Abstract
Steroids have numerous physiological functions associated with cellular signaling or modulation of the lipid membrane structure and dynamics, and as such, they have found broad pharmacological applications. Steroid–membrane interactions are relevant to multiple steps of steroid biosynthesis and action, as steroids are known to interact with neurotransmitter or membrane steroid receptors, and steroids must cross lipid membranes to exert their physiological functions. Therefore, rationalizing steroid function requires understanding of steroid–membrane interactions. We combined molecular dynamics simulations and isothermal titration calorimetry to characterize the conformations and the energetics of partitioning, in addition to the kinetics of flip–flop transitions and membrane exit, of 26 representative steroid compounds in a model lipid membrane. The steroid classes covered in this study include birth control and anabolic drugs, sex and corticosteroid hormones, neuroactive steroids, as well as steroids modulating the lipid membrane structure. We found that the conformational ensembles adopted by different steroids vary greatly, as quantified by their distributions of tilt angles and insertion depths into the membrane, ranging from well-defined steroid conformations with orientations either parallel or normal to the membrane, to wide conformational distributions. Surprisingly, despite their chemical diversity, the membrane/water partition coefficient is similar among most steroids, except for structural steroids such as cholesterol, leading to similar rates for exiting the membrane. By contrast, the rates of steroid flip–flop vary by at least 9 orders of magnitude, revealing that flip–flop is the rate-limiting step during cellular uptake of polar steroids. This study lays the ground for a quantitative understanding of steroid–membrane interactions, and it will hence be of use for studies of steroid biosynthesis and function as well as for the development and usage of steroids in a pharmacological context.
Short abstract
Interactions of 26 steroid compounds with lipid membranes were derived using molecular dynamics simulations and isothermal titration calorimetry.
Introduction
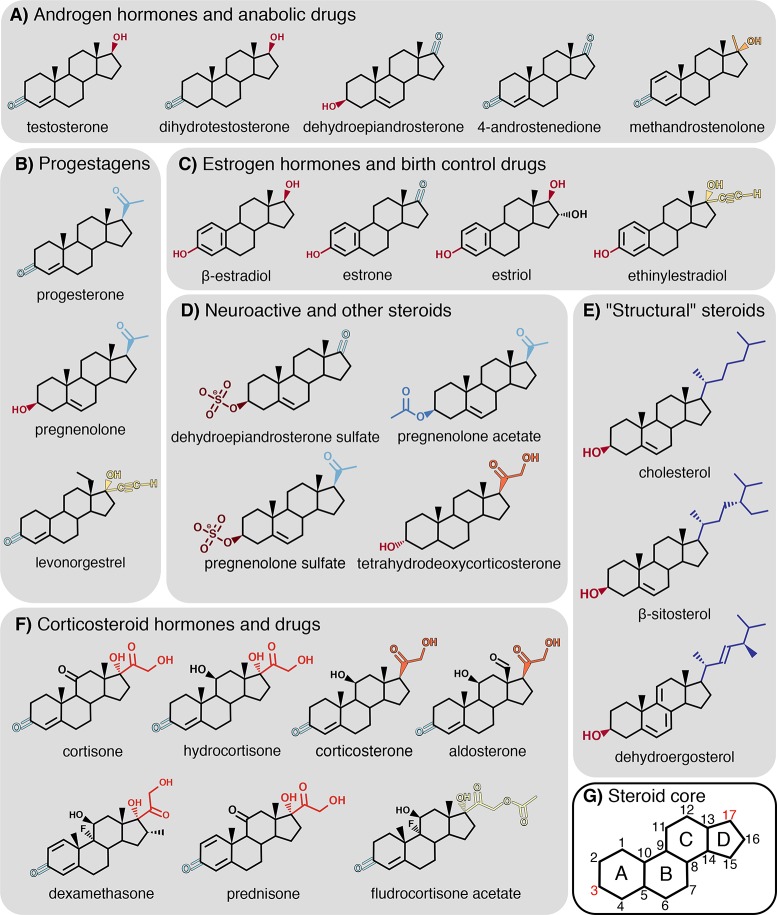
Steroids are a heterogeneous group of typically hydrophobic organic compounds characterized by a tetracyclic fused-ring core (Figure 1). Steroids have various functions in cells and are involved in numerous metabolic pathways. Certain steroid compounds modulate the structure of biological membranes, typical examples including cholesterol in animals, β-sitosterol in plants, and ergosterol in fungi.1,2 Other steroids function as signaling molecules, such as corticosteroid and sex hormones. Steroids have found wide pharmacological applications in, among others, anti-inflammatory drugs, birth control, anesthetics, and cancer treatment, and they are frequently abused to improve performance in work or sports.3−7
Figure 1.
(A–F) Steroids considered in this study. The groups on C-3 (“head”) and C-17 (“tail”) atoms are color-coded as in the following figures. (G) Nomenclature of the tetracyclic steroid core.
The interactions of steroid hormones with biological membranes are relevant to many aspects of their functions. The classical action of steroid hormones entails binding to intracellular steroid receptors, which ultimately results in changes in gene expression.8,9 To this end, steroids have to be internalized into cells. According to the free hormone hypothesis, because of their hydrophobicity, steroids are able to freely diffuse across lipid bilayers; however, megalin-dependent endocytosis has been shown to be at least partly responsible for the uptake of sex hormones and vitamin D3.10,11 In addition to their classical genomic action, also nongenomic mechanisms of action are known for certain steroids. This includes neuroactive steroid compounds, which interact with neurotransmitter receptors and modulate neuronal excitability, as well as steroid actions mediated by membrane steroid receptors.12−16 Further, the biosynthesis of steroid molecules may be influenced by their interactions with lipid membranes.17
Hence, rationalizing the metabolic functions of steroids requires understanding of steroid–membrane interactions. The best-studied steroids are probably the long-tailed sterols, such as cholesterol. Using both experimental and computational approaches, the effects of cholesterol on membrane structure, the conformations and partitioning of cholesterol in bilayers, cholesterol–lipid interactions, and cholesterol flip–flop transitions have been described in great detail.1,17−29 By contrast, the literature on other steroids is less abundant. Partition coefficients have often been reported for octanol or bilayers from cell extracts, while data on partitioning in model lipid bilayers of controlled composition are limited.30−38 Computational studies on the properties of steroids in bilayers have usually been done for only a few steroids, some using short simulations, coarse-grained models, or implicit solvent.26,39−43
Here, we provide a comprehensive overview of the conformational, energetic, and kinetic characteristics of steroids in a model lipid membrane of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). We used all-atom molecular dynamics (MD) simulations to compare the steroid–membrane interactions of 26 steroid compounds, as quantified by their positions in the membrane and distributions of tilt angles, as well as by their membrane/water partition coefficients, and kinetics of flip–flop and membrane exiting (Figure 1). To this end, we derived force field parameters for 26 steroids, and we refined these parameters against membrane/water partition coefficients obtained from isothermal titration calorimetry (ITC) or the literature. The all-atom simulations, complemented by calorimetric data, provide an atomic-level view of the conformations, energetics, and kinetics of the steroids in a lipid membrane.
