Abstract
Meropenem–vaborbactam is a fixed-dose combination product of a carbapenem and a cyclic boronic acid β-lactamase inhibitor with potent in vitro activity against Klebsiella pneumoniae carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CRE). The efficacy of meropenem–vaborbactam for the treatment of complicated urinary tract infections and acute pyelonephritis was demonstrated in a Phase III trial (TANGO I). Preliminary data from TANGO II, a separate Phase III study, support the efficacy of meropenem–vaborbactam for the treatment of infections caused by CRE. Overall, meropenem–vaborbactam appears to be safe and well tolerated. It has favorable toxicity, pharmacokinetic and pharmacodynamic profiles compared with other antibiotics with activity against CRE. Meropenem–vaborbactam is an important addition to the current armamentarium of antimicrobial agents with activity against K. pneumoniae carbapenemase-producing CRE.
Keywords: : β-lactam, β-lactamase, β-lactamase inhibitor, carbapenem, carbapenem-resistant Enterobacteriaceae, Gram-negative, Klebsiella pneumoniae carbapenemase, meropenem and vaborbactam
The threat of antimicrobial resistance is increasing worldwide at alarming rates, posing a significant menace to patients [1–3]. In particular, treatment of infections caused by multidrug-resistant Gram-negative bacteria can be quite challenging. In response to the emergence of carbapenem resistance in Enterobacteriaceae, international health organizations have alerted clinicians regarding the risks associated with infections due to these pathogens. The CDC has categorized carbapenem-resistant Enterobacteriaceae (CRE) as the highest threat level of ‘urgent’, and the WHO has deemed CRE as one of the three critical pathogens in need of new antimicrobial options [2,4,5]. Rates of mortality in patients with invasive infections caused by CRE have historically been reported to be as high as 70% [6]. Antimicrobial agents with activity against CRE are few in number and often associated with significant toxicities and/or suboptimal pharmacokinetic parameters, such as aminoglycosides, polymyxins and tigecycline. Although use of carbapenem-containing combination regimens with these agents improved outcomes as compared with monotherapy, mortality rates are still in excess of 30% [7–10]. Furthermore, rates of acute kidney injury with the polymyxins, a common backbone agent of combination regimens for CRE, approach, or in some reports, exceed 50% of treated patients [11]. These data underscore the need for novel antimicrobial therapies with activity against carbapenemase-producing organisms and a more favorable toxicity profile.
The US government's response to this public health crisis was in part the passing of the Generating Antibiotic Incentive Now (GAIN) Act as a part of the US FDA Safety and Innovation Act. Under this legislation, economic incentives and expedited review are granted to pharmaceutical companies for Qualified Infectious Disease Products. Ceftazidime–avibactam, a β-lactam combined with a first-in-class, non-β-lactam, β-lactamase inhibitor, was the first agent to become available through this expedited pathway in February 2015 [12]. The superior efficacy of ceftazidime-–avibactam over historical regimens has been demonstrated in multiple, retrospective cohort studies evaluating the clinical outcomes of patients with CRE infections [13,14]. Shields et al. demonstrated higher rates of clinical success in patients who received ceftazidime–avibactam compared with those who received combination therapy with a carbapenem and aminoglycoside (85 vs 48%, p = 0.04) or carbapenem and colistin (85 vs 40%, p = 0.009), for bloodstream infections due to Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae [13]. Likewise, in an adjusted analysis by van Duin et al., 30-day all-cause in-hospital mortality was significantly lower in patients with CRE infection who received treatment with ceftazidime–avibactam compared with those who received colistin (9 vs 32%, p = 0.001) [14]. While these data are encouraging, numerous cases of resistance to ceftazidime–avibactam have already been published, underlining the need for newer therapies [15–19].
Meropenem–vaborbactam (Vabomere™), a carbapenem and first-in-class boronic acid-based β-lactamase inhibitor combination product with potent in vitro activity against CRE mediated by KPC production, received priority review and approval from the FDA under the GAIN Act [20]. The purpose of this manuscript is to describe the chemistry and pharmacology of meropenem-vaborbactam and to focus on the in vitro antimicrobial activity and currently available clinical data for this agent. Additionally, the role and niche for meropenem–vaborbactam within the current armamentarium of antimicrobial agents with activity against Gram-negative bacteria are discussed.
Chemistry & clinical pharmacology
Chemistry
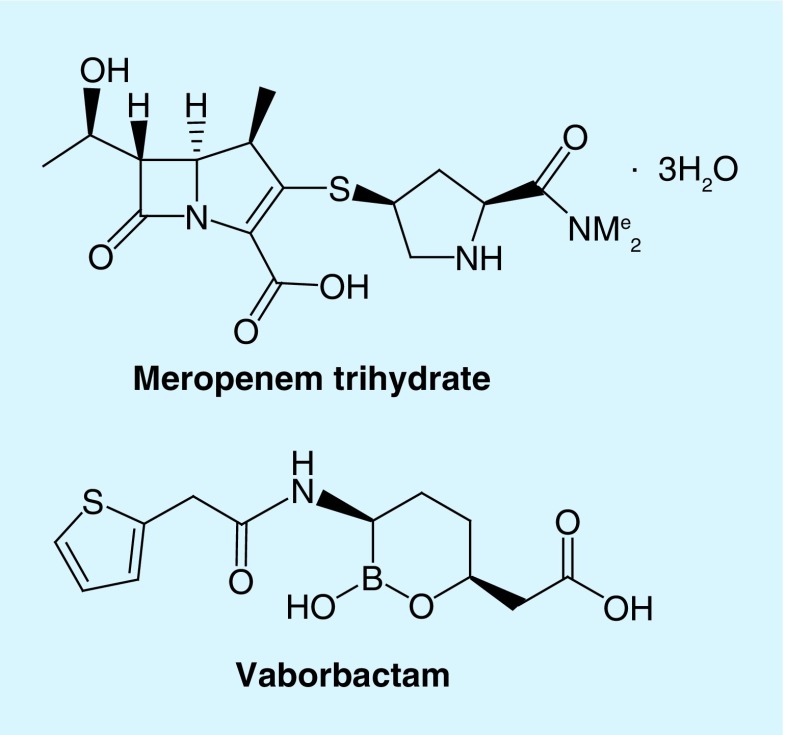
Meropenem–vaborbactam is a fixed-dose combination product of a carbapenem antibiotic and a cyclic boronic acid β-lactamase inhibitor. Figure 1 illustrates the chemical structures of these compounds. Meropenem is a member of the carbapenem class of antimicrobials [20]. Carbapenems are structurally unique from other β-lactams, with the substitution of a carbon atom for sulfur at position 1 of the 4:5 fused thiazolidine ring structure and presence of an unsaturated bond between C-2 and C-3. The 6-trans-hydroxyethyl group accounts for the relative stability against β-lactamases seen with meropenem. Moreover, the dimethylcarbamylpyrrolidinethio substituent chain found at C-2 is responsible for enhanced activity against Gram-negative organisms [21,22]. Notably, the methyl group at C-1 prevents the degradation of meropenem by the kidney dehydropeptidase found in the proximal tubule, thus co-administration of an inhibitor is unnecessary [23,24].
