Abstract
Aim:
Tuberculosis meningitis (TBM) diagnosis is difficult, new biomarkers are needed. We evaluated the diagnostic utility of delta-like 1 protein (DLL1), vitamin D binding protein (VDBP) and fetuin.
Methods:
Biomarker concentrations were measured by ELISA in cryopreserved cerebrospinal fluid from 139 HIV-infected Ugandans with suspected meningitis. TBM was diagnosed by GeneXpert MTB/Rif or culture. Cohort diagnoses included TBM (n = 22), cryptococcal (n = 71), or aseptic meningitis (n = 16) and no meningitis (n = 30).
Results:
DLL1 (cut-off value 1150 pg/ml) provided 32% sensitivity and 98% specificity. Adding fetuin, cryptococcal antigen and IFN-γ resulted in sensitivities of 36, 63 and 76% with specificities of 98, 90 and 92%, respectively. VDBP (cut-off value 2.0 μg/ml) provided 81% sensitivity and 68% specificity while fetuin (cut-off value 2 μg/ml) provided a sensitivity of 86% and specificity of 68%.
Conclusion:
CSF DLL1, VDBP and fetuin exhibited fair diagnostic performance for TBM diagnosis.
Keywords: : biomarkers, Mycobacterium tuberculosis, TB meningitis
Meningitis due to Mycobacterium tuberculosis (TB) is the deadliest form of TB. In settings where TB-HIV co-infection is common, mortality is often >50% and survivors frequently experience significant neurologic disability [1]. Diagnostic delay drives mortality in TB meningitis (TBM) [2–5]. Ziehl–Neelsen staining for acid-fast bacilli (AFB) is rapid but sensitivity is poor (∼10–15%) [6,7]. Mycobacterial culture demonstrates approximately 60% sensitivity compared with the uniform clinical case definition, but slow growth precludes rapid clinical decision-making [6,8]. GeneXpert MTB/Rif and GeneXpert MTB/Rif Ultra are rapid with accuracy at least as good as culture; however, up to a third of cases are likely still missed [9,10]. A rapid, accurate diagnostic method of detecting TB in the cerebrospinal fluid (CSF) would likely improve clinical outcomes.
DLL1 is a transmembrane protein involved, as a part of the Notch-ligand family, in the differentiation of multiple human cell lines, including adipocytes [11–13]. Mycolic acid chains (fatty acid chains) are key cell wall components of M. tuberculosis [14]. Additionally, DLL1 has been found to selectively drive antigen-specific Th1 cell responses [15] and DLL1 polymorphisms increase susceptibility to other intracellular (i.e., Th1 response inducing) organisms, such as leishmaniasis [16]. DLL1 may selectively drive similar Th1 responses in persons infected with TB [17]. Prior studies in China have raised DLL1 as a possible TBM diagnostic marker however, case definitions were unclear and investigation in other populations are needed [18–21].
A second, host-derived biomarker is VDBP. Vitamin D is thought to influence the immune response to M. tuberculosis and VDBP plays a role in macrophage activation, which is important in the immune response to TB [22,23]. VDBP has shown promise as a biomarker in studies of bovine tuberculosis [24,25]. No studies have systematically analyzed VDBP for use as a biomarker in TBM.
Third, the protein fetuin inhibits release of the proinflammatory ‘high mobility group box one’ which is released from macrophages and monocytes when stimulated by IFN-γ, TNF-α, (cytokines associated with TB infection) [26,27]. Fetuin has shown promise as a biomarker of bovine tuberculosis in two studies [24,25], and Tanaka and colleagues found lower levels of fetuin in persons with active TB versus controls in Japan and Vietnam by whole blood electrophoresis, immunoblot and plasma ELISA detection [28]. Fetuin has not been studied in TBM.
Given data regarding the use of DLL1 for TBM diagnosis are inadequate and that no such data exist for VDBP or fetuin, we evaluated the diagnostic utility of these biomarkers in differentiating TBM from other forms of meningitis in an HIV-infected African population. We hypothesized that one of more of these biomarkers would provide adequate diagnostic accuracy for use as adjunct tools to TBM diagnosis.