Results and Discussion
Orientational Diversity of Steroids in the Membrane
Steroids are overall hydrophobic because of their common tetracyclic hydrocarbon core (Figure 1G). However, they carry different functional groups at various positions, which influence the orientation and position of the steroid in the membrane (Figure 1A–F). For instance, the conformation of cholesterol is imposed by the polar hydroxyl group at the C-3 atom and the aliphatic tail attached to C-17 (Figure 1 E). This specific configuration of functional groups imposes a vertical orientation (i.e., parallel to the membrane normal), positioned such that the aliphatic tail is solvated by the lipid tails, whereas the hydroxyl group can form hydrogen bonds with the polar lipid head groups or water.26
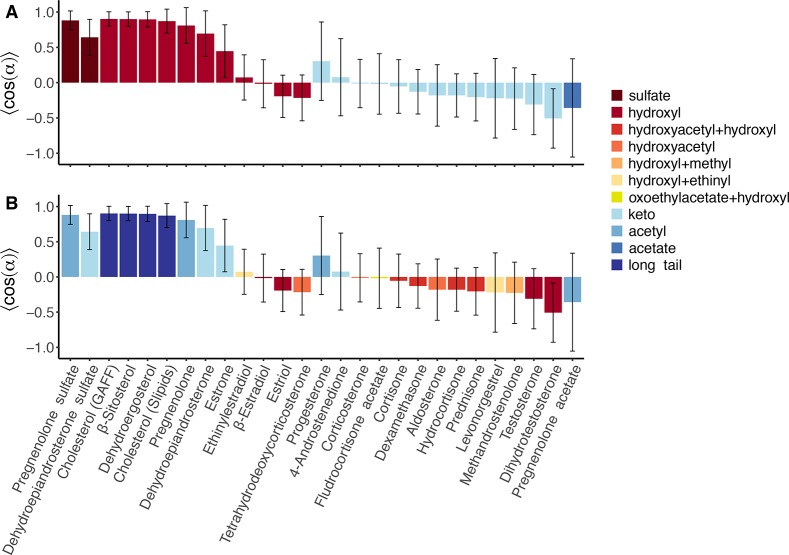
Analogously to cholesterol, we defined atoms C-3 and C-17 as “head” and “tail” atoms, respectively, and derived the steroid orientation in the membrane (Figure 2). Figure 3 presents the average of the cosine of the tilting angle between (i) the steroid axis, connecting the head and tail atoms, and (ii) the z-axis, normal to the membrane plane. Hence, cos(α) = 1 denotes a vertical orientation in the membrane, with the steroid core oriented as in cholesterol; cos(α) = 0 indicates that the steroid is oriented horizontally, and cos(α) = −1 indicates an inverted vertical orientation, with the A-ring toward the membrane core (see Figure 1G). Values between these three special cases denote a tilted orientation of the steroid with respect to the membrane normal.
Figure 2.
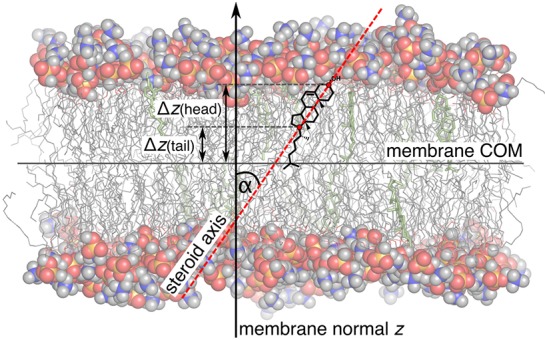
Typical simulation system. Lipid head groups are shown in sphere representation, lipid tails as gray sticks, and steroids as green sticks. Water is omitted for clarity. The steroid axis (red dashed line), membrane normal (black arrow), steroid tilt angle α, and vertical positions of the steroid head and tail atoms relative to the membrane center of mass (COM) are indicated.
Figure 3.
Tilting of the steroids with respect to the membrane normal, quantified by the mean of the cosine of the tilting angle between the steroid axis and the membrane normal (mean and SD over 500 ns and 14 steroid molecules). The bar plots are colored according to the functional group on (A) the C-3 (“head”) atom and on (B) the C-17 (“tail”) atom (see legend and Figure 1). Values of cos(α) of 1, 0, and −1 indicate a vertical orientation (as in cholesterol), a horizontal orientation, and an inverted vertical orientation, respectively. For cholesterol, results from two different force fields are shown (GAFF and Slipids, see the Methods section in the SI).
Figure 3 demonstrates that a steroid core alone does not impose any consensus orientation in the membrane shared by all steroids. Instead, different steroids adopt different orientations (Figure 4), depending on the functional chemical groups (Figure 3, colored bars). In addition, large standard deviations of cos(α) found for many steroids suggest that they do not assume a single well-defined orientation but instead a wide distribution of orientations (Figure 3, error bars). Indeed, the complete cos(α) distributions presented in Figure S1 reveal wide orientational distributions of, for instance, estrogen and corticosteroid hormones (see also β-estradiol and hydrocortisone in Figure 4A,I). In some cases, the cos(α) distribution exhibits multiple peaks, indicating that these steroids can adopt multiple distinct orientations, as found for testosterone, 4-androstenedione (Figure S1, Figure 4C,D), dihydrotestosterone, and levonorgestrel.
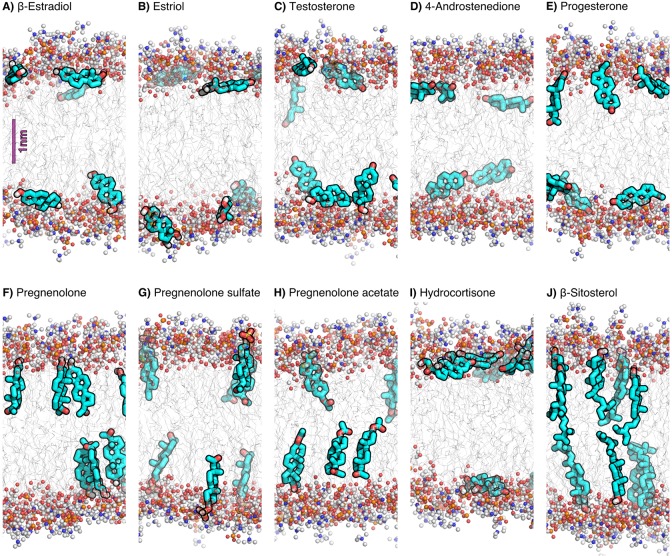
Figure 4.
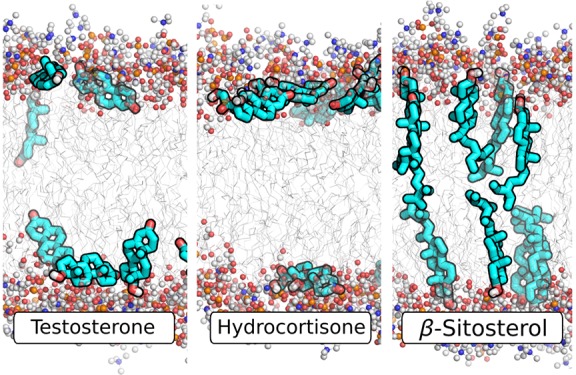
(A–J) Simulation snapshots (fragments) of 10 representative steroids. (A) β-estradiol, (B) estriol, (C) testosterone, (D) 4-androstenedione, (E) progesterone, (F) pregnenolone, (G) pregnenolone sulfate, (H) pregnenolone acetate, (I) hydrocortisone, and (J) β-sitosterol. Polar lipid head groups are shown in ball and stick representation, lipid tails as gray lines, and steroids as cyan sticks (only polar hydrogen atoms are shown). Water molecules are omitted for clarity.
A well-defined vertical orientation is observed for steroids with a clear distinction between the hydrophilicity of the head and tail functional groups, respectively (Figure 3, left bars). These include primarily the sterols with a hydroxyl head group and a long aliphatic tail (i.e., cholesterol, β-sitosterol, and dehydroergosterol, see also Figure 4J). Pregnenolone and pregnenolone sulfate are likewise oriented vertically because of a combination of a hydroxyl or negatively charged head group, respectively, with a relatively hydrophobic tail group with limited capabilities of forming hydrogen bonds (Figure 4F,G). On the other hand, steroids with identical or similar head and tail groups tend to lie horizontally in the membrane, such as β-estradiol with a hydroxyl group on each end, or 4-androstenedione with a keto group on each end (Figure 3, middle bars; Figure 4A,D).