Figure 1. . Chemical structures of meropenem and vaborbactam.
Vaborbactam is a novel non-β-lactam, cyclic boronic acid inhibitor of β-lactamases [20]. The cyclic boronate ester was configured to restrict the inhibitor to a preferred conformation to improve substrate–enzyme interactions. Additionally, the cyclic boronic ester ring enhances the selectivity of the complex against serine β-lactamases compared with other serine hydrolases that may be produced by mammalian cells. Because of the excellent activity against serine β-lactamases, namely KPC enzymes, the inhibitor was ultimately designed to potentiate carbapenem activity. Structure–activity relationships were determined to identify the N-acyl substituent that restored the activity of a broad-spectrum carbapenem, biapenem. Compared with the addition of a N-acetyl group, addition of a 2-thienyl acetyl group significantly decreased the concentration of β-lactamase inhibitor required to reduce the biapenem MIC from 32 to 1 μg/ml for a KPC-producing strain of K. pneumoniae. Moreover, experiments conducted with vaborbactam and Ambler class A (ex. CTX-M) and C (ex. AmpC) enzymes demonstrated the importance of the amide and carboxylate moieties in maintaining effective substrate–enzyme interactions [25].
Mechanism of action
Meropenem exerts its antimicrobial activity by binding to penicillin-binding proteins, thereby inhibiting the cross-linking of peptidoglycan chains which ultimately leads to cell lysis and death due to the inability to form intact cell walls [22].
Vaborbactam restores the activity of meropenem by inhibiting the activity of serine β-lactamases. The boron atom of vaborbactam forms a covalent bond with the catalytic serine side chain of enzymes, mimicking the tetrahedral transition state on the acylation or deacylation reaction pathway seen in β-lactam hydrolysis. Further, while the interactions between vaborbactam and β-lactamases are reversible, the rate of dissociation of the vaborbactam–β-lactamase complex can vary based on the enzyme. For example, the residence time for KPC-2 was 992 min compared with 19 min for CTX-M-15 and 3 min for AmpC. Vaborbactam exhibited an even faster on and off interaction with SHV-12 and TEM-43 and did not appear to inactivate these enzymes. These data highlight the favorable interaction between vaborbactam and KPC enzymes, and suggest that a carbapenem is the ideal partner for this inhibitor [25,26].
Antimicrobial spectrum of activity
Meropenem–vaborbactam displays potent in vitro activity against a variety of Gram-negative organisms [27–29]. At the FDA-approved clinical breakpoint of ≤4/8 μg/ml, 99.5% (10.374/10.426) of clinical Enterobacteriaceae isolates tested susceptible to meropenem–vaborbactam in a multicenter and multinational evaluation using broth microdilution methods [28]. Although meropenem alone has broad in vitro activity against Gram-negative organisms, particularly Enterobacteriaceae, its activity is significantly reduced in the presence of certain β-lactamases, such as carbapenemases [22,26,30]. With the addition of vaborbactam, the activity of meropenem is restored against CRE isolates producing Ambler class A β-lactamases, such as KPCs, as shown in Table 1 [26–29]. In an evaluation of 991 clinical isolates of KPC-producing Enterobacteriaceae, 99% (n = 981) were considered susceptible using a clinical breakpoint of ≤4/8 μg/ml [29]. Further, the potentiation of meropenem by vaborbactam is evidenced by the 32–533-fold reduction in MICs seen among KPC-producing Enterobacteriaceae when vaborbactam is added to meropenem [29]. When activity of meropenem–vaborbactam was stratified by KPC variant, no notable differences were identified. The MIC50 and MIC90 of organisms producing KPC-2 (n = 610) and KPC-3 (n = 373) were 0.06 and 1 μg/ml and 0.12 and 1 μg/ml, respectively, similar to the MIC50 and MIC90 identified when MICs for all KPC producers were examined (Table 1) [29]. Additionally, the activity of meropenem–vaborbactam was determined for a small number of ceftazidime–avibactam-resistant isolates (n = 18, MIC ≥16 μg/ml) and 14 were considered susceptible using the clinical breakpoint of ≤4/8 μg/ml [29].
Table 1. . Comparative in vitro susceptibility of Gram-negative organisms with varying resistance to meropenem and meropenem–vaborbactam.
| Organism | Sample size | Meropenem | Meropenem–vaborbactam | ||||
|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC range | MIC50 | MIC90 | MIC range | ||
| KPC producers | |||||||
| All Enterobacteriaceae | 991 | 32 | >32 | 2–>32 | 0.06 | 1 | ≤0.03–>32 |
| Klebsiella pneumoniae | 878 | >32 | >32 | 2–>32 | 0.12 | 1 | ≤0.03–>32 |
| Escherichia coli | 35 | 4 | 16 | 2–32 | ≤0.03 | ≤0.03 | ≤0.03–0.12 |
| Enterobacter spp. | 29 | 8 | >32 | 2–>32 | ≤0.03 | 0.12 | ≤0.03–0.12 |
| Klebsiella oxytoca | 19 | 4 | 32 | 2–32 | ≤0.03 | 0.25 | ≤0.03–0.25 |
| Serratia marcescens | 16 | 16 | >32 | 2–>32 | 0.06 | 1 | ≤0.03–2 |
| Citrobacter spp. | 13 | 4 | 8 | 2–32 | ≤0.03 | 0.06 | ≤0.03–0.12 |
| Non-KPC-producing carbapenem-resistant Enterobacteriaceae | 129 | 8 | >32 | 0.25–>32 | 4 | >32 | ≤0.015–>32 |
| OXA-48-like producers | 25 | 16 | >32 | 0.5–>32 | 16 | >32 | 0.5–>32 |
| MBL producers | 41 | 32 | >32 | 1–>32 | 32 | >32 | 1–>32 |
| Pseudomonas aeruginosa | 2604 | 0.5 | 8 | ≤0.015–>32 | 0.5 | 8 | ≤0.015–>32 |
| Acinetobacter spp. | 708 | ND | ND | ND | 32 | >32 | 0.03–>32 |
| Stenotrophomonas maltophilia | 353 | ND | ND | ND | >32 | >32 | ≤0.015–>32 |
KPC: Klebsiella pneumoniae carbapenemase; MBL: Metallo-β-lactamases; ND: Not determined.
Data taken from [29].
Meropenem has good stability against hydrolysis by extended-spectrum β-lactamases including SHV, TEM and CTX-M type, and the addition of vaborbactam did not reduce the MICs of genetically engineered strains of Escherichia coli as compared with meropenem alone (meropenem and meropenem–vaborbactam MICs were both ≤0.03 μg/ml) [26]. In an evaluation of Enterobacteriaceae strains (n = 346) that co-produced both a KPC and ESBL enzyme, the MIC90 of meropenem–vaborbactam was 1 μg/ml, the same MIC90 identified among producers of the KPC enzyme alone [29].