Methods
CSF samples were collected as a part of two clinical trials, the Cryptococcal Optimal ART Timing (COAT) trial (clinicaltrials.gov identifier NCT01075152) [29] and the Adjunctive Sertraline for the treatment of HIV-Associated Cryptococcal Meningitis (ASTRO-CM) trial (clinicaltrials.gov identifier NCT01802385) [30]. Both trials screened HIV-infected individuals with presumed meningitis at Mulago National Referral Hospital in Kampala, Uganda. Eligibility criteria were clinical suspicion of meningitis, and all participants or their surrogates (in case of altered subject mental status) provided written informed consent. Both trials received institutional review board approvals via the University of Minnesota, Mulago Hospital, and the Uganda National Council for Science and Technology. Samples were selected based on convenience of sample availability.
Diagnostic testing
Cryptococcal meningitis was diagnosed by cryptococcal antigen lateral flow assay (CRAG; IMMY Inc, OK, USA) [31]. TB microbiological diagnosis was obtained by automated PCR via GeneXpert MTB/Rif – Xpert (Cepheid, CA, USA) or by modified growth tube inhibitor culture (Becton Dickinson, NJ, USA) [10]. All subjects had bacterial gram stain and culture completed. DLL1, VDBP and fetuin concentrations were measured on cryopreserved CSF (-80°C) using the human soluble DLL1 ELISA (AdipoGen Inc., Incheon, South Korea), and VDBP and Fetuin ELISA kits (R&D Systems, MN, USA). Luminex magnetic bead technology (Bio-Rad Laboratories, CA, USA) measured 17 cytokines and chemokines IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, G-CSF, GM-CSF, IFN-γ, TNF-α, MCP-1 (or CCL2), MIP-1β (or CCL4) on cryopreserved specimens. Data collection was prospective aside from measurement of DLL-1, VDBP, fetuin, and chemokine/cytokine measurements which were performed on cryopreserved specimens. Indeterminate tests were deemed negative. Missing data on the index tests and/or reference tests were not included in analysis of the biomarker in question.
Case definitions
Subjects with positive CSF CRAG were determined to have cryptococcal meningitis. Subjects with a positive Xpert or culture were determined to have TB meningitis (definite TB meningitis per consensus case definitions) [32]. Subjects with negative bacterial, Cryptococcus, and TB meningitis testing but with substantial CSF inflammation (protein ≥100 mg/dl or white cells ≥10 cells/μl) were deemed to have aseptic meningitis. Those subjects with negative TB and cryptococcal testing and no substantial inflammation were determined to have ‘no meningitis’.
Statistical analysis
Clinical and demographic data were grouped by diagnosis (TBM, cryptococcal meningitis, aseptic meningitis or no meningitis) and compared using ANOVA, and χ2 tests as appropriate. Age, CD4 count, CSF protein, CSF white blood cell count, headache duration, DLL1, VDBP and fetuin were log2-transformed for use in baseline characteristic comparisons and logistic regression. Area under the curve (AUC) was calculated using a receiver operator characteristic curve. Logistic regression was used to evaluate combined and separate utilities of CSF measurement of DLL1, VDBP and fetuin as well as all 17 chemokines and cytokines as diagnostic tests for TBM. Spearman correlation was used to test correlation between chemokine/cytokines and concentrations of DLL1, VDBP and fetuin. Multiple cut-off values for each of DLL1, VDBP and fetuin were used to optimize AUC for sensitivity. Statistical analysis was done using SPSS version 21 (IBM Inc., NY, USA).
Results
A total of 139 participants were recruited and had a full diagnostic workup carried out. All subjects had gram stain and bacterial culture completed, none were positive. DLL1 was tested in 136 specimens, while fetuin and VDBP were tested in 130 specimens, based on CSF specimen volume availability. In total, 71 participants were diagnosed with cryptococcal meningitis, 22 participants with TB meningitis, 16 participants with aseptic meningitis (negative CRAG and TB testing but significant inflammation) and 30 with no meningitis (negative CRAG, TB testing and no significant inflammation).