The most frequent substitutions at the head C-3 atom are hydroxyl and keto groups (Figure 3A). The hydroxyl group can form hydrogen bonds with the ester moiety of the POPC lipid heads as well as with water molecules found in this region. Therefore, a hydroxyl group in this position tends to favor a vertical or tilted orientation, such that ring A points toward the membrane surface. The only exceptions are the steroids where the tail atoms also carry hydroxyl groups, leading to a horizontal or even slightly downward-tilted orientation, as seen for estriol with two hydroxyl groups on ring D as opposed to only one hydroxyl group in ring A (Figure 4B). On the other hand, a keto group on the C-3 atom leads to a slight “inverted” tilt, such that ring A points to the membrane core (Figure 3A, light blue bars). This is rationalized by the fact that keto groups are purely hydrogen-bond acceptors but not donors, leading to reduced possibilities of forming hydrogen bonds. Therefore, when coupled with a hydroxyl group at the steroid tail, the head tends to sink deeper into the membrane than the tail, leading to an inverted orientation. Of course, when a similar or more hydrophobic group is present at the tail, such as in 4-androstenedione or progesterone, an average horizontal orientation or noninverted tilted orientation is found (Figure 4D,E). Corticosteroids, which in addition to the hydroxyl-containing group on C-17 and the keto group on C-3 have an additional polar group on atom C-11, similarly adopt a horizontal orientation (Figure 4I).
Insertion Depth of Steroids in the Membrane
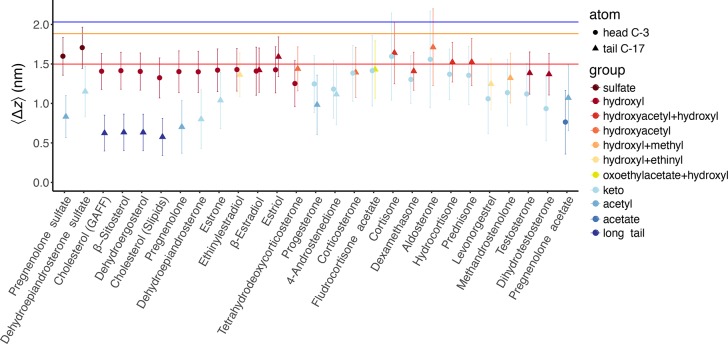
In addition to the orientation, another major degree of freedom is given by the vertical position of the steroid relative to the membrane center of mass, that is, the insertion depth in the membrane (Figure 2). Figure 5 shows the mean vertical position ⟨Δz⟩ of the head and tail atoms, where Δz = 0 denotes the membrane center of mass (COM; Figure 5, dots and triangles). As expected, because of their overall amphiphilic nature, many steroids tend to localize below or near the ester groups of POPC (Figure 5, red horizontal line), at the interface between the polar and apolar regions of the membrane. However, the vertical position of the steroid is clearly modulated by the chemical modifications: steroids that have no hydrogen-bond donors sink deeper into the membrane than steroids that carry hydroxyl groups. For instance, pregnenolone acetate with zero hydroxyl groups is localized closer to the membrane COM than estriol with three hydroxyl groups (Figure 4H,B).
Figure 5.
Distance Δz of the C-3 “head” atoms (dots) and C-17 “tail” atoms (triangles) from the membrane center of mass (mean and SD over 500 ns and 14 steroid molecules). The horizontal blue, orange, and red lines represent the approximate positions of the POPC choline, phosphate, and ester moieties, respectively. The color indicates the chemical modification at the head and tail atoms (see legend and Figure 1).
Likewise, the vertical positions of the individual head and tails atoms are strongly modulated by the chemical modifications, in line with the orientational diversity of steroids (previous section). For instance, the negatively charged sulfate group resides higher in the polar region of the bilayer, as it may form salt-bridges with the POPC choline group (Figure 5, two left dots; Figure 4G). Head and tail atoms with substitutions that contain hydroxyl groups as hydrogen-bond donors are typically located around the POPC ester group (Figure 5, red horizontal line and red to yellow symbols). Head and tail atoms carrying long hydrophobic tails, acetate, or acetyl groups tend to penetrate deeper into the membrane core at distances of ∼0.6, 0.75, and 0.9 nm from the membrane COM, respectively (Figure 5, darker blue symbols). Keto groups cannot form hydrogen bonds with the POPC ester moiety but are moderately polar, allowing them to locate within a wide range of distances between 0.8 and 1.6 nm from the membrane COM (Figure 5, light blue symbols).
Finally, we note that although averages of the tilting angle and depth in the membrane are instructive for detecting the general trends discussed above, the conformation of a specific steroid is more accurately captured by the respective distributions as shown for reference in Figures S1 and S2 in the Supporting Information. Moreover, in this study, we discuss the role of the most common functional groups at atoms C-3 and C-17, but other factors, such as the saturation of the steroid core and modification at other atoms of the core, might also affect the conformations of the steroid in the membrane.
Water/Membrane Partitioning of Steroids
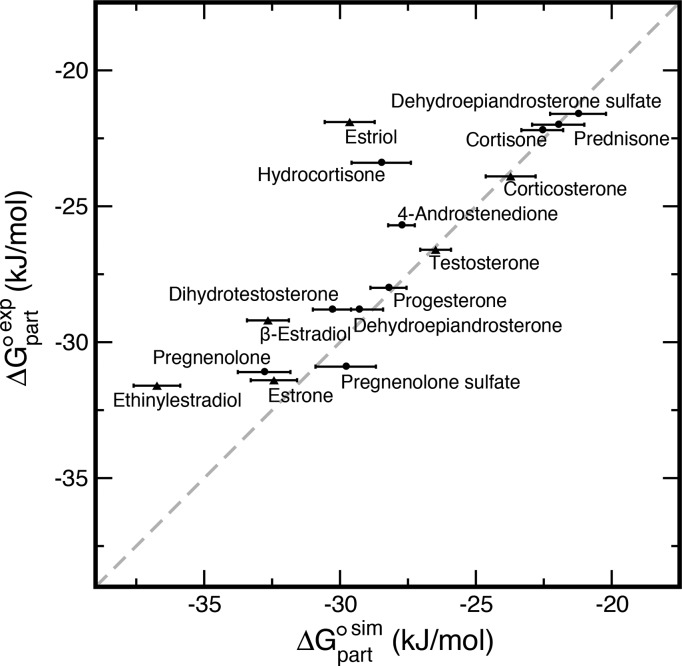
For each steroid, we calculated the standard molar free energy of partitioning ΔGpart◦ between water and a POPC bilayer using potential of mean force (PMF) calculations along the membrane normal. Free energies of partitioning between different phases are often used to validate force field parameters. We collected several available experimental water/POPC partition coefficients from the literature30,32,34,35 and, furthermore, measured the coefficients for another 10 steroid compounds using ITC. Indeed, comparison of the PMF-derived values (ΔGpart) to the experimentally derived values (ΔGpart◦,exp) suggested that partial atomic charges based on quantum-mechanical calculations in vacuum, as often used for force field parametrization, did not yield the correct polarity for all steroids (Figure S3 in the Supporting Information). Therefore, we refined the partial charges using complementary quantum-mechanical calculations in solvent (see the Methods section in the SI). With the refined force field parameters for the steroids, we achieved reasonable agreement between ΔGpart and ΔGpart◦,exp (Figure 6). The only exceptions are a few relatively polar steroids (estriol, hydrocortisone, and ethinylestradiol), for which ΔGpart is ∼6 kJ mol–1 more negative than ΔGpart◦,exp.