Although robust analyses have not yet been published, meropenem–vaborbactam also has activity against organisms producing Ambler class C β-lactamases, which can largely be attributed to the inherent stability of meropenem to these types of β-lactamases [26,29]. In E. coli strains producing DHA-1, MIR-1, FOX-5 and AmpC-ECL, both the meropenem and meropenem–vaborbactam MICs were ≤0.03 μg/ml [26]. Furthermore, in an analysis of clinical isolates that co-produced AmpC and KPC enzymes, meropenem–vaborbactam demonstrated potent in vitro activity (MIC90 = 0.06 μg/ml, n = 34) [29].
Although meropenem–vaborbactam has activity against nonfermenting Gram-negative bacilli such as Pseudomonas aeruginosa and Acinetobacter spp., it appears to be similar to that of meropenem alone, as the addition of vaborbactam does not significantly potentiate meropenem activity against these organisms. As seen in Table 1, the MIC50 (0.5 μg/ml) and MIC90 (8 μg/ml) of meropenem–vaborbactam was the same as meropenem alone in an evaluation of 2604 isolates of P. aeruginosa [28]. Carbapenem resistance in P. aeruginosa can be caused by a number of mechanisms that would not be impacted by vaborbactam, including reduced outer membrane permeability (commonly due to the loss of the OprD porin channel), overexpression of efflux pumps (particularly MexAB-OprM or MexEF-OprN) and production of Ambler class B β-lactamases (metallo-β-lactamases or MBL) [31]. Likewise, carbapenem resistance in Acinetobacter spp. is not commonly due to the production of KPCs but instead more commonly due to production of Ambler class D enzymes (oxacillinases) and thus the addition of vaborbactam would not be expected to improve the activity of meropenem against Acinetobacter spp. [32]. More comprehensive evaluations of meropenem–vaborbactam activity against other nonfermenting Gram-negative bacilli are warranted.
There are limited published data regarding the activity of meropenem–vaborbactam against Gram-positive bacteria and anaerobic bacteria [33,34]. However, in vitro activity against Gram-positive bacteria would be expected to be similar to that of meropenem alone because β-lactam resistance is largely mediated by alterations in penicillin-binding proteins leading to reduced binding affinity [22,35]. Thus, the addition of vaborbactam should not potentiate meropenem activity against Gram-positive organisms. Notably, meropenem has activity against methicillin-sensitive Staphylococcus aureus, Streptococcus pyogenes, Streptococcus agalactiae, penicillin-sensitive Streptococcus pneumoniae, and some strains of Enterococcus faecalis and Enterococcus faecium. Meropenem is also active against a number of anaerobic bacteria, including Bacteroides fragilis and Fusobacterium [22]. Furthermore, the activity of biapenem–vaborbactam (then RPX7009) was similar to biapenem alone for common anaerobic bacteria including Bacteroides spp., Fusobacterium spp. and Prevotella spp. including isolates with elevated carbapenem MICs, as evidenced by similar MIC50 and MIC90 values [34]. Therefore, it would be expected that the anaerobic activity of meropenem–vaborbactam should be similar to that of meropenem alone.
Mechanisms of resistance
Although the development of resistance to meropenem–vaborbactam has not been reported clinically, in vitro investigations have been conducted evaluating the impact of known β-lactam resistance mechanisms on the activity of meropenem–vaborbactam in Enterobacteriaceae [26,30]. Mechanisms of antibiotic resistance found in Gram-negative pathogens are innumerable and often complex. Generally, they can be classified as production of hydrolytic enzymes, reduction in antibiotic permeability by alteration in outer membrane porin channel expression, overexpression of efflux pumps or target site mutations.
Although vaborbactam restores the activity of meropenem against class A carbapenemases, there are notable gaps in the spectrum of β-lactamase inhibition, including Ambler class B and class D enzymes. Thus, the activity of meropenem–vaborbactam would not be expected to differ from that of meropenem alone in the presence of MBL and/or oxacillinase producers. However, in vitro antimicrobial activity may appear enhanced due to different clinical susceptibility breakpoints between meropenem–vaborbactam and meropenem alone for organisms that commonly produce these enzymes (≤4 μg/ml for meropenem–vaborbactam and Enterobacteriaceae compared with ≤1 μg/ml for meropenem and Enterobacteriaceae and ≤2 μg/ml for meropenem and nonfermenting Gram-negative rods). In an experiment with genetically engineered strains of E. coli-producing OXA-48, an Ambler class D enzyme, the meropenem MIC was unchanged (and remained elevated) even when a fixed concentration of vaborbactam was added. Likewise, in the presence of E. coli strains producing NDM-1 or VIM-1, Ambler class B enzymes, vaborbactam was unable to potentiate the activity of meropenem as evidenced by no reduction in the MIC [26]. However, no mutations in the KPC enzyme have been identified to date that result in the decreased activity of meropenem–vaborbactam [30].
To determine the impact of efflux and outer membrane porin mutations on the activity of meropenem–vaborbactam, experiments with KPC-3-producing strains of K. pneumoniae have been conducted. Maximum potentiating concentrations (MPCmax), defined as the concentration of vaborbactam required to achieve maximal reduction of the meropenem MIC, were used to compare activity against strains with various mutations and wild-type strains. Decreased activity of meropenem–vaborbactam was observed against strains with mutations leading to the inactivation of ompK35 and ompK36 as evidenced by a fourfold and 64-fold increase, respectively, in vaborbactam MPCmax. In line with these results, inactivation of both of these porins was associated with a 512-fold increase in vaborbactam MPCmax. Thus, decreased outer membrane porin expression appears to result in reduced cell membrane permeability and activity of vaborbactam. Further, in a strain expressing OmpK36 with the GD repeat or glycine-aspartic acid duplication, similar to reports from clinical practice settings, vaborbactam potency was reduced when compared with a similar strain without the GD repeat [26]. However, the clinical significance of these mechanisms of resistance remains unknown because in the presence of a fixed concentration of vaborbactam (8 μg/ml), the meropenem MIC is ≤2 μg/ml for pathogens expressing these porin changes and producing KPC-3 [26]. Thus, using a dose of meropenem–vaborbactam 2 g/2 g given every 8 h should allow adequate concentrations of both meropenem and vaborbactam to be achieved such that pharmacodynamic targets are attained. It remains to be determined if hyperproduction of the KPC enzyme in combination with porin mutations, most notably inactivation or modification of both ompK35 and ompK36, will lead to clinical resistance (MICs ≥4/8 μg/ml). Strains with meropenem–vaborbactam MICs of 8–64 μg/ml have been described where KPC and porin mutations are present, often in combination with additional β-lactamases. It is noteworthy that all of these isolates have high-level resistance to meropenem (MICs >64 μg/ml) [36].
Interestingly, published data suggest that AcrAB-mediated efflux is not a major contributor to meropenem–vaborbactam resistance [26]. In a strain with significant mutations in ompK35 and ompK36, vaborbactam potency was unchanged in the presence of AcrAB efflux overexpression. Additionally, the MPCmax for a strain with inactivation of ramR, resulting in acrAB overexpression and ompK35 downregulation, was only twofold higher when compared with the MPCmax for a strain with ompK35 inactivated and normal expression of acrAB [26].