Participant characteristics
Participant baseline characteristics are compared by diagnosis in Table 1. Total protein and white blood cell count were higher in patients with TBM or aseptic meningitis than in the patients with cryptococcal meningitis or no meningitis. The percentage of lymphocytes was higher in the patients with cryptococcal meningitis or TBM than those with aseptic meningitis. Percent male was higher, and CD4 count lower in those with cryptococcal meningitis compared with all other groups. Glucose was not measured in enough samples to analyze. Mortality at hospital discharge was similar between the groups though this study was not powered to detect mortality differences.
Table 1. . Participant characteristics compared between subjects with cryptococcal meningitis, tuberculosis meningitis, aseptic meningitis and no meningitis.
| Characteristic | Cryptococcal meningitis (n = 71) | Tuberculosis meningitis (n = 22) | Aseptic meningitis (n = 16) | No meningitis (n = 30) | p-value |
|---|---|---|---|---|---|
| Sex, n (%) males | 48 (68) | 12 (55) | 7 (44) | 12 (40) | 0.046 |
| Age in years | 36 (31–42) | 37 (34–44) | 34 (30–43) | 36 (29–45) | 0.782 |
| CSF total protein, mg/dl† | 76 (34–114) | 132 (71–213) | 190 (84–278) | 30 (20–47) | <0.001 |
| CSF white blood cells, cells/μl | <5 (<5–40) | 70 (<5–178) | 58 (26–138) | <5 (<5–<5) | <0.001 |
| CSF% lymphocytes‡ | 100% (100–100%) | 100% (100–100%) | 96% (78–100%) | NA | 0.001 |
| Alive at hospital discharge, % | 63% | 58% | 75% | 89% | 0.075 |
| CD4 T-cell count, cells/μl§ | 15 (7–52) | 55 (46–150) | 55 (12–1305) | 63 (13–197) | 0.001 |
| Headache duration, days¶ | 14 (7–28) | 14 (8–28) | 14 (8–30) | 14 (7–30) | 0.782 |
| CSF DLL1, pg/ml# | 422 (304–544) | 870 (558–1233) | 671 (463–780) | 233 (172–379) | <0.001 |
| CSF VDBP μg/ml†† | 1.55 (0.74–2.71) | 4.72 (2.97–8.96) | 3.48 (1.30–9.11) | 0.62 (0.34–1.10) | <0.001 |
| CSF Fetuin μg/ml†† | 1.55 (0.82–2.37) | 3.30 (2.19–4.55) | 3.10 (1.42–4.24) | 0.51 (0.29–0.76) | <0.001 |
Numeric values given in medians with interquartile range or n (%). p-values are from ANOVA tests, comparing log2 transformed mean values or χ2 test as applicable.
†29 subjects with no meningitis had this measurement.
‡Only conducted when >5 WBCs/μl. 23 subjects with cryptococcal meningitis, 15 with TB meningitis, 12 for aseptic meningitis and 0 for no meningitis had this measurement.
§68 subjects with cryptococcal meningitis, 12 with TB meningitis, six with aseptic meningitis and 13 with no meningitis had this measurement.
¶20 subjects with TB meningitis, 15 with aseptic meningitis and 24 with no meningitis had this measurement.
#27 subjects for no meningitis had this measurement.
††15 subjects for aseptic meningitis and 22 for no meningitis had this measurement.
CSF: Cerebrospinal fluid; NA: Not available.
DLL1 CSF concentrations
Geometric mean DLL1, Fetuin and VDBP values were statistically compared by diagnosis and median numeric values with interquartile ranges (IQRs) presented in Table 1. The patients with TBM (n = 22) or aseptic meningitis (n = 16) had a significantly higher geometric mean DLL1 levels than did patients with cryptococcal meningitis (n = 71) or no meningitis (n = 27) (Table 1). DLL1 concentrations were also compared between patients with TBM (n = 22) and those with any other diagnosis (e.g., Cryptococcus, aseptic meningitis or no meningitis; n = 114). Non-TBM subjects had a significantly lower median DLL1 concentration of 412 pg/ml (IQR: 269–617 pg/ml) as compared with those with TBM 870 pg/ml (IQR: 558–1233 pg/ml; p < 0.001).