Figure 6.
Experimental (ΔGpart◦,exp) vs calculated (ΔGpart) standard molar free energies of partitioning between water and a POPC bilayer. Bars represent calculated standard errors. Experimental standard errors were ≤0.5 kJ mol–1 (see Table S1). Experimental values for estrone, β-estradiol, and ethinylestradiol from ref (35), for estriol from ref (34), for testosterone from ref (30), and for corticosterone from ref (32) (triangles). Experimental values obtained in this work shown as circles.
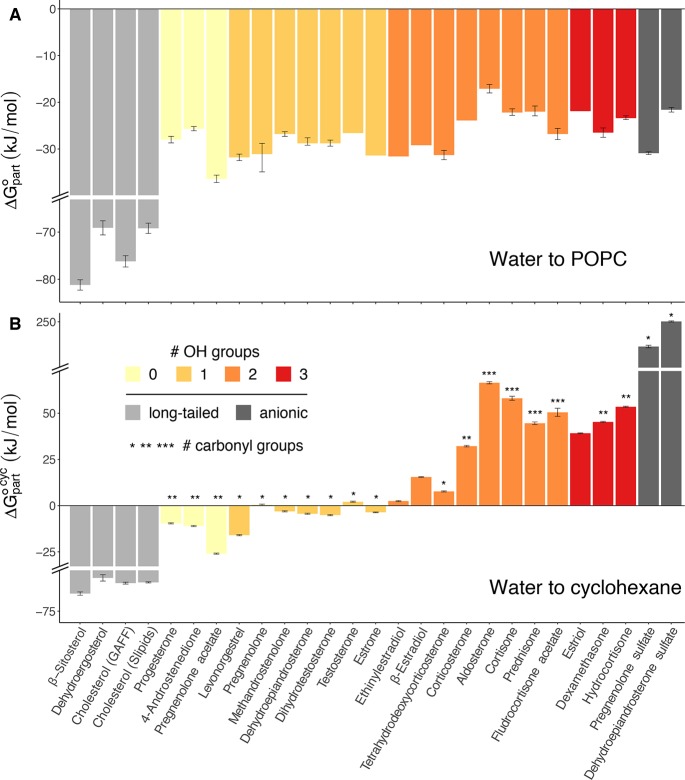
Figure 7A presents ΔGpart◦ from water to POPC for all steroids considered in this study. If available, experimental values were taken from the literature (Figure 6, triangles) or determined by ITC in this study (Figure 6, circles). All other values were taken from calculated PMFs. For an interpretation of the ΔGpart values, it is instructive to compare ΔGpart◦ with the standard molar free energy of partitioning between water and an apolar solvent. To this end, Figure 7B shows the water/cyclohexane standard molar free energy of partitioning, ΔGpart, computed using thermodynamic integration (TI, see the Methods section in the SI). As expected, ΔGpart◦,cyc strongly depends on the chemical modifications of the steroid. For instance, long-tailed steroids strongly favor the apolar environment (Figure 7B, light gray bars), whereas charged steroids strongly favor the aqueous phase (Figure 7B, dark gray bars). For all other steroids, the partitioning between water and cyclohexane roughly correlates with the number of hydroxyl groups. Namely, steroids without hydroxyl groups exhibit negative ΔGpart, indicating a preference for the apolar solvent (Figure 7B, bright yellow), while steroids with only one hydroxyl yield mostly ΔGpart◦,cyc ≈ 0 (Figure 7B, bright orange). Steroids with two and three hydroxyl groups mostly prefer water over cyclohexane, with corticosteroids, which have additional polar groups (such as carbonyl/keto groups), showing the most positive ΔGpart values (Figure 7B, orange and red). The number of carbonyl groups on the steroid further modulates ΔGpart◦,cyc, as indicated by the number of asterisks in Figure 7B. Compared with hydroxyl groups, however, carbonyl groups have a smaller effect on ΔGpart owing to their lower polarity.
Figure 7.
Free energies of partitioning (A) from water to a POPC bilayer, ΔGpart◦, and (B) from water to cyclohexane, ΔGpart. Where available, experimentally determined values are shown in part A (all steroids shown in Figure 6); for the rest of the steroids, PMF-derived values are shown. Values in part B were obtained by TI. Error bars represent 95% confidence intervals for the ITC-derived values, and standard errors for the PMF- and TI-derived values. Bars without error bars represent experimental data from the literature for which no errors were available.30,32,34,35 The coloring indicates the number of hydroxyl groups, long-tailed, or anionic steroids (see legend). The number of asterisks on top of the bars in part B indicates the number of carbonyl groups.
Compared with the water/cyclohexane partition free energies, water/membrane ΔGpart◦ values are much less dependent on the chemical modifications (Figure 7A). The only exceptions are long-tailed sterols that exhibit more negative ΔGpart than all other steroids (Figure 7A, dark gray), indicating a strong preference for the membrane. For all other steroids, however, despite their chemical diversity, most ΔGpart◦ values vary within ∼10 kJ mol–1, corresponding to variation of the partition coefficient by a factor of only ∼50. This finding is rationalized by the steroid location at the interface between the polar and apolar regions of the membrane. Here, upon changing chemical modifications on the steroid ring, rearrangements of the steroid’s orientation and depth are sufficient to maintain hydrogen bonds of polar groups, while keeping large parts of the apolar surface in contact with the apolar lipid tails. Consequently, chemical modifications have a much smaller effect on ΔGpart as compared with ΔGpart◦,cyc. This demonstrates that ΔGpart is not explained by simple determinants such as the number of carbon atoms or number of hydroxyl groups. Instead, the finer modulations of ΔGpart◦ may depend on a combination of determinants, including configurational flexibility and specific steroid–lipid interactions, in addition to the overall hydrophobicity of the molecule.
Kinetics of Steroid Flip–Flop and Membrane Exit
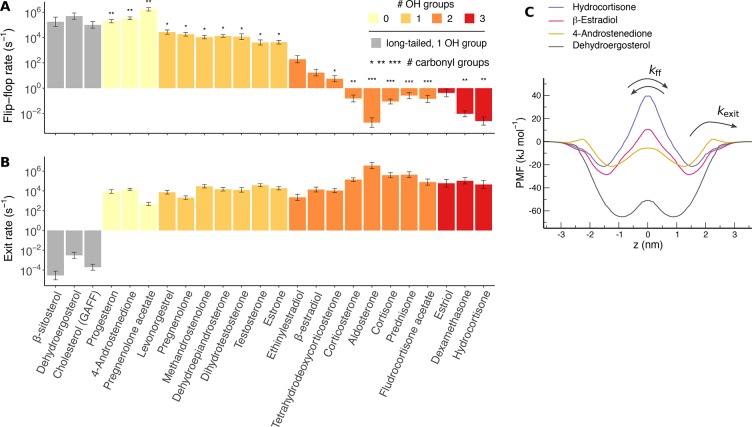
To obtain the kinetics of steroid transitions at a POPC membrane, we computed transversal diffusion coefficients of the steroids (Figure S5) (see the Methods section in the SI). Rates for steroid flip–flop and for exiting the membrane were estimated following Kramers’ theory (Figure 8C, arrows).44Figure 8A presents flip–flop rates, kff, for all steroids considered in this study except for the anionic steroids, revealing that kff for steroids may span at least 9 orders of magnitude. Evidently, kff anticorrelates with the number of polar groups in the steroid, in particular with the number of hydroxyl groups (Figure 8A, color code). The number of carbonyl groups has only a smaller effect on kff (Figure 8A, asterisks). For steroids with zero or one hydroxyl group, including the long-tailed structural steroids, we found large kff values in the rage 104–106 s–1, corresponding to rapid flip–flop events on the time scale of microseconds up to hundreds of microseconds. These values are in reasonable agreement with previous reports for cholesterol flip–flop.28,29 For steroids with two or three hydroxyl groups, by contrast, kff spans the range 102–10–3 s–1, corresponding to flip–flop events on the time scale of milliseconds up to many minutes. As discussed in the Methods section in the SI, for the most polar steroids we cannot exclude the possibility that the PMFs underestimate the true flip–flop barrier. Hence, the flip–flop events of the most polar steroids, such as aldosterone or hydrocortisone, could also occur on the time scale of hours or even longer.
