si Y.; Yang F.; Guo Z. Bio-Inspired One-Pot Route to Prepare Robust and Repairable Micro-Nanoscale Superhydrophobic Coatings. J. Colloid Interface Sci. 2017, 498, 182–193. 10.1016/j.jcis.2017.03.063. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Ding B.; Cheng R.; Dixon S. C.; Lu Y. Computational Intelligence-Assisted Understanding of Nature-Inspired Superhydrophobic Behavior. Adv. Sci. 2018, 5, 1700520–1700528. 10.1002/advs.201700520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev A. Y.; Guo C. Laser Turns Silicon Superwicking. Opt. Express 2010, 18, 6455–6460. 10.1364/OE.18.006455. [DOI] [PubMed] [Google Scholar]
- Li Y.; Sasaki T.; Shimizu Y.; Koshizaki N. A Hierarchically Ordered TiO2 Hemispherical Particle Array with Hexagonal-Non-Close-Packed Tops: Synthesis and Stable Superhydrophilicity without UV Irradiation. Small 2008, 4, 2286–2291. 10.1002/smll.200800428. [DOI] [PubMed] [Google Scholar]
- Zheng S.; Wang D.; Tian Y.; Jiang L. Superhydrophilic Coating Induced Temporary Conductivity for Low-Cost Coating and Patterning of Insulating Surfaces. Adv. Funct. Mater. 2016, 26, 9018–9025. 10.1002/adfm.201602843. [DOI] [Google Scholar]
- Wang R.; Hashimoto K.; Fujishima A.; Chikuni M.; Kojima E.; Kitamura A.; Shimohigoshi M.; Watanabe T. Light-induced Amphiphilic Surfaces. Nature 1997, 388, 431–432. 10.1038/41233. [DOI] [Google Scholar]
- Kang H.; Liu Y.; Lai H.; Yu X.; Cheng Z.; Jiang L. Under-Oil Switchable Superhydrophobicity to Superhydrophilicity Transition on TiO2 Nanotube Arrays. ACS Nano 2018, 12, 1074–1082. 10.1021/acsnano.7b05813. [DOI] [PubMed] [Google Scholar]
- Zhu Z.; Tian Y.; Chen Y.; Gu Z.; Wang S.; Jiang L. Superamphiphilic Silicon Wafer Surfaces and Applications for Uniform Polymer Film Fabrication. Angew. Chem., Int. Ed. 2017, 56, 5720–5724. 10.1002/anie.201700039. [DOI] [PubMed] [Google Scholar]
- Zheng S.; Du M.; Miao W.; Wang D.; Zhu Z.; Tian Y.; Jiang L. 2d Prior Spreading Inspired from Chinese Xuan Papers. Adv. Funct. Mater. 2018, 1800832–1800838. 10.1002/adfm.201800832. [DOI] [Google Scholar]
- Xue Z.; Wang S.; Lin L.; Chen L.; Liu M.; Feng L.; Jiang L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. 10.1002/adma.201102616. [DOI] [PubMed] [Google Scholar]
- Bai H.; Tian X.; Zheng Y.; Ju J.; Zhao Y.; Jiang L. Direction Controlled Driving of Tiny Water Drops on Bioinspired Artificial Spider Silks. Adv. Mater. 2010, 22, 5521–5525. 10.1002/adma.201003169. [DOI] [PubMed] [Google Scholar]
- Ju J.; Xiao K.; Yao X.; Bai H.; Jiang L. Bioinspired Conical Copper Wire with Gradient Wettability for Continuous and Efficient Fog Collection. Adv. Mater. 2013, 25, 5937–5942. 10.1002/adma.201301876. [DOI] [PubMed] [Google Scholar]
- Rossi M. P.; Ye H. H.; Gogotsi Y.; Babu S.; Ndungu P.; Bradley J. C. Environmental Scanning Electron Microscopy Study of Water in Carbon Nanopipes. Nano Lett. 2004, 4, 989–993. 10.1021/nl049688u. [DOI] [Google Scholar]
- Li C.; Li N.; Zhang X.; Dong Z.; Chen H.; Jiang L. Uni-Directional Transportation on Peristome-Mimetic Surfaces for Completely Wetting Liquids. Angew. Chem., Int. Ed. 2016, 55, 14988–14992. 10.1002/anie.201607514. [DOI] [PubMed] [Google Scholar]
- Dong Z.; Wu L.; Li N.; Ma J.; Jiang L. Manipulating Overflow Separation Directions by Wettability Boundary Positions. ACS Nano 2015, 9, 6595–6602. 10.1021/acsnano.5b02580. [DOI] [PubMed] [Google Scholar]
- Yu C.; Li C.; Gao C.; Dong Z.; Wu L.; Jiang L. Time-Dependent Liquid Transport on a Biomimetic Topological Surface. ACS Nano 2018, 12, 5149–5157. 10.1021/acsnano.8b01800. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Song X.; Li X.; Chen Z.; Zhou C.; Zhou Q.; Chen Y. Recent Progress in Biomimetic Additive Manufacturing Technology: From Materials to Functional Structures. Adv. Mater. 2018, 1706539–1706572. 10.1002/adma.201706539. [DOI] [PubMed] [Google Scholar]