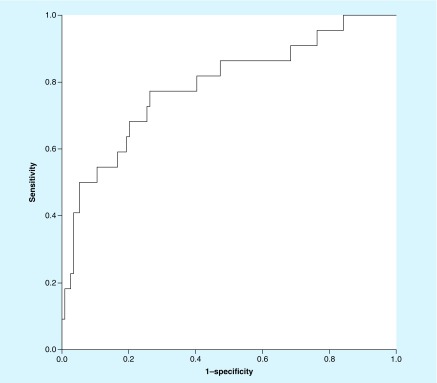
DLL1 was also assessed for accuracy in diagnosis of TB meningitis. AUC as measured by receiver operator characteristic curve was 0.789 (95% CI: 0.676–0.902) (Figure 1). Using a DLL1 cutoff of 600 pg/ml, we found a sensitivity of 73% (16/22; 95% CI: 50–89%), specificity of 74% (84/114; 95% CI: 65–81%), positive predictive value (PPV) of 35% (16/46; 95% CI: 26–44%) and negative predictive value (NPV) of 93% (84/90; 95% CI: 88–97%) for TBM diagnosis. Using a cutoff of 800 pg/ml, we found a specificity of 92% (105/114; 95% CI: 86–96%) and PPV of 55% (11/20; 95% CI: 37–72%). When presence of cryptococcal meningitis were excluded by negative CRAG and a cutoff of 600 pg/ml was used to compare 21 TBM patients with 43 patients aseptic or no meningitis, DLL1 gave a 71% (95% CI: 48–89%) sensitivity, 67% (95% CI: 51–81%) specificity, 52% (95% CI: 39–64%) PPV and 83% (95% CI: 70–91%) NPV. Using a higher cut-off value of 800 pg/ml in this comparison yielded a sensitivity of 48% (95% CI: 26–70%), specificity of 88% (95% CI: 75–96%), PPV of 67% (95% CI: 44–84%) and NPV of 78% (95% CI: 69–84%). Thus, though DLL1 levels were higher with TB meningitis, no cutoff could be identified for use as a suitable biomarker.
Figure 1. . Receiver operator characteristic curve.
The ROC above shows an AUC of 0.789 (95% CI: 0.676–0.902) for DLL1.
AUC: Area under the curve.
VDBP CSF concentrations
VDBP was also assessed among subject groups. Participants with TBM (n = 22) or aseptic meningitis (n = 16) had a significantly higher median VDBP CSF level than did patients with Cryptococcus (n = 71) or no meningitis (n = 22) (Table 1). Subjects with TBM (n = 22) had significantly higher mean VDBP concentration of 4.72 μg/ml (IRQ 2.97–8.96 μg/ml as compared with those without TBM [n = 108] as defined above) of 1.31 μg/ml (IQR: 0.55–2.86 μg/ml; p < 0.001).
VDBP was then assessed as a diagnostic biomarker. AUC as measured by receiver operator characteristic curve was 0.794 (95% CI: 0.701–0.887). Using a cutoff of 1.0 μg/ml we found a sensitivity of 91% (20/22; 95% CI: 71–99%), specificity of 38% (41/107; 95% CI: 29–48%), PPV of 23% (20/86; 95% CI: 20–27%) and NPV of 95% (41/43; 84–99%). With a cut-off value of 2.0 μg/ml, the sensitivity of VDBP was 81% (18/22; 95% CI: 60–95%), specificity 68% (73/108; 95% CI: 58–76%), PPV 34% (18/53; 95% CI: 27–42%) and NPV 95% (73/77; 95% CI: 88–98%). When cryptococcal meningitis subjects were excluded and a cutoff of 1.0 μg/ml used to compare 21 TBM subjects with 37 subjects with aseptic meningitis or no meningitis, VDBP yielded a sensitivity of 90% (95% CI: 70–99%), specificity of 46% (95% CI: 29–63%), PPV of 49% (95% CI: 41–57%), and NPV of 89% (95% CI: 68–97%). When a cut-off value of 2.0 μg/ml was used, sensitivity was 81% (95% CI: 58–95%), specificity 65% (95% CI: 47–80%), PPV 57% (95% CI: 45–68) and NPV 86% (95% CI: 71–94%). As with DLL1, though VDBP levels were more elevated in subjects with TBM than those without TBM, no cutoff had sufficient accuracy for use as a lone biomarker.