Figure 8.
Rates (A) for steroid flip–flop and (B) for exiting the membrane. The coloring indicates the number of hydroxyl groups or long-tailed steroids (see legend). The number of asterisks on top of the bars in part A indicates the number of carbonyl groups. (C) PMFs for four selected steroids along the membrane normal z, where z = 0 is the membrane center (see legend for color code). Arrows illustrate the transitions for flip–flop and membrane exiting.
The wide range of kff is readily explained by the wide range of water/cyclohexane partition free energies ΔGpart◦,cyc presented above (Figure 7B). Starting from a membrane-bound state (Figure 8C, z ≈ ±1.2 nm), steroid flip–flop requires the transition across the hydrophobic membrane core, which involves the removal of most of the steroid–water contacts, similar to a transition from water to cyclohexane. Hence, the free-energy cost for steroid flip–flop correlates with the cost for translocating a steroid from water to cyclohexane (Figure S4). More quantitatively, kff is dictated by the height of the free-energy barrier in the transmembrane PMFs shown in Figure 8C. Here, the barrier height is the difference between (i) the free energy at the hydrophobic core (Figure 8C, z ≈ 0 nm) approximately given via ΔGpart, and (ii) the free-energy minimum at the membrane-bound state (Figure 8C, z ≈ ±1.2 nm), approximately given via ΔGpart◦. Hence, kff correlates with exp[−β(ΔGpart – ΔGpart◦)], where β = 1/RT is the inverse temperature. Further, since ΔGpart is similar among most steroids, kff is primarily dictated by ΔGpart◦,cyc (compare Figure 7B with Figure 8A). Notable exceptions are the long-tailed structural steroids; for these steroids, large negative ΔGpart and ΔGpart◦ compensate each other, leading to similar flip–flop rates as compared to steroids with one or without any hydroxyl group (Figures 7A,B and 8A, light gray bars).
In contrast with the wide range of kff values, the rates kexit for exiting the membrane are highly similar among most of the steroids (Figure 8B). Most kexit values are in the order of 104 s–1 corresponding to rapid exit events on the time scale of only hundreds of microseconds. Hence, steroids bind to membranes in a highly transient manner. Exceptions are again the long-tailed steroids that exhibit kexit in the rage 10–3–10–5 s–1, indicating that the long-tailed steroids bind tightly to the membranes for minutes up to many hours, in excellent agreement with experimental findings for cholesterol.45 Since exiting the membrane requires overcoming the free energy of membrane/water partitioning, kexit strongly correlates with exp(−βΔGpart◦) (compare Figure 7A with Figure 8B). Hence, the similarity of kexit among the nonstructural steroids is a consequence of the similarity of ΔGpart.
Kinetics of Membrane Permeation
Membrane permeation requires (i) steroid entering the membrane, (ii) followed by at least one flip–flop event, and (iii) membrane exiting in the opposite direction as compared with membrane entry. We estimated rates for membrane entry to be in the order of 101 s–1 for eukaryotic cellular environments, 10–1 s–1 for planar membranes with pronounced unstirred layers, and 105 s–1 for large unilamellar vesicles (LUVs) with a typical radius of 0.1 μm (see the Methods section in the SI). Comparing kentry with kff and kexit shown in Figure 8A,B reveals that different transitions may become limiting for steroid permeation (Table 1). Namely, membrane permeation for structural long-tailed steroids is limited by slow membrane exit (kexit). Permeation for steroids with 2 or 3 OH groups is limited by flip–flop. Permeation of steroids with 0 or 1 OH groups may be limited by entry (i.e., by unstirred layers) in cells or planar membranes, and by exit in LUVs.
Table 1. Rate-Limiting Steps (Membrane Entry, Flip–Flop, or Membrane Exit) for Membrane Permeation Depending on the Steroid Structure and the Type of Membrane.
| cell | planar membrane | LUV (radius 0.2 μm) | |
|---|---|---|---|
| long-tailed | exit | exit | exit |
| 0–1 OH groups | entry | entry | exit |
| 2–3 OH groups | flip–flop | flip–flop | flip–flop |
In addition, the ratio kff/kexit determines the average number of flip–flop events before the steroid exits the membrane, and consequently, kff/kexit further determines the probabilities for the two possible directions of membrane exit. A rate ansatz (see the Methods section it the SI) shows that the probability for a full permeation event per membrane entry event is given by
| 1 |
while the probability that the steroid returns to its original water compartment is Pret = 1 – Pperm. In the case of kff ≫ kexit, the steroid typically carries out multiple flip–flop events before its exit with equal probability in each direction (Pperm ≈ Pret ≈ 1/2). By contrast, in the case of kff ≪ kexit, the steroid will mostly return to its original water compartment before the first flip–flop event occurs (Pperm ≈ 0, Pret ≈ 1), which may strongly reduce cellular uptake rates (see discussion below).
Among all steroids, kff/kexit varies in the range 10–9–1010, and among the nonstructural steroids in the range 10–9–103, demonstrating that the probability for permeation after membrane binding, Pperm, greatly varies. Specifically, for steroids without hydroxyl groups we obtain Pperm ≈ 1/2. For steroids with one hydroxyl group, Pperm drops to values between 0.1 and 0.45. For steroids with two or three hydroxyl groups, Pperm may take values as low as 10–10, demonstrating that many membrane binding events are needed before the most polar steroids permeate the membrane.
Significance of Membrane Permeation for Steroid Function
At first sight, the wide range of flip–flop rates might seem at odds with the textbook assertion that steroid hormones pass biological membranes “freely” or “unhindered”. Experimentally, it has indeed been observed that membrane crossing of the classical steroid hormones is a fairly rapid process. For instance, the movement of intracellular mineralocorticoid receptors after steroid binding into the cell nucleus can be detected within 3 min after extracellular aldosterone application.46 Furthermore, nonclassical effects such as an aldosterone-induced rise of intracellular Ca2+ concentration have been observed even within seconds.47,48 In either case, complete membrane traversal of aldosterone is necessary, as the presumed target molecules of aldosterone, whether they be mineralocorticoid receptors or nonclassical targets, are located intracellularly. The rapid action of aldosterone is particularly remarkable in this respect, as our simulations indicated that the flip–flop rate of aldosterone is the lowest among all steroids tested (apart from the anionic, sulfonated steroids that would require either protonation or an aqueous defect to flip–flop).
To resolve the apparent discrepancy between (i) experimentally observed rapid responses of cells to steroid exposure and (ii) computationally derived slow flip–flop rates for polar steroids, it is important to notice that such experiments are typically conducted at constant steroid concentration in the bulk solvent. Consequently, because of the hydrophobicity of the steroids, the steroids are greatly enriched in the outer membrane leaflet as quantified by the membrane/water partition coefficients. More quantitatively, using the ΔGpart◦ definition shown in the Methods section of the SI, the partition free energies, ΔGpart, of −20 to −35 kJ mol–1 (Figure 7) suggest that steroids are enriched in the membrane by a factor between 75 and 30 000 as compared with the bulk. This enrichment largely compensates for low flip–flop rates, thus leading to high permeabilities and hence to cell entry of a significant number of steroid molecules within seconds. As such, rapid entry of steroids into the cell is, for polar steroids, not a consequence of “unhindered” diffusion over the membrane, but instead a consequence of steroid enrichment in the outer membrane leaflet.