- Ahmad Nor Y.; Niu Y.; Karmakar S.; Zhou L.; Xu C.; Zhang J.; Zhang H.; Yu M.; Mahony D.; Mitter N.; Cooper M. A.; Yu C. Shaping Nanoparticles with Hydrophilic Compositions and Hydrophobic Properties as Nanocarriers for Antibiotic Delivery. ACS Cent. Sci. 2015, 1, 328–334. 10.1021/acscentsci.5b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z.; Feng Y.; Seeger S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem., Int. Ed. 2015, 54, 2328–2338. 10.1002/anie.201405785. [DOI] [PubMed] [Google Scholar]
- Li N.; Yu C.; Si Y.; Song M.; Dong Z.; Jiang L. Janus Gradient Meshes for Continuous Separation and Collection of Flowing Oils under Water. ACS Appl. Mater. Interfaces 2018, 10, 7504–7511. 10.1021/acsami.8b00044. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhang Z.; Wang P. Smart Surfaces with Switchable Superoleophilicity and Superoleophobicity in Aqueous Media: Toward Controllable Oil/Water Separation. NPG Asia Mater. 2012, 4, e8. 10.1038/am.2012.14. [DOI] [Google Scholar]
- Hou X.; Hu Y.; Grinthal A.; Khan M.; Aizenberg J. Liquid-Based Gating Mechanism with Tunable Multiphase Selectivity and Antifouling Behaviour. Nature 2015, 519, 70–73. 10.1038/nature14253. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhao Y.; Tian Y.; Jiang L. A General Strategy for the Separation of Immiscible Organic Liquids by Manipulating the Surface Tensions of Nanofibrous Membranes. Angew. Chem., Int. Ed. 2015, 54, 14732–14737. 10.1002/anie.201506866. [DOI] [PubMed] [Google Scholar]
- Li C.; Wu L.; Yu C.; Dong Z.; Jiang L. Peristome-Mimetic Curved Surface for Spontaneous and Directional Separation of Micro Water-in-Oil Drops. Angew. Chem., Int. Ed. 2017, 56, 13623–13628. 10.1002/anie.201706665. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Liu N.; Zhang Q.; Qu R.; Liu Y.; Li X.; Wei Y.; Feng L.; Jiang L. Thermo-Driven Controllable Emulsion Separation by a Polymer-Decorated Membrane with Switchable Wettability. Angew. Chem., Int. Ed. 2018, 57, 5740–5745. 10.1002/anie.201801736. [DOI] [PubMed] [Google Scholar]
- Song Y.; Zhou J.; Fan J.-B.; Zhai W.; Meng J.; Wang S. Hydrophilic/Oleophilic Magnetic Janus Particles for the Rapid and Efficient Oil-Water Separation. Adv. Funct. Mater. 2018, 28, 1802493–1802500. 10.1002/adfm.201802493. [DOI] [Google Scholar]
- Si Y.; Guo Z. Superwetting Materials of Oil–Water Emulsion Separation. Chem. Lett. 2015, 44, 874–883. 10.1246/cl.150223. [DOI] [Google Scholar]
- Sheng Z.; Wang H.; Tang Y.; Wang M.; Huang L.; Min L.; Meng H.; Chen S.; Jiang L.; Hou X. Liquid Gating Elastomeric Porous System with Dynamically Controllable Gas/Liquid Transport. Sci. Adv. 2018, 4, eaao6724. 10.1126/sciadv.aao6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z.; Feng X.; Guo Z.; Niu S.; Ren L. Flourishing Bioinspired Antifogging Materials with Superwettability: Progresses and Challenges. Adv. Mater. 2018, 30, 1704652–1704683. 10.1002/adma.201704652. [DOI] [PubMed] [Google Scholar]
- Gao X.; Yan X.; Yao X.; Xu L.; Zhang K.; Zhang J.; Yang B.; Jiang L. The Dry-Style Antifogging Properties of Mosquito Compound Eyes and Artificial Analogues Prepared by Soft Lithography. Adv. Mater. 2007, 19, 2213–2217. 10.1002/adma.200601946. [DOI] [Google Scholar]