Fetuin CSF concentrations
Finally, fetuin was assessed for use as a marker of TBM. The patients with TBM (n = 22) or aseptic meningitis (n = 16) had a significantly higher median fetuin concentration than did patients with Cryptococcus (n = 71) or no meningitis (n = 22), Table 1. Subjects with TBM (n = 22) had a significantly higher median fetuin concentration of 3.30 μg/ml (IQR: 32.19–4.55 μg/ml) as compared with non-TBM participants (n = 108) whose median concentration was 1.44 μg/ml (IQR: 0.57–2.38 μg/ml; p < 0.001). AUC as measured by receiver operator characteristic curve was 0.824 (95% CI: 0.739–0.909).
Using a cutoff of 1 μg/ml for CSF fetuin, we found a sensitivity of 95% (21/22; 95% CI: 77–100%), specificity of 38% (41/108; 95% CI: 29–48%), PPV of 24% (21/88; 95% CI: 21–27%) and NPV of 98% (41/42; 95% CI: 86–100%). Using a cutoff of 2 μg/ml, we found a sensitivity of 86% (19/22; 95% CI: 65–97%), specificity of 68% (73/108; 95% CI: 58–76%), PPV of 35% (19/54; 95% CI: 28–43%) and NPV of 96% (73/76; 95% CI: 89–99%). When cryptococcal patients were excluded and a cutoff of 1 μg/ml was used to compare 21 TBM patients with 37 patients with aseptic meningitis or no meningitis fetuin gave a sensitivity of 95% (95% CI: 76–100%), specificity of 57% (95% CI: 40–73%), PPV of 56% (95% CI: 46–65%) and NPV of 95% (95% CI: 75–99%). Using a cut-off value of 2 μg/ml in this comparison yielded a sensitivity of 86% (95% CI: 64–97%, specificity of 63% (95% CI: 56–86%), PPV of 53% (95% CI: 51–76%) and NPV of 90% (95% CI: 76–96%). As with fetuin and DLL1, though fetuin levels were more elevated in TBM as compared with those without TBM, the marker was not a suitable lone diagnostic marker for TBM.
Correlation
Spearman correlation showed moderate correlation between DLL1 and both VDBP (Rho = 0.521) and fetuin (Rho = 0.593). VDBP was strongly correlated with fetuin (Rho = 0.754). In assessing correlation with CSF cytokines/chemokines, nearly all cytokines and chemokines positively correlated with DLL1, VDBP and fetuin (Table 2), except for IL-5 and MCP-1 (CCL2).
Table 2. . Correlation of 17 cerebrospinal fluid cytokines/chemokines with DLL1, vitamin D binding protein and fetuin.
| Biomarker | n | DLL1 | VDBP | Fetuin |
|---|---|---|---|---|
| DLL1 | 136 | – | 0.521 (<0.001) | 0.593 (<0.001) |
| VDBP | 130 | 0.521 (<0.001) | – | 0.754 (<0.001) |
| Fetuin | 130 | 0.593 (<0.001) | 0.754 (<0.001) | – |
| IL-β | 112 | 0.579 (<0.001) | 0.494 (<0.001) | 0.602 (<0.001) |
| IL-2 | 123 | 0.356 (<0.001) | 0.344 (0.001) | 0.400 (<0.001) |
| IL-4 | 123 | 0.384 (<0.001) | 0.342 (0.005) | 0.470 (<0.001) |
| IL-5 | 112 | 0.173 (0.068) | 0.100 (0.296) | 0.261 (0.005) |
| IL-6 | 123 | 0.568 (<0.001) | 0.579 (<0.001) | 0.578 (<0.001) |
| IL-7 | 112 | 0.332 (<0.001) | 0.289 (0.002) | 0.330 (<0.001) |
| IL-8 | 123 | 0.578 (<0.001) | 0.500 (<0.001) | 0.517 (<0.001) |
| IL-10 | 123 | 0.415 (<0.001) | 0.397 (<0.001) | 0.465 (<0.001) |
| IL-12 | 112 | 0.408 (0.001) | 0.217 (0.021) | 0.361 (<0.001) |
| IL-13 | 112 | 0.493 (<0.001) | 0.542 (<.001) | 0.623 (<0.001) |
| IL-17 | 112 | 0.375 (<0.001) | 0.258 (0.006) | 0.386 (<0.001) |
| G-CSF | 112 | 0.467 (<0.001) | 0.391 (<0.001) | 0.507 (<0.001) |
| GM-CSF | 123 | 0.182 (0.044) | 0.347 (<0.001) | 0.225 (0.012) |
| MIP-1β | 130 | 0.407 (<0.001) | 0.230 (0.015) | 0.404 (<0.001) |
| MCP-1 | 112 | 0.101 (0.291) | -0.116 (0.224) | -0.114 (0.230) |
| IFN-γ | 123 | 0.455 (<0.001) | 0.450 (<0.001) | 0.579 (<0.001) |
| TNF-α | 123 | 0.426 (<0.001) | 0.287 (0.004) | 0.389 (<0.001) |
Correlation by nonparametric Spearman correlation, Rho (ρ). Values are Rho (p-value).