Pharmacokinetics
Published pharmacokinetic data generated from Phase I studies of meropenem–vaborbactam conducted in healthy adults are summarized in Table 2 [37,38]. In a sequential single and multiple dose-escalating study, the pharmacokinetics of vaborbactam were defined in healthy adult volunteers following the administration of a 3-h intravenous infusion of 250 mg to 2 g of vaborbactam. Plasma and urine samples were obtained at various time points following the start of infusion and analyses were conducted using HPLC–tandem mass spectrometry. Exposure variables including Cmax and area under the curve (AUC) increased proportionally with dose. There was no evidence of accumulation of vaborbactam following administration of multiple doses given every 8 h for 7 days. Additionally, these data demonstrate the extensive renal clearance of vaborbactam with >90% excreted unchanged in the urine following administration of 2 g. The average plasma protein binding for vaborbactam was 33% [37]. The renal clearance (70%) and low protein binding (2%) of meropenem have been previously described [20,22]. In a separate multiple dose Phase I study, the pharmacokinetics of both agents were determined following the intravenous administration of a fixed-dose combination product of meropenem (2 g) and vaborbactam (2 g) [38]. Collectively, data from these Phase I studies suggest that meropenem and vaborbactam display similar pharmacokinetics in plasma as evidenced by exposure characteristics. An average total plasma Cmax of 58.2 μg/ml and 40.9–59.0 μg/ml, total plasma AUC of 186 μg·h/ml and 140–204 μg·h/ml, volume of distribution of 16.3 l and 17.6–21.8 l, and half-life of 1 h and 1.3–1.7 h for meropenem and vaborbactam, respectively, were determined at steady state following the administration of meropenem–vaborbactam 2 g/2 g given as a 3-h intravenous infusion [37,38]. With a protein binding of 2% for meropenem and 33% for vaborbactam, the estimated free-drug AUC0–24 h is 547 and 290–410 μg·h/ml, respectively, from Phase I pharmacokinetic data [37,38]. Although pharmacokinetic data from Phase III studies have not yet been published, preliminary reports suggest even higher estimated free-drug AUC0–24 h of 560 μg·h/ml for vaborbactam were observed [39,40].
Table 2. . Pharmacokinetic parameters of meropenem and vaborbactam determined in healthy adults.
| Study | Sample size | Dose | Cmax‡ (μg/ml) | T1/2 (h) | AUC0–8 h‡ (μg·h/ml) | Estimated AUC0–24 h‡ (μg·h/ml) | CL (l/h) | V (l) | Urinary recovery (%) | Protein binding (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meropenem† | |||||||||||
| Wenzler | 25 | 2000 mg every 8 h × 3 doses | 58.2 ± 10.8 | 1.03 ± 0.15 | 186 ± 33.6 | 558 | 11.1 ± 2.1 | 16.3 ± 2.6 | ND | ND | [38] |
| Vaborbactam† | |||||||||||
| Griffith | 6 | 2000 mg × 1 | 41.60 ± 4.75 | 1.52 ± 0.08 | 140.00 ± 13.50 | NA | 14.00 ± 1.40 | 21.8 ± 2.26 | 105.00 ± 15.10 | 33 | [37] |
| 6 | 2000 mg every 8 h × 7 days | 40.90 ± 4.68 | 1.66 ± 0.10 | 145.00 ± 15.80 | 435 | 14.00 ± 1.78 | ND | 91.60 ± 5.36 | 33 | ||
| Wenzler | 25 | 2000 mg every 8 h × 3 doses | 59.0 ± 8.4 | 1.27 ± 0.2 | 204 ± 34.6 | 612 | 10.1 ± 1.9 | 17.6 ± 2.6 | ND | ND | [38] |
The pharmacokinetics of meropenem–vaborbactam has also been studied in patients with renal impairment. Meropenem–vaborbactam 1 g/1 g was administered to 41 patients with normal renal function, mild impairment (estimatd glomerular filtration rate [eGFR] of 60 to 89 ml/min/1.73 m2), moderate impairment (eGFR of 30 to <60 ml/min/1.73 m2), severe impairment (eGFR <30 ml/min/1.73 m2), or end-stage renal disease requiring hemodialysis. For both meropenem and vaborbactam, exposures (Cmax and AUC) were greater and the elimination half-life was longer in patients with worsening renal impairment. eGFR correlated well with the total plasma clearance of both compounds. Both meropenem and vaborbactam are removed by hemodialysis as evidenced by the mean 2.21-fold and 5.11-fold increase in total plasma clearance in patients receiving hemodialysis, respectively. The median percentage of the dose recovered in dialysate during the hemodialysis session was 38.3 and 52.9% for meropenem and vaborbactam, respectively [41]. Dose adjustments for patients with renal impairment are recommended (Table 3) [20].
Table 3. . Renal dose adjustments for meropenem–vaborbactam.
| eGFR† (ml/min/1.73 m2) | Recommended dose for meropenem–vaborbactam‡ |
|---|---|
| >50 | 2000 mg–2000 mg every 8 h |
| 30–49 | 1000 mg–1000 mg every 8 h |
| 15–29 | 1000 mg–1000 mg every 12 h |
| <15 | 500 mg–500 mg every 12 h |
Data taken from [20].
†eGFR is calculated using the Modification of Diet in Renal Disease formula.
‡Administered as a 3-h infusion.
eGFR: Estimatd glomerular filtration rate.
Wenzler et al. assessed intrapulmonary penetration of meropenem–vaborbactam by measuring steady-state concentrations in epithelial lining fluid (ELF) and alveolar macrophages following administration of three doses of the 4 g (2 g meropenem/2 g vaborbactam) combination product (given over 3 h) in 25 healthy adults. Multiple respiratory samples were obtained by bronchoscopy with bronchoalveolar lavage during various time points within the dosing interval (8 h). The concentration versus time profile of meropenem was similar to that of vaborbactam in ELF. Using mean AUC, the ratios of ELF to unbound plasma concentrations of meropenem and vaborbactam were 65 and 79%, respectively, suggesting good intrapulmonary penetration of meropenem–vaborbactam [38].
Pharmacodynamics
The pharmacodynamics of meropenem has been previously described elsewhere [42]. Similar to other β-lactam antibiotics, meropenem displays time-dependent bactericidal activity. Thus, the percentage of time that free-drug concentrations are above the MIC (%fT >MIC) in the dosing interval is the best predictor of antimicrobial activity [42]. More specifically, animal infectivity models suggest a %fT >MIC of 40 or greater should be achieved to maximize bactericidal activity with meropenem [42,43].