- Wang S.; Liu K.; Liu J.; Yu Z. T.; Xu X.; Zhao L.; Lee T.; Lee E. K.; Reiss J.; Lee Y. K.; Chung L. W.; Huang J.; Rettig M.; Seligson D.; Duraiswamy K. N.; Shen C. K.; Tseng H. R. Highly Efficient Capture of Circulating Tumor Cells by Using Nanostructured Silicon Substrates with Integrated Chaotic Micromixers. Angew. Chem., Int. Ed. 2011, 50, 3084–3088. 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J.; Zhang H.; Yang Q.; Li M.; Jiang L.; Song Y. Hydrophilic-Hydrophobic Patterned Molecularly Imprinted Photonic Crystal Sensors for High-Sensitive Colorimetric Detection of Tetracycline. Small 2015, 11, 2738–2742. 10.1002/smll.201403640. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Lee D.; Rubner M. F.; Cohen R. E. Structural Color in Porous, Superhydrophilic, and Self-Cleaning SiO2/TiO2 Bragg Stacks. Small 2007, 3, 1445–1451. 10.1002/smll.200700084. [DOI] [PubMed] [Google Scholar]
- Tian D.; Song Y.; Jiang L. Patterning of Controllable Surface Wettability for Printing Techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. 10.1039/c3cs35501b. [DOI] [PubMed] [Google Scholar]
- Prakash M.; Quere D.; Bush J. W. Surface Tension Transport of Prey by Feeding Shorebirds: The Capillary Ratchet. Science 2008, 320, 931–934. 10.1126/science.1156023. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Wang L.; Hou Y.; Shi W.; Feng S.; Zheng Y. Controlled Smart Anisotropic Unidirectional Spreading of Droplet on a Fibrous Surface. Adv. Mater. 2015, 27, 5057–5062. 10.1002/adma.201502143. [DOI] [PubMed] [Google Scholar]
- Li C.; Yu C.; Hao D.; Wu L.; Dong Z.; Jiang L. Smart Liquid Transport on Dual Biomimetic Surface Via Temperature Fluctuation Control. Adv. Funct. Mater. 2018, 1707490–1707497. 10.1002/adfm.201707490. [DOI] [Google Scholar]
- Jiang T.; Guo Z.; Liu W. Biomimetic Superoleophobic Surfaces: Focusing on Their Fabrication and Applications. J. Mater. Chem. A 2015, 3, 1811–1827. 10.1039/C4TA05582A. [DOI] [Google Scholar]
- Liu H.; Wang Y.; Huang J.; Chen Z.; Chen G.; Lai Y. Bioinspired Surfaces with Superamphiphobic Properties: Concepts, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1707415–1707441. 10.1002/adfm.201707415. [DOI] [Google Scholar]
- Chu Z.; Seeger S. Superamphiphobic Surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. 10.1039/C3CS60415B. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhou X.; Li J.; Che L.; Yao J.; McHale G.; Chaudhury M. K.; Wang Z. Topological Liquid Diode. Sci. Adv. 2017, 3, eaao3530. 10.1126/sciadv.aao3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.; Ju J.; Luo S.; Han Y.; Dong Z.; Wang Y.; Gu Z.; Zhang L.; Hao R.; Jiang L. Controlling Liquid Splash on Superhydrophobic Surfaces by a Vesicle Surfactant. Sci. Adv. 2017, 3, e1602188. 10.1126/sciadv.1602188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z.; Wang H.; Tang Y.; Wang M.; Huang L.; Min L.; Meng H.; Chen S.; Jiang L.; Hou X. Liquid Gating Elastomeric Porous System with Dynamically Controllable Gas/Liquid Transport. Sci. Adv. 2018, 4, eaao6724. 10.1126/sciadv.aao6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Zhang L.; Wang J.; Wang X.; Duan L.; Wang N.; Xiao F.; Xie Y.; Zhao J. Inorganic Surface Coating with Fast Wetting-Dewetting Transitions for Liquid Manipulations. ACS Appl. Mater. Interfaces 2018, 10, 19182–19188. 10.1021/acsami.8b02537. [DOI] [PubMed] [Google Scholar]
- Walsh E. J.; Feuerborn A.; Wheeler J. H. R.; Tan A. N.; Durham W. M.; Foster K. R.; Cook P. R. Microfluidics with Fluid Walls. Nat. Commun. 2017, 8, 816–824. 10.1038/s41467-017-00846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.; Jin X.; Zheng Y.; Han D.; Liu K.; Jiang L. Direct Imaging of Superwetting Behavior on Solid-Liquid-Vapor Triphase Interfaces. Adv. Mater. 2017, 29, 1703009–1703020. 10.1002/adma.201703009. [DOI] [PubMed] [Google Scholar]