Partitioning between the Extracellular Bulk Solution and the Plasma Membrane Can Be an Important Determinant of Steroid Potency
Several steroids are known to influence the function of transmembrane proteins. In most cases where evidence has been obtained, e.g., in the metabotropic CB1 receptors,49 the bacterial channel GLIC50 and the ionotropic GABAA,51,52 nicotinic ACh,53 and NMDA receptors,54,55 it has been shown that steroids interact with these targets on transmembrane helices. For many other steroid receptor transmembrane proteins, a binding site in the transmembrane region also seems likely, although the location of the binding site has not yet been established with certainty. To reach their target, steroids supplied with the bloodstream must first partition into the membrane and then, by lateral diffusion, reach the transmembrane receptors. Therefore, the partitioning of the steroids into the membrane is an important determinant of the interaction and partly determines the kinetics of steroid binding to transmembrane receptors.56
For instance, the effects of the structurally very similar steroids pregnenolone sulfate and dehydroepiandrosterone sulfate (DHEAS) have been studied in GABAA receptors and TRPM3 channels. While in TRPM3 channels, the EC50 for pregnonolone sulfate is 13–25 times lower than for DHEAS,57 in GABAA receptors, DHEAS has been reported to be approximately equally efficient in inhibiting Cl– currents through these receptors.58 Because our results indicate that the concentration of pregnenolone sulfate in the plasma membrane is about 40 times larger than the DHEAS concentration (at the same bulk concentration in the extracellular solution), these findings indicate that the binding site of steroids on TRPM3 channels59 is only poorly discriminating between pregnenolone sulfate and DHEAS. On the other hand, our data suggest that membrane-bound DHEAS has stronger effects on GABAA compared to pregnenolone sulfate. This example demonstrates that a quantitative understanding of steroid–membrane interactions, as derived in this work, is needed for a detailed interpretation of the experimentally observed receptor response.
Functional Consequences of the Position and Orientation of Steroids in the Membrane
Before binding to a proteinaceous binding site on transmembrane segments, steroids must adopt an orientation and an insertion depth that matches the binding site. In addition, if the binding site is located at the intracellular membrane leaflet, at least one flip–flop event is required for binding. Previously, these requirements have complicated a molecular interpretation of experiments. For instance, if a certain steroid shows no (or weak) activity on a receptor, it remains unclear whether (i) the affinity for the steroid is low, (ii) steroid binding does not trigger a relevant conformational transition of the protein, or (iii) whether the steroid does not reach the binding site because of unfavorable orientations adopted in the membrane. Our simulations showed that most (but not all) steroids adopt wide conformational distributions (Figures S1 and S2), in terms of both steroid orientation and insertion depth. Hence, unfavorable conformations may, for most steroids, be excluded as an underlying reason for weak steroid activity.
To illustrate this, it is instructive to pick two extreme examples: Pregnenolone acetate is completely inactive on TRPM3 channels, while pregnenolone sulfate is a strong agonist.57,59 Pregnenolone sulfate is predominantly oriented perpendicular to the plasma membrane, whereas pregnenolone acetate has a strong preference for the orientation parallel to the phospholipid bilayer, but also samples other orientations. Also, these molecules can be found at various depths inside the membrane. These observations indicate that pregnenolone acetate is incapable of activating TRPM3 channels not only because of its unfavorable orientation and position within the membrane. Rather, because this substance does not have any appreciable effect on TRPM3,59 either this steroid cannot bind to TRPM3 proteins, or its binding does not induce channel opening. For agonist activity on TRPM3 channels, either bulky or negatively charged (or both) head groups on the C3 position of pregnenolone appear to be indispensable.59 More generally, though, the observed rapid and wide-ranging movements observed for many of the steroids indicate that these molecules are capable of approaching and docking to membrane-embedded binding sites regardless of their average orientation. Unfavorably oriented binding sites, however, would exhibit reduced rates of binding.
Conclusions
The broad spectrum of steroid compounds encompassed by this study provides a global view of the range of steroid–membrane interactions, highlighting similarities and differences among the family of steroids. Although steroids share a common structural core, they reveal greatly different conformational ensembles in a lipid membrane, imposed by the chemical modifications on the tetracyclic steroid core. Namely, certain steroids adopt well-defined conformations, by orienting strictly either parallel or normal to the membrane, whereas other steroids reveal high orientational flexibility, hence adopting wide conformational ensembles.
For steroids that are neither long-tailed nor anionic, free-energy calculations revealed that the cyclohexane/water partition coefficients vary by 16 orders of magnitude. By contrast, membrane/water partition coefficients are surprisingly similar, varying by only 2–3 orders of magnitude. Further, we derived the kinetics of steroids in membranes, that is, the rates of steroid flip–flop and membrane exiting. We found that rates of membrane exiting are remarkably similar among many steroids, whereas flip–flop rates vary by many orders of magnitude. These trends for steroid flip–flop and exiting rates are rationalized by the trends of cyclohexane/water and membrane/water partition coefficients.
Exceptions are given by the long-tailed steroids such as cholesterol or dehydroergosterol, as well by the anionic steroids such as pregnenolone sulfate; namely, long-tailed steroids exhibit greatly increased membrane affinity and greatly decreased membrane exiting rates, but they display similar flip–flop rates compared to most other steroids. Anionic steroids exhibit greatly reduced flip–flop rates because flip–flop would either involve translocation of the anionic group across the hydrophobic core or require protonation of the steroid; however, anionic steroids show similar membrane/water partitioning compared to most other steroids.
This study provides quantitative understanding of steroid–bilayer interactions, relevant to steroid permeation across the bilayer, as well as for steroid binding to transmembrane receptors and to other membrane proteins. The topologies for all steroids with refined partial atomic charges are available for download at https://biophys.uni-saarland.de/steroids.html.
Acknowledgments
K.A. and J.S.H. acknowledge support through the Deutsche Forschungsgemeinschaft (Grants HU 1971/1-1, HU 1971/1-3) and by the International Max Planck Research School—Physics of Biological and Complex Systems. J.K. and S.K. thank Markus Fleisch (TU München) for assistance with ITC experiments and acknowledge support by the Carl Zeiss Foundation through the Centre for Lipidomics (CZSLip). Computing time at the Gesellschaft für wissenschaftliche Datenverarbeitung (GWDG) and Norddeutscher Verbund fuer Hoch- und Hoechstleistungsrechnen (HLRN) centers is acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.8b00332.