Few studies have been published that evaluate pharmacodynamics of vaborbactam. In an in vitro hollow-fiber model, when exposures of meropenem and vaborbactam were adjusted to mimic those seen in humans receiving 2 g/2 g administered every 8 h by a 3-h intravenous infusion, bactericidal activity and suppression of resistance were observed against KPC-producing Enterobacteriaceae [36]. Further, in unpublished data presented at a scientific conference, the ratio of free-drug AUC to meropenem–vaborbactam (vaborbactam fixed concentration of 8 mg/l) MIC (fAUC:MIC) was the pharmacodynamic target of vaborbactam that best predicted restoration of meropenem antimicrobial activity in both an in vitro hollow-fiber model and a neutropenic murine thigh model. In the in vitro hollow-fiber model, fAUC:MIC ratios of 12 and 18 were associated with bacteriostasis and 1-log10 kill, whereas in the neutropenic murine thigh model, fAUC:MIC ratios of 9 and 38 were the targets associated with bacteriostasis and 1-log10 kill [44]. Furthermore, fAUC:MIC ratios >24 were associated with resistance suppression in the hollow-fiber model (resistance did not develop in the murine thigh model). Therefore, with a meropenem–vaborbactam clinical breakpoint of 4/8 μg/ml and a fAUC:MIC ratio of 38 (the highest pharmacodynamic target for 1-log10 kill, identified from the murine thigh model), vaborbactam fAUC(0–24) ≥152 μg h/ml would be required to ensure bactericidal activity. Although robust Monte Carlo simulations have yet to be published relating exposures seen in the Phase III trials to these targets, rough estimates from the aforementioned pharmacokinetic analyses are encouraging. The Phase I data suggest mean vaborbactam fAUC(0–24) from 290 to 410 μg h/ml following administration of meropenem–vaborbactam 2 g/2 g every 8 h (Table 2), and the unpublished Phase III pharmacokinetic data suggest even higher mean exposures (∼560 μg h/ml) [39,40]. Therefore, it would be expected that most patients attain the target of a vaborbactam fAUC(0–24) ≥152 μg h/ml. Of note, evaluation of pharmacodynamic targets of vaborbactam in a lung infection model is warranted to ensure targets are similar to those determined in the neutropenic murine thigh model and achievable in patients.
Clinical efficacy
The clinical efficacy of meropenem–vaborbactam has been explored in two Phase III clinical trials [33,45]. TANGO I was a multicenter, multinational, randomized, double-blind, double-dummy, active-control Phase III trial of meropenem–vaborbactam for the treatment of complicated urinary tract infection (cUTI) or acute pyelonephritis. cUTI was defined as having at least two clinical criteria (chills/rigors/fever, elevated white blood cell count, nausea/vomiting, dysuria/increased urinary frequency/urinary urgency or lower abdominal/pelvic pain), presence of pyuria and at least one risk factor (indwelling catheter, neurogenic bladder, obstructive uropathy, azotemia, urinary retention in men due to benign prostatic hypertrophy). Adult patients received either meropenem–vaborbactam 2 g/2 g intravenously every 8 h over a 3-h infusion or piperacillin–tazobactam 4 g/0.5 g intravenously every 8 h over a 30-min infusion and had an option to switch to oral levofloxacin 500 mg daily if clinically improving after receipt of at least 15 doses of intravenous therapy to complete a 10-day total treatment duration. The primary efficacy end point for the FDA was a composite of clinical cure, defined as complete resolution or significant improvement of baseline signs and symptoms of infection, and microbiological cure, defined as reduction in bacteria to <104 CFU/ml at the end of intravenous treatment. The primary efficacy end point for the EMA was microbiological cure, defined as reduction in bacteria to <103 CFU/ml at the test-of-cure visit [33].
A total of 545 patients were included in the modified intention to treat (mITT) analysis with 272 receiving meropenem–vaborbactam and 273 receiving piperacillin–tazobactam. Of these, 68.6% (374) had at least 105 CFU/ml of bacteria isolated from the urine or the same pathogen in both urine and blood cultures, and made up the microbiologic mITT population. More than 50% of the patients included in the study presented with acute pyelonephritis with no significant differences between treatment arms. Of those with cUTI, about half had a removable source of infection. There were no significant differences in other baseline characteristics among the mITT population. Escherichia coli was the most common pathogen identified in the microbiologic mITT population. No isolates of E. coli were resistant to meropenem, whereas 5.2% (n = 6) of isolates in piperacillin–tazobactam-treated patients were resistant to piperacillin–tazobactam. In the microbiologic mITT population, among isolates of K. pneumoniae, only one isolate in the meropenem–vaborbactam treatment arm was resistant to meropenem. Additionally, 33.3% (n = 9) of K. pneumoniae isolates were resistant to piperacillin–tazobactam in the piperacillin–tazobactam treatment arm. Average total duration of antibiotic therapy was similar in both treatment groups, 10.1 days (range: 1–17) in the meropenem–vaborbactam arm and 9.9 days (range: 2–15) in the piperacillin–tazobactam arm. Furthermore, duration of intravenous therapy was similar between both groups with an average of 8 days [33].
Results from TANGO I demonstrated that meropenem–vaborbactam was superior to piperacillin–tazobactam for the treatment of cUTI and acute pyelonephritis according to the FDA primary end point of overall success at the end of intravenous therapy in the microbiologic mITT population. High rates of overall clinical success were observed in both treatment groups in the microbiologic mITT population, 98.4% with meropenem–vaborbactam and 94.0% with piperacillin–tazobactam (difference of 4.5%, [95% CI: 0.7–9.1%]; p < 0.001 for noninferiority; p = 0.01 for superiority). In patients with bloodstream infections (n = 27), overall success rates were 10/12 (83.3%) with meropenem–vaborbactam and 15/15 (100%) with piperacillin–tazobactam. The two treatment failures in the meropenem–vaborbactam group were due to adverse events leading to early discontinuation of study drug. In the microbiologic mITT population, rates of microbiological eradication at test-of-cure (EMA primary end point) were similar between the two treatment arms, 66.7% in the meropenem–vaborbactam group and 57.7% in the piperacillin–tazobactam group (difference of 9.0%, [95% CI: -0.9%–18.7%]; p < 0.001 for noninferiority). Clinical cure remained high at the test-of-cure visit, and was similar between groups (90.6% in the meropenem-vaborbactam group and 86.3% in the piperacillin-tazobactam group, difference of 4.4%, [95% CI: -2.2–11.1%]) [33].