Multivariable analysis
We performed logistic regression to assess the performance of combinations of CSF biomarkers (n = 127). Using logistic regression, DLL1 alone, with a cut-off value of approximately 1150 pg/ml, provided 32% (95% CI: 14–55%) sensitivity, 98% (95% CI: 93–100%) specificity, 78% (95% CI: 44–94%) PPV and 87% (95% CI: 84–90%) NPV. When analyzing the combined accuracy of both the DLL1 and fetuin assays for diagnosis of TB meningitis, sensitivity improved slightly to 36% (95% CI: 17–59%). Further incorporating negative cryptococcal antigen testing improved sensitivity to 63% (95% CI: 41–83%) though with decreased specificity (90%, 95% CI: 83–95%). Adding IFN-γ measurement improved sensitivity to 76% (95% CI: 53–92%) with 92% (95% CI: 85–97%) specificity, 67% (95% CI: 50–80%) PPV and 95% (95% CI: 90–98%) NPV. DLL1 combined with cryptococcal antigen testing and IFN-γ provided 71% (95% CI: 48–89%) sensitivity, 93% (95% CI: 86–97%) specificity, 68% (95% CI: 50–82%) PPV and 94% (95% CI: 89–97%) NPV. Further adding IL-1β slightly improved specificity to 95% (95% CI: 88–98%) and PPV to 75% (95% CI: 55–88%). Other cytokines/chemokines were analyzed separately but were not predictive of TBM.
For every twofold increase in DLL1, the odds of TBM increased (odds ratio [OR]: 2.9; 95% CI: 1.3–6.5; p = .013). A similar but not-statistically significant relationship was noted with fetuin (OR: 1.8; 95% CI: .99–3.4; p = .054). Incorporating VDBP, CSF white cell count, or CSF protein into a predictive model did not improve diagnostic performance.
Among 117 persons with known outcome, in-hospital mortality was positively associated with DLL1 CSF levels (OR: 2.0 per twofold increase, 95% CI: 1.2–3.4; p = .008) and inversely associated with fetuin (OR: 0.67; 95% CI: 0.47–0.96; p = 0.030).
Discussion
We have shown that concentrations of DLL1, VDBP and fetuin measured on CSF in an HIV-infected Ugandan population are significantly higher in patients with microbiologically proven TBM or aseptic meningitis than in the patients with cryptococcal meningitis or no meningitis. DLL1, VDBP and fetuin concentrations were also higher in subjects with TBM compared with all patients without TBM in the same population. DLL1 alone was statistically associated with TBM diagnosis, but there was no single threshold that provided good diagnostic performance, as higher levels provided a very specific biomarker with poor sensitivity and lower thresholds of DLL increased sensitivity but lost specificity. Combining DLL1 and fetuin, or subsequently adding cryptococcal antigen testing did not adequately improve sensitivity to allow for routine use. DLL1, fetuin and VDBP have a statistical association with TBM but lack clinical utility.