Additional experimental and computational methods, data, and figures including cos(α) distributions, Δz distributions, experimental vs calculated free energies, cyclohexane/water partition free energies vs the transfer free energies for steroids between the bulk and the membrane center, and transversal diffusion coefficients (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yeagle P. L. Cholesterol and the Cell Membrane. Biochim. Biophys. Acta, Rev. Biomembr. 1985, 822, 267–287. 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Bernsdorff C.; Winter R. Differential Properties of the Sterols Cholesterol, Ergosterol, β-Sitosterol, Trans-7-Dehydrocholesterol, Stigmasterol and Lanosterol on DPPC Bilayer Order. J. Phys. Chem. B 2003, 107, 10658–10664. 10.1021/jp034922a. [DOI] [Google Scholar]
- Orme M. L.; Back D. J.; Breckenridge A. M. Clinical Pharmacokinetics of Oral Contraceptive Steroids. Clin. Pharmacokinet. 1983, 8, 95–136. 10.2165/00003088-198308020-00001. [DOI] [PubMed] [Google Scholar]
- Haupt H. A.; Rovere G. D. Anabolic Steroids: A Review of the Literature. Am. J. Sports Med. 1984, 12, 469–484. 10.1177/036354658401200613. [DOI] [PubMed] [Google Scholar]
- Ericson-Neilsen W.; Kaye A. D. Steroids: Pharmacology, Complications, and Practice Delivery Issues. Ochsner J. 2014, 14, 203–207. [PMC free article] [PubMed] [Google Scholar]
- Blickenstaff R. T.Antitumor Steroids; Academic Press, 2012. [Google Scholar]
- Sjöqvist F.; Garle M.; Rane A. Use of Doping Agents, Particularly Anabolic Steroids, in Sports and Society. Lancet 2008, 371, 1872–1882. 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The Steroid and Thyroid Hormone Receptor Superfamily. Science 1988, 240, 889–895. 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M.; Chávez S.; Truss M. Transcriptional Regulation by Steroid Hormones. Steroids 1996, 61, 240–251. 10.1016/0039-128X(96)00030-X. [DOI] [PubMed] [Google Scholar]
- Nykjaer A.; Dragun D.; Walther D.; Vorum H.; Jacobsen C.; Herz J.; Melsen F.; Christensen E. I.; Willnow T. E. An Endocytic Pathway Essential for Renal Uptake and Activation of the Steroid 25-(OH) Vitamin D3. Cell 1999, 96, 507–515. 10.1016/S0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Hammes A.; Andreassen T. K.; Spoelgen R.; Raila J.; Hubner N.; Schulz H.; Metzger J.; Schweigert F. J.; Luppa P. B.; Nykjaer A.; Willnow T. E. Role of Endocytosis in Cellular Uptake of Sex Steroids. Cell 2005, 122, 751–762. 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Rupprecht R.; Holsboer F. Neuroactive Steroids: Mechanisms of Action and Neuropsychopharmacological Perspectives. Trends Neurosci. 1999, 22, 410–416. 10.1016/S0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Falkenstein E.; Tillmann H. C.; Christ M.; Feuring M.; Wehling M. Multiple Actions of Steroid Hormones–a Focus on Rapid, Nongenomic Effects. Pharmacol Rev. 2000, 52, 513–556. [PubMed] [Google Scholar]
- Losel R. M.; Falkenstein E.; Feuring M.; Schultz A.; Tillmann H.-C.; Rossol-Haseroth K.; Wehling M. Nongenomic Steroid Action: Controversies, Questions, and Answers. Physiol. Rev. 2003, 83, 965–1016. 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Norman A. W.; Mizwicki M. T.; Norman D. P. G. Steroid-Hormone Rapid Actions, Membrane Receptors and a Conformational Ensemble Model. Nat. Rev. Drug Discovery 2004, 3, 27–41. 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- Levin E. R.; Hammes S. R. Nuclear Receptors Outside the Nucleus: Extranuclear Signalling by Steroid Receptors. Nat. Rev. Mol. Cell Biol. 2016, 17, 783–797. 10.1038/nrm.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfsen A. Biophysical Aspects of the Mechanism of Action of Steroid Hormones. Prog. Biophys. Mol. Biol. 1983, 42, 79–93. 10.1016/0079-6107(83)90004-4. [DOI] [PubMed] [Google Scholar]
- Bloch K. E. Sterol Structure and Membrane Function. CRC Crit Rev. Biochem 1983, 14, 47–92. 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Ipsen J. H.; Mouritsen O. G.; Bloom M. Relationships Between Lipid Membrane Area, Hydrophobic Thickness, and Acyl-Chain Orientational Order. the Effects of Cholesterol. Biophys. J. 1990, 57, 405–412. 10.1016/S0006-3495(90)82557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen T. P.; McElhaney R. N. Physical Studies of Cholesterol-Phospholipid Interactions. Curr. Opin. Colloid Interface Sci. 1996, 1, 83–90. 10.1016/S1359-0294(96)80048-3. [DOI] [Google Scholar]
- Chiu S. W.; Jakobsson E.; Mashl R. J.; Scott H. L. Cholesterol-Induced Modifications in Lipid Bilayers: A Simulation Study. Biophys. J. 2002, 83, 1842–1853. 10.1016/S0006-3495(02)73949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch S. L.; Keller S. L. Organization in Lipid Membranes Containing Cholesterol. Phys. Rev. Lett. 2002, 89, 268101. 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- Ohvo-Rekilä H.; Ramstedt B.; Leppimäki P.; Slotte J. P. Cholesterol Interactions with Phospholipids in Membranes. Prog. Lipid Res. 2002, 41, 66–97. 10.1016/S0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G.; Zuckermann M. J. What’s So Special About Cholesterol?. Lipids 2004, 39, 1101–1113. 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- de Meyer F.; Smit B. Effect of Cholesterol on the Structure of a Phospholipid Bilayer. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 3654–3658. 10.1073/pnas.0809959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róg T.; Pasenkiewicz-Gierula M.; Vattulainen I.; Karttunen M. Ordering Effects of Cholesterol and Its Analogues. Biochim. Biophys. Acta, Biomembr. 2009, 1788, 97–121. 10.1016/j.bbamem.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Wennberg C. L.; van der Spoel D.; Hub J. S. Large Influence of Cholesterol on Solute Partitioning into Lipid Membranes. J. Am. Chem. Soc. 2012, 134, 5351–5361. 10.1021/ja211929h. [DOI] [PubMed] [Google Scholar]
- Bennett W. F. D.; MacCallum J. L.; Hinner M. J.; Marrink S. J.; Tieleman D. P. Molecular View of Cholesterol Flip-Flop and Chemical Potential in Different Membrane Environments. J. Am. Chem. Soc. 2009, 131, 12714–12720. 10.1021/ja903529f. [DOI] [PubMed] [Google Scholar]
- Jo S.; Rui H.; Lim J. B.; Klauda J. B.; Im W. Cholesterol Flip-Flop: Insights from Free Energy Simulation Studies. J. Phys. Chem. B 2010, 114, 13342–13348. 10.1021/jp108166k. [DOI] [PubMed] [Google Scholar]
- Tipping E.; Ketterer B.; Christodoulides L. Interactions of Small Molecules with Phospholipid Bilayers. Binding to Egg Phosphatidylcholine of Some Uncharged Molecules (2-Acetylaminofluorene, 4-Dimethylaminoazobenzene, Oestrone and Testosterone) That Bind to Ligandin and Aminoazo-Dye-Binding Protein A. Biochem. J. 1979, 180, 319–326. 10.1042/bj1800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato B.; Huseby R. A.; Matsumoto K.; Samuels L. T. Molecular Nature of Interaction of Steroids with Biomembranes Related to Androgen Biosynthesis. J. Steroid Biochem. 1979, 11, 1353–1359. 10.1016/0022-4731(79)90106-7. [DOI] [PubMed] [Google Scholar]