TANGO II was a randomized, open-label trial in patients with cUTI, acute pyelonephritis, hospital-acquired or ventilator-associated bacterial pneumonia, bloodstream infection or complicated intra-abdominal infection due to known or suspected CRE. Patients were randomized 2:1 to receive monotherapy with meropenem–vaborbactam 2 g/2 g administered every 8 h over 3-h intravenous infusion or best available therapy (BAT) as determined by the site primary investigator, and included monotherapy or combination therapy of a carbapenem, aminoglycoside, polymyxin B, colistin, tigecycline or ceftazidime–avibactam (monotherapy only) for 7–14 days. Patients with infection due to pathogens known to be harboring NDM, VIM, IMP, or OXA carbapenemases were excluded. Additionally, patients who received >24 h of potentially effective antimicrobials prior to enrollment (unless deemed a clinical failure) and those with immediate life-threatening diseases were also excluded. The primary efficacy end point was clinical cure defined as complete resolution of signs and symptoms of infection where no further antimicrobial therapy was warranted. Although the results of TANGO II are not yet published, preliminary data were presented at a scientific conference. A total of 72 patients were enrolled including 50 (69%) who were infected with a Gram-negative organism and 43 (60%) with CRE. Of these 43 patients with CRE, 20 (46%) had bloodstream infections, 15 (35%) had cUTI or acute pyelonephritis, 5 (12%) had hospital-acquired or ventilator-associated pneumonia, and 3 (7%) had complicated intra-abdominal infections. Of the isolated CRE, the most common organism was K. pneumoniae (86%) and 80% of all CRE harbored a KPC. Although a number of different regimens were used in the BAT group, the majority received combination therapy, which commonly included a carbapenem (47%, n = 7). There were also high rates of colistin (53%, n = 8), aminoglycoside (47%, n = 7) and tigecycline (33%, n = 5) use as both monotherapy and combination therapy. Only one patient received treatment with ceftazidime–avibactam, and it was administered as monotherapy. More patients receiving meropenem–vaborbactam (n = 28), compared with patients receiving BAT (n = 15), for the treatment of CRE infections achieved a clinical cure at the end of therapy (64.3 vs 33.3%; p = 0.04) and test-of-cure visit (57.1 vs 26.7%; p = 0.04), respectively. A 28-day mortality was statistically similar in the patients receiving meropenem–vaborbactam as compared with those receiving BAT (17.9 vs. 33.3%; p = 0.3); however, this study was underpowered to detect a difference in mortality. Meropenem–vaborbactam was well tolerated in the study [45]. The full publication of TANGO II data is highly anticipated as patients included in this study more closely resemble the anticipated real-world use of meropenem–vaborbactam than do patients who were enrolled in TANGO I.
Safety & tolerability
Overall, meropenem–vaborbactam appears to be well tolerated as demonstrated by safety data generated from TANGO I. Among patients receiving meropenem–vaborbactam and piperacillin–tazobactam, the percentages experiencing any adverse event were 39.0 and 35.5%, study drug-related adverse events were 15.1 and 12.8%, severe adverse events were 2.6 and 4.8% and life-threatening adverse events were 1.1 and 0%, respectively. The proportion of patients who died or experienced an adverse event leading to study or drug discontinuation was low in both treatment groups. The most common adverse effect reported in patients who received meropenem–vaborbactam was headache. Table 4 details common adverse events experienced by patients enrolled in TANGO I. Although not reported with meropenem–vaborbactam, C. difficile-associated diarrhea and neurotoxicities including seizures have been reported with meropenem and thus caution should be exercised with use of meropenem–vaborbactam [20,22,33,46].
Table 4. . Common adverse events experienced by ≥1.5% of patients receiving meropenem-vaborbactam in TANGO I.
| Adverse event | Meropenem–vaborbactam (n = 272) | Piperacillin–tazobactam (n = 273) |
|---|---|---|
| Headache | 24 (8.8) | 12 (4.4) |
| Diarrhea | 9 (3.3) | 12 (4.4) |
| Nausea | 5 (1.8) | 4 (1.5) |
| Asymptomatic bacteriuria | 4 (1.5) | 4 (1.5) |
| Catheter site phlebitis | 5 (1.8) | 3 (1.1) |
| Infusion site phlebitis | 6 (2.2) | 2 (0.7) |
| Urinary tract infection | 4 (1.5) | 4 (1.5) |
| Alanine aminotransferase increased | 5 (1.8) | 1 (0.4) |
| Aspartate aminotransferase increased | 4 (1.5) | 2 (0.7) |
| Pyrexia | 4 (1.5) | 2 (0.7) |
Data are presented as n (%).
Data taken from [33].
Conclusion
The addition of vaborbactam to meropenem restores the activity of meropenem against Enterobacteriaceae that produces Ambler class A enzymes. In particular, meropenem–vaborbactam is especially potent against KPC-producing organisms. Although this β-lactam/β-lactamase inhibitor combination has expanded Enterobacteriaceae activity, it adds little to meropenem with regards to in vitro activity against P. aeruginosa, Acinetobacter spp. and most organisms producing Ambler class B and D carbapenemases. Following administration of meropenem–vaborbactam 2 g/2 g every 8 h as a 3-h infusion, successful clinical outcomes in patients with cUTI and acute pyelonephritis caused by Enterobacteriaceae were demonstrated in Tango I. Preliminary data from Tango II regarding the efficacy of meropenem–vaborbactam for the treatment of invasive KPC-producing CRE infections are encouraging. These data add to the growing body of literature demonstrating that novel therapies, such as meropenem–vaborbactam, ceftazidime–avibactam and plazomicin, are superior to colistin-based therapy for serious CRE infections [13,14,45,47]. Overall, meropenem–vaborbactam appears to be well tolerated with low rates of serious adverse events reported in controlled trials. Additional clinical outcomes data in patients with CRE infection are necessary to further delineate the role of this agent in real-world practice.
Although meropenem–vaborbactam has displayed potent in vitro activity against Enterobacteriaceae and was an effective treatment option for patients with cUTI and acute pyelonephritis in the TANGO I trial, antimicrobial stewardship programs will likely reserve this agent for patients with infections due to known or highly suspected CRE, and in particular KPC producers. In this context, the efficacy of meropenem–vaborbactam in TANGO II in preliminary reports and its potent in vitro activity displayed against clinical isolates of KPC-producing organisms in large surveillance studies are encouraging. Recently approved ceftazidime–avibactam also has good in vitro activity against KPC-producing CRE and less toxicity than polymyxins and aminoglycosides, and represents an important treatment alternative. However, recent reports of resistance to ceftazidime–avibactam among KPC-producing Enterobacteriaceae highlight the need for additional options for CRE [15–19]. Data presented at a scientific conference evaluating the activity of meropenem–vaborbactam against one strain of CRE with high level resistance to ceftazidime–avibactam via a point mutation in the D179Y amino acid in the blaKPC-2 gene suggest meropenem–vaborbactam may retain susceptibility against these strains; however, this was largely driven by residual activity of meropenem against this strain and further investigation is warranted [48]. Real-world data, such as those evaluated in the Tango II trial, and ultimately comparative data with ceftazidime/avibactam, will help to further define the role of meropenem/vaborbactam in clinical practice.
Executive summary.
Chemistry & clinical pharmacology
Meropenem–vaborbactam is a fixed-dose combination product of a carbapenem antibiotic and a cyclic boronic acid β-lactamase inhibitor.
Meropenem exerts its antimicrobial activity by binding to penicillin-binding proteins, thereby inhibiting the crosslinking of peptidoglycan chains causing cell lysis and death.
Vaborbactam restores the activity of meropenem by inhibiting the activity of serine β-lactamases.
Antimicrobial spectrum of activity
With the addition of vaborbactam, the activity of meropenem is restored against carbapenem-resistant Enterobacteriaceae isolates producing Ambler class A β-lactamases, such as Klebsiella pneumoniae carbapenemases.
Meropenem–vaborbactam has activity against nonfermenting Gram-negative bacilli such as Pseudomonas aeruginosa and Acinetobacter spp., but it appears to be similar to that of meropenem alone.
In vitro activity against Gram-positive and anaerobic bacteria is expected to be similar to that of meropenem alone.
Mechanisms of resistance
Meropenem–vaborbactam does not have activity against Gram-negative organisms that produce Ambler class B (metallo-β-lactamases) and class D enzymes (oxacillinases).
Decreased activity of meropenem–vaborbactam was observed against strains with mutations in outer membrane porins, OmpK35 and OmpK36. However, they usually, but not always, remained in the susceptible range.
AcrAB-mediated efflux does not appear to be a major contributor to meropenem–vaborbactam resistance.
Pharmacokinetics
Phase I pharmacokinetic data demonstrate that exposure variables including Cmax and area under the curve (AUC) increase proportionally with dose.
An average total plasma Cmax of 58.2 and 40.9–59.0 μg/ml, total plasma AUC of 186 μg·h/ml and 140–204 μg·h/ml, volume of distribution of 16.3 l and 17.6–21.8 l, and half-life of 1 h and 1.3–1.7 h for meropenem and vaborbactam, respectively, were determined at steady state following the administration of meropenem–vaborbactam 2 g/2 g given as a 3-h intravenous infusion.
eGFR correlated well with the total plasma clearance of meropenem and vaborbactam, and both compounds are removed by hemodialysis.
Pharmacodynamics
Animal infectivity models suggest a %fT>MIC of 40 or greater should be achieved to maximize bactericidal activity with meropenem.
The pharmacodynamic target of vaborbactam that best predicted restoration of meropenem antimicrobial activity is fAUC:MIC (fAUC:MIC ratio of 38 to achieve a 1-log10 kill was identified from a neutropenic murine thigh model).
With a clinical breakpoint of 4/8 μg/ml, vaborbactam fAUC(0–24) ≥152 μg·h/ml would be required to ensure bactericidal activity. Pharmacokinetic data estimate vaborbactam fAUC(0–24) to range from 290 to 560 μg·h/ml.
Clinical efficacy
TANGO I was a Phase III trial of meropenem–vaborbactam compared with piperacillin–tazobactam for the treatment of complicated urinary tract infection or acute pyelonephritis. High rates of overall clinical success were observed in both treatment groups, 98.4% with meropenem–vaborbactam and 94.0% with piperacillin–tazobactam (difference of 4.5%, [95% CI: 0.7–9.1%]; p < 0.001 for noninferiority; p = 0.01 for superiority).
TANGO II was a randomized, open-label trial comparing meropenem–vaborbactam to best available therapy for the treatment of complicated urinary tract infection, acute pyelonephritis, hospital-acquired or ventilator-associated bacterial pneumonia, bloodstream infection or complicated intra-abdominal infection due to known or suspected carbapenem-resistant Enterobacteriaceae. More patients receiving meropenem–vaborbactam, compared with patients receiving best available therapy, achieved a clinical cure at the end of therapy (64.3 vs 33.3%; p = 0.04) and test-of-cure visit (57.1 vs 26.7%; p = 0.04), respectively.
Safety & tolerability
Overall, meropenem–vaborbactam appears to be well tolerated as demonstrated by safety data generated from TANGO I.
The most common adverse effect reported in patients who received meropenem–vaborbactam was headache.
Footnotes
Company review
In addition to the peer-review process, with the author's consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made by the author at their discretion and based on scientific or editorial merit only. The author maintained full control over the manuscript, including content, wording and conclusions.
Financial & competing interests disclosure
KS Kaye and JM Pogue are funded by the National Institute of Allergy and Infectious Diseases (DMID Protocol Number: 10-0065 and R01-AI119446-01). JM Pogue is a paid consultant, has received research support, and/or has served on the Speaker's Bureau for The Medicines Company, Melinta, Merck, Allergand, Achaogen, Tetraphase, Shionogi, and Zavante. KS Kaye is a paid consultant and advisor for Melinta Therapeutics, The Medicines Company and Allergan. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Thaden JT, Lewis SS, Hazen KC, et al. Rising rates of carbapenem-resistant enterobacteriaceae in community hospitals: a mixed methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect. Control. Hosp. Epidemiol. 2014;35:978–983. doi: 10.1086/677157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Antimicrobial resistance global report on surveillance. 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1
- 3.Zilberberg MD, Shorr AF. Prevalence of multidrug-resistant Pseudomonas aeruginosa and carbapenem resistant Enterobacteriaceae among specimens from hospitalized patients with pneumonia and bloodstream infections in the United States from 2000 to 2009. J. Hosp. Med. 2013;8:559–563. doi: 10.1002/jhm.2080. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. GA, USA: 2013. Antibiotic resistance threats in the United States, 2013.www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013–2508.pdf#pageD11 [Google Scholar]
- 5.Tacconelli E, Magrini N, Carmeli Y, et al. WHO; 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics.www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 [Google Scholar]
- 6.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 2011;17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob. Agents Chemother. 2014;58(4):2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin. Infect. Dis. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 11.Pogue JM, Ortwine JK, Kaye KS. Are there any ways around the exposure-limiting nephrotoxicity of the polymyxins? Int. J. Antimicrob. Agents. 2016;48(6):622–626. doi: 10.1016/j.ijantimicag.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Generating antibiotic incentives now. Food and Drug Administration Safety and Innovation Act. 2018. www.fda.gov/downloads/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm595188.pdf
- 13.Shields RK, Nguyen MH, Chen L, et al. Ceftazidime–avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob. Agents Chemother. 2017;61(8) doi: 10.1128/AAC.00883-17. pii:e00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime–avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin. Infect. Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime–avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin. Infect. Dis. 2016;63(12):1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime–avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob. Agents Chemother. 2017;61(3) doi: 10.1128/AAC.02097-16. pii:e02097h16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humphries RM, Yang S, Hemarajata P, et al. First report of ceftazidime–avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob. Agents Chemother. 2015;59(10):6605–6607. doi: 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giddins MJ, Macesic N, Annavajhala MK, et al. Successive emergence of ceftazidime–avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae ST307. Antimicrob. Agents Chemother. 2017;62(3) doi: 10.1128/AAC.02101-17. pii:e02101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. Mutations in blaKPC-3 that confer ceftazidime–avibactam resistance encode novel KPC-3 variants that function as extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 2017;61(5) doi: 10.1128/AAC.02534-16. pii:e02534h16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Medicines Company; NJ, USA: Meropenem–vaborbactam, package insert. [Google Scholar]
- 21.Mouton JW, van den Anker JN. Meropenem clinical pharmacokinetics. Clin. Pharmacokinet. 1995;28:275–286. doi: 10.2165/00003088-199528040-00002. [DOI] [PubMed] [Google Scholar]
- 22.Fish DN, Singletary TJ. Meropenem, a new carbapenem antibiotic. Pharmacotherapy. 1997;17(4):644–669. [PubMed] [Google Scholar]
- 23.Hikida M, Kawashima K, Yoshida M, et al. Inactivation of new carbapenem antibiotics by dehydropeptidase-1 from porcine and human renal cortex. J. Antimicrob. Chemother. 1992;30:129–134. doi: 10.1093/jac/30.2.129. [DOI] [PubMed] [Google Scholar]
- 24.Fukasawa M, Sumita Y, Harabe ET, et al. Stability of meropenem and effect of 1 β-methyl substitution on its stability in the presence of renal dehydropeptidase I. Antimicrob. Agents Chemother. 1992;36:1577–1579. doi: 10.1128/aac.36.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecker SJ, Reddy KR, Totrov M, et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J. Med. Chem. 2015;58(9):3682–3692. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 26.Lomovskaya O, Sun D, Rubio-Aparicio D, et al. Vaborbactam: spectrum of β-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017;24(61) doi: 10.1128/AAC.01443-17. pii:e01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Summarizes the activity of meropenem–vaborbactam against various strains of E. coli and K. pneumoniae with known resistance mechanisms including production of various β-lactamases and efflux and porin mutations.
- 27.Castanheira M, Rhomberg PR, Flamm RK, Jones RN. Effect of the β-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2016;60(9):5454–5458. doi: 10.1128/AAC.00711-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castanheira M, Huband MD, Mendes RE, Flamm RK. Meropenem–vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017;61(9) doi: 10.1128/AAC.00567-17. pii:e00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hackel MA, Lomovskaya O, Dudley MN, Karlowsky JA, Sahm DF. Activity of meropenem–vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob. Agents Chemother. 2017;21(62) doi: 10.1128/AAC.01904-17. pii:e01904-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Evaluates the in vitro activity of meropenem–vaborbactam against a large collection of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae.
- 30.Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. Meropenem–vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae . Antimicrob. Agents Chemother. 2001;22(61) doi: 10.1128/AAC.01694-17. pii:e01694-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai H, Kim J-W, Kim J, Lee JH, Choe KW, Gotoh N. Carbapenem resistance mechanisms in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2001;45(2):480–484. doi: 10.1128/AAC.45.2.480-484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viehman JA, Nguyen M-H, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 2014;74(12):1315–1333. doi: 10.1007/s40265-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem–vaborbactam vs piperacillin–tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319(8):788–799. doi: 10.1001/jama.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Presents the results of the Phase III trial comparing the efficacy and safety of meropenem–vaborbactam to piperacillin–tazobactam for the treatment of complicated urinary tract infections including acute pyelonephritis.
- 34.Goldstein EJ, Citron DM, Tyrrell KL, Merriam CV. In vitro activity of Biapenem plus RPX7009, a carbapenem combined with a serine β-lactamase inhibitor, against anaerobic bacteria. Antimicrob. Agents Chemother. 2013;57(6):2620–2630. doi: 10.1128/AAC.02418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakenbeck R, Brückner R, Denapaite D, Maurer P. Molecular mechanisms of β-lactam resistance in Streptococcus pneumoniae . Future Microbiol. 2012;7(3):395–410. doi: 10.2217/fmb.12.2. [DOI] [PubMed] [Google Scholar]
- 36.Sabet M, Tarazi Z, Rubio-Aparicio D, et al. Activity of simulated human dosage regimens of meropenem and vaborbactam against carbapenem-resistant Enterobacteriaceae in an in vitro hollow-fiber model. Antimicrob. Agents Chemother. 2018;25(62) doi: 10.1128/AAC.01969-17. pii:e01969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffith DC, Loutit JS, Morgan EE, Durso S, Dudley MN. Phase I study of the safety, tolerability, and pharmacokinetics of the β-lactamase inhibitor vaborbactam (RPX7009) in healthy adult subjects. Antimicrob. Agents Chemother. 2016;60(10):6326–6332. doi: 10.1128/AAC.00568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identifies key pharmacokinetic parameters of vaborbactam in healthy adult volunteers.
- 38.Wenzler E, Gotfried MH, Loutit JS, et al. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob. Agents Chemother. 2015;59(12):7232–7239. doi: 10.1128/AAC.01713-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identifies key pharmacokinetic parameters of meropenem–vaborbactam in plasma as well as the intrapulmonary penetration of this agent.
- 39.Trang M, Griffith DC, Bhavnani SM, et al. 2nd ASM Microbe. New Orleans, LA, USA: 1–5 June 2017. Population pharmacokinetics of meropenem and vaborbactam in healthy volunteers and infected patients. Presented at. [Google Scholar]
- 40.Bhavnani SM, Hammel JP, Rubino CM, et al. 2nd ASM Microbe. New Orleans, LA, USA: 1–5 June 2017. Meropenem–vaborbactam pharmacokinetic pharmacodynamic analyses for efficacy based on data from patients enrolled in Phase III studies. Presented at. [Google Scholar]
- 41.Rubino CM, Bhavnani SM, Loutit JS, Lohse B, Dudley MN, Griffith DC. Meropenem–vaborbactam: single-dose pharmacokinetics and safety in subjects with chronic renal impairment. Antimicrob. Agents Chemother. 2018 doi: 10.1128/AAC.02103-17. pii:AAC.02103-02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin. Infect. Dis. 2008;15(47, Suppl. 1):S32–S40. doi: 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 43.Ong CT, Tessier PR, Li C, Nightingale CH, Nicolau DP. Comparative in vivo efficacy of meropenem, imipenem, and cefepime against Pseudomonas aeruginosa expressing MexA-MexB-OprM efflux pumps. Diagn. Microbiol. Infect. Dis. 2007;57(2):153–161. doi: 10.1016/j.diagmicrobio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Griffith DC, Sabet M, Tarazi Z, Lomovskaya O, Dudley MN. 2nd ASM Microbe. New Orleans, LA, USA: 1–5 June 2017. Pharmacodynamics of vaborbactam when administered in combination with meropenem. Presented at. [Google Scholar]
- 45.Kaye KS, Vazquez J, Mathers A, et al. ID Week. San Diego, CA, USA: 4–8 October 2017. Meropenem–vaborbactam (vabomere) vs best available therapy for carbapenem-resistant Enterobacteriaceae infections in TANGO II: pooled population analysis across infection types. Presented at. [Google Scholar]; •• Presents the results of the Phase III trial comparing the efficacy and safety of meropenem–vaborbactam to best available therapy for the treatment of invasive carbapenem-resistant Enterobacteriaceae infections.
- 46.AstraZeneca; DE, USA: Meropenem, package insert. [Google Scholar]
- 47.McKinnell JA, Connolly L, Pushkin R, Jubb AM, et al. IDWeek. San Diego, CA, USA: 4–8 October 2017. Improved outcomes with plazomicin compared with colistin in patients with bloodstream infections caused by carbapenem-resistant Enterobacteriaceae (CRE): results from the CARE study. Presented at. [Google Scholar]
- 48.Lomovskaya O, Tsivkovski R. 2nd ASM Microbe. New Orleans, LA, USA: 1–5 June 2017. Vaborbactam (VAB) is not affected by KPC-2 and KPC-3 variants containing Asp179Tyr amino acid substitution that are resistant to ceftazidime (CAZ) potentiation with avibactam. Presented at. [Google Scholar]