Perhaps most encouraging was the use of only cryptococcal status, along with DLL1 and IFN-γ – this array of diagnostics provided nearly equivalent sensitivity compared with when fetuin was included as well, but increased specificity, PPV with stable NPV. Similarly, the advantage of adding IL-1β was that there was slight improvement in PPV and specificity; however, in either case, using one less test would save cost and time. Adding CSF VDBP to the model did not improve diagnostic performance. Using particular lower cut-off values rather than higher cutoff used in logistic regression improved performance of all three tests in terms of sensitivity and NPV but with large sacrifices in PPV and specificity.
Though these novel biomarkers were not adequate to rely on by themselves, they may hold promise when used with other markers as part of a diagnostic algorithm. When subjects with cryptococcal meningitis were excluded from the analysis, sensitivity improved though specificity decreased slightly. That said, when IFN-γ was added, the sensitivity improved further and some specificity was recovered. Given the impressive accuracy, speed and low cost of the cryptococcal antigen lateral flow assay [31] exclusion of Cryptococcus should always be considered prior to TBM testing [33]. Our analysis would suggest that doing so would improve the likelihood of a positive result being correct, while worsening the specificity; giving this test less utility as a ‘rule-out’ test, but allowing for more confidence in its use to detect TBM. Adding IFN-γ, measurement allows for even more sensitivity and improved overall accuracy but adds further cost.
Importantly, we used culture or Xpert as our TBM gold standard. Culture has approximately 60% sensitivity, while GeneXpert MTB/Rif has been shown to have approximately 40–70% sensitivity [10,34,35] when testing CSF specimens to detect TBM. The gold standard tests (e.g., culture or nucleic acid amplification tests) one might compare a new diagnostic test to clearly do not have adequate sensitivity. Clinical criteria have been developed and tested to attempt to remedy this issue, but they generally include as probable cases of TBM, cases which cannot be microbiologically confirmed. Thus, whether a study uses microbiology testing or clinical criteria; the new diagnostic test is compared with an imperfect standard. Though not available at the time of this study, Xpert MTB/Rif Ultra has shown higher sensitivity in one study (70% vs clinical criteria and 95% vs microbiologic composite standard) [9].
In our study, we used microbiological confirmation with a very specific test as our gold standard so that we could be certain that all tests we designated as TBM were in fact TBM. Yet, it is likely that among the aseptic meningitis group in our study, some patients may have actually had TBM. Given our relatively small sample size, a small number of patients with high DLL1 values (who tested negative by Xpert but who had TBM) would be enough to draw the group’s mean DLL1 concentration closer to that of the TBM group. Table 3 describes such patients that we suspect may have had TBM though we were not able to confirm this microbiologically. The same issue should be considered with VDBP and fetuin.
Table 3. . Selected subjects with high DLL1 values and without microbiologic evidence of tuberculosis meningitis or Cryptococcus.
| Clinical meningitis diagnosis | DLL1 pg/ml | VDBP μg/ml | Fetuin μg/ml | CSF WBC cells/μl | CSF protein mg/dl | IFN-γ pg/ml | Alive at hospital discharge |
|---|---|---|---|---|---|---|---|
| Unknown, EBV+ | 744 | 3.5 | 3.5 | 50 | 229 | 9.0 | No |
| Cryptococcus, pulmonary TB | 710 | 7.3 | 4.2 | 60 | 150 | 60 | Yes |
| S. pneumoniae | 782 | 18.7 | 3.1 | 800 | 276 | – | Yes |
| Unknown | 1009 | 17.3 | 3.2 | <5 | 173 | – | Left AMA |
| Unknown | 1364 | 9.6 | 2.0 | 2580 | 120 | – | No |
| Viral | 655 | 3.0 | 3.5 | 45 | 279 | 310 | Unknown |
| Unknown | 649 | 3.4 | 2.7 | 100 | 443 | 1.1 | Left AMA |
| Putative TBM | 622 | 5.8 | 4.9 | 352 | 283 | 8.5 | No |
| Putative TBM | 886 | 9.1 | 5.4 | 310 | 250 | 10.4 | Yes |
| Unknown, EBV+ | 1404 | 14.1 | 17.8 | 75 | 20 | 1.3 | Unknown |
| Unknown | 687 | 0.36 | 1.1 | 25 | 40 | 140 | No |
Participants selected for this analysis when DLL1 was >600 pg/ml without confirmed TBM. Clinical diagnosis was made by the treating physicians with information available at the time.
AMA: Against medical advice; CSF: Cerebrospinal fluid; EVB: Epstein–Barr virus; TB: Mycobacterium tuberculosis; TBM: Tuberculosis meningitis; WBC: White blood cell.
Interestingly, one study of patients with pulmonary M. tuberculosis infection found lower levels of VDBP in the patients of African ancestry as opposed to those of Eurasian ancestry [36]. Thus, it may be possible that VDBP may be more sensitive in the Eurasian population as opposed to our African population, although it should be noted that this analysis measured serum VDBP as opposed to CSF. Similarly, our population was HIV-infected Ugandan subjects, possibly contributing to difference in DLL-1 performance compared against largely HIV noninfected Asian patients [18–21]. Fundamentally, this speaks to the need to validate putative biomarkers in diverse genetic populations with and without HIV infection.
Limitations of this study include its retrospective design, relatively small numbers of subjects with TBM and the limited sensitivity of our gold standard with potential for misclassification bias. Though, it should be noted that the main limitation of these potential biomarkers was lack of sensitivity, not lack of specificity as would be influenced by misclassification bias. Additionally, all three tests would require further study including external validation, and the current study cannot state whether or not DLL1, VDBP and fetuin levels (alone or in combination) predictably decrease with antimycobacterial therapy. Finally, most patients were HIV-infected, and so the study is not generalizable to HIV-uninfected populations.
In summary, CSF DLL1, VDBP and fetuin appear to have statistical association with tuberculosis meningitis individually, but lack clinical utility. AUC for DLL1 was 0.789, 0.794 for VDBP, and 0.824 for fetuin. When logistic regression was used, we found 71% sensitivity, 93% specificity, 68% PPV and 94% NPV by using a combination of DLL1, IFN-γ and cryptococcal antigen testing. Furthermore, the unique mechanism by which DLL1, VDBP and fetuin might serve as a method for diagnosing TBM may allow for the possibility of an additive effect with current or future diagnostic tests.
Summary points.
TB meningitis is difficult to diagnose due, in large part, to the presence of relatively few TB bacilli in the cerebrospinal fluid (CSF).
Novel biomarkers DLL1, VDBP, and fetuin were evaluated in CSF for TB meningitis.
Stored CSF samples were analyzed from 139 HIV-infected Ugandans suspected to have meningitis.
Diagnoses included TB meningitis (n = 22), cryptococcal meningitis (n = 71), aseptic meningitis (n = 16) and no meningitis (n = 30).
DLL1 at a cut-off value of 1150 pg/ml provided 32% sensitivity and 98% specificity.
DLL1, fetuin, negative cryptococcal antigen and IFN-γ in combination provided 76% sensitivity and 92% specificity.
VDBP (cut-off value 2.0 μg/ml) provided 81% sensitivity and 68% specificity.
Fetuin (cut-off value 2 μg/ml) provided a sensitivity of 86% and specificity of 68%.
No single novel marker or combination provided adequate diagnostic accuracy to warrant large scale study for diagnosis of TB meningitis.
Footnotes
Financial & competing interests disclosure
This research was made possible through support from the Fogarty International Center (R25TW009345), the National Institute of Neurologic Diseases and Stroke (R01NS086312) and the National Institute of Allergy and Infectious Diseases (U01AI089244). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Vinnard C, MacGregor RR. Tuberculous meningitis in HIV-infected individuals. Curr. HIV/AIDS Rep. 2009;6(3):139–145. doi: 10.1007/s11904-009-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thwaites GE, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010. doi: 10.1016/S1474-4422(13)70168-6. [DOI] [PubMed] [Google Scholar]
- 3.Verdon R, Chevret S, Laissy JP, Wolff M. Tuberculous meningitis in adults: review of 48 cases. Clin. Infect. Dis. 1996;22(6):982–988. doi: 10.1093/clinids/22.6.982. [DOI] [PubMed] [Google Scholar]
- 4.Youssef FG, Afifi SA