- Golden G.; Rubin R.; Mason R. Steroid Hormones Partition to Distinct Sites in a Model Membrane Bilayer: Direct Demonstration by Small-Angle X-Ray Diffraction. Biochim. Biophys. Acta, Biomembr. 1998, 1368, 161–166. 10.1016/S0005-2736(97)00227-7. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. In Situ Determination of Partition and Diffusion Coefficients in the Lipid Bilayers of Stratum Corneum. Pharm. Res. 2000, 17, 1026–1029. 10.1023/A:1007547809430. [DOI] [PubMed] [Google Scholar]
- Yamamoto H.; Liljestrand H. M. Partitioning of Selected Estrogenic Compounds Between Synthetic Membrane Vesicles and Water: Effects of Lipid Components. Environ. Sci. Technol. 2004, 38, 1139–1147. 10.1021/es034311w. [DOI] [PubMed] [Google Scholar]
- Kwon J.-H.; Liljestrand H. M.; Katz L. E.; Yamamoto H. Partitioning Thermodynamics of Selected Endocrine Disruptors Between Water and Synthetic Membrane Vesicles: Effects of Membrane Compositions. Environ. Sci. Technol. 2007, 41, 4011–4018. 10.1021/es0618200. [DOI] [PubMed] [Google Scholar]
- Estronca L. M. B. B.; Moreno M. J.; Vaz W. L. C. Kinetics and Thermodynamics of the Association of Dehydroergosterol with Lipid Bilayer Membranes. Biophys. J. 2007, 93, 4244–4253. 10.1529/biophysj.107.112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokajová J.; Tiala H.; Viitala T.; Riekkola M.-L.; Wiedmer S. K. Covalent Binding of Phospholipid Vesicles on Fused Silica Capillaries for Electrochromatography. Soft Matter 2011, 7, 6041–6050. 10.1039/c1sm05372h. [DOI] [Google Scholar]
- Modi S.; Anderson B. D. Bilayer Composition, Temperature, Speciation Effects and the Role of Bilayer Chain Ordering on Partitioning of Dexamethasone and Its 21-Phosphate. Pharm. Res. 2013, 30, 3154–3169. 10.1007/s11095-013-1143-z. [DOI] [PubMed] [Google Scholar]
- Oren I.; Fleishman S. J.; Kessel A.; Ben-Tal N. Free Diffusion of Steroid Hormones Across Biomembranes: A Simplex Search with Implicit Solvent Model Calculations. Biophys. J. 2004, 87, 768–779. 10.1529/biophysj.103.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan R.; Biggin P. C. A Steroid in a Lipid Bilayer: Localization, Orientation, and Energetics. Biophys. J. 2008, 95, L45–L47. 10.1529/biophysj.108.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi M.; Essex J. W. Permeability of Drugs and Hormones Through a Lipid Bilayer: Insights from Dual-Resolution Molecular Dynamics. Soft Matter 2010, 6, 3797–3808. 10.1039/c0sm00136h. [DOI] [Google Scholar]
- Parisio G.; Sperotto M. M.; Ferrarini A. Flip-Flop of Steroids in Phospholipid Bilayers: Effects of the Chemical Structure on Transbilayer Diffusion. J. Am. Chem. Soc. 2012, 134, 12198–12208. 10.1021/ja304007t. [DOI] [PubMed] [Google Scholar]
- Khelashvili G.; Harries D. How Sterol Tilt Regulates Properties and Organization of Lipid Membranes and Membrane Insertions. Chem. Phys. Lipids 2013, 169, 113–123. 10.1016/j.chemphyslip.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi P.; Talkner P.; Borkovec M. Reaction-Rate Theory: Fifty Years After Kramers. Rev. Mod. Phys. 1990, 62, 251. 10.1103/RevModPhys.62.251. [DOI] [Google Scholar]
- Fugler L.; Clejan S.; Bittman R. Movement of Cholesterol Between Vesicles Prepared with Different Phospholipids or Sizes. J. Biol. Chem. 1985, 260, 4098–4102. [PubMed] [Google Scholar]
- Grossmann C.; Ruhs S.; Langenbruch L.; Mildenberger S.; Strätz N.; Schumann K.; Gekle M. Nuclear Shuttling Precedes Dimerization in Mineralocorticoid Receptor Signaling. Chem. Biol. 2012, 19, 742–751. 10.1016/j.chembiol.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Harvey B. J.; Higgins M. Nongenomic Effects of Aldosterone on Ca2+ in M-1 Cortical Collecting Duct Cells. Kidney Int. 2000, 57, 1395–1403. 10.1046/j.1523-1755.2000.00981.x. [DOI] [PubMed] [Google Scholar]
- Haseroth K.; Gerdes D.; Berger S.; Feuring M.; Günther A.; Herbst C.; Christ M.; Wehling M. Rapid Nongenomic Effects of Aldosterone in Mineralocorticoid-Receptor-Knockout Mice. Biochem. Biophys. Res. Commun. 1999, 266, 257–261. 10.1006/bbrc.1999.1771. [DOI] [PubMed] [Google Scholar]
- Vallée M.; et al. Pregnenolone Can Protect the Brain from Cannabis Intoxication. Science 2014, 343, 94–98. 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W. W. L.; Chen Z.-W.; Bracamontes J. R.; Budelier M. M.; Krishnan K.; Shin D. J.; Wang C.; Jiang X.; Covey D. F.; Akk G.; Evers A. S. Mapping Two Neurosteroid-Modulatory Sites in the Prototypic Pentameric Ligand-Gated Ion Channel GLIC. J. Biol. Chem. 2018, 293, 3013–3027. 10.1074/jbc.RA117.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty D.; Thomas P.; Field M.; Andersen O. J.; Gold M. G.; Biggin P. C.; Gielen M.; Smart T. G. Crystal Structures of a GABAA-Receptor Chimera Reveal New Endogenous Neurosteroid-Binding Sites. Nat. Struct. Mol. Biol. 2017, 24, 977–985. 10.1038/nsmb.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S.; Scott S.; Masiulis S.; De Colibus L.; Pardon E.; Steyaert J.; Aricescu A. R. Structural Basis for GABAA Receptor Potentiation by Neurosteroids. Nat. Struct. Mol. Biol. 2017, 24, 986–992. 10.1038/nsmb.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier C. J.; Fantini J.; Barrantes F. J. Disclosure of Cholesterol Recognition Motifs in Transmembrane Domains of the Human Nicotinic Acetylcholine Receptor. Sci. Rep. 2011, 1, 69. 10.1038/srep00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostakis E.; Jang M.-K.; Russek S. J.; Gibbs T. T.; Farb D. H. A Steroid Modulatory Domain in NR2a Collaborates with NR1 Exon-5 to Control NMDAR Modulation by Pregnenolone Sulfate and Protons. J. Neurochem. 2011, 119, 486–496. 10.1111/j.1471-4159.2011.07442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovska J.; Vyklicky V.; Stastna E.; Kapras V.; Slavikova B.; Horak M.; Chodounska H.; Vyklicky L. Access of Inhibitory Neurosteroids to the NMDA Receptor. Br. J. Pharmacol. 2012, 166, 1069–1083. 10.1111/j.1476-5381.2011.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerklotz H.; Keller S. How Membrane Partitioning Modulates Receptor Activation: Parallel Versus Serial Effects of Hydrophobic Ligands. Biophys. J. 2013, 105, 2607–2610. 10.1016/j.bpj.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T. F. J.; Loch S.; Lambert S.; Straub I.; Mannebach S.; Mathar I.; Düfer M.; Lis A.; Flockerzi V.; Philipp S. E.; Oberwinkler J. Transient Receptor Potential M3 Channels Are Ionotropic Steroid Receptors in Pancreatic Beta Cells. Nat. Cell Biol. 2008, 10, 1421–1430. 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Park-Chung M.; Malayev A.; Purdy R. H.; Gibbs T. T.; Farb D. H. Sulfated and Unsulfated Steroids Modulate Gamma-Aminobutyric AcidA Receptor Function Through Distinct Sites. Brain Res. 1999, 830, 72–87. 10.1016/S0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Drews A.; Mohr F.; Rizun O.; Wagner T. F. J.; Dembla S.; Rudolph S.; Lambert S.; Konrad M.; Philipp S. E.; Behrendt M.; Marchais-Oberwinkler S.; Covey D. F.; Oberwinkler J. Structural Requirements of Steroidal Agonists of Transient Receptor Potential Melastatin 3 (TRPM3) Cation Channels. Br. J. Pharmacol. 2014, 171, 1019–1032. 10.1111/bph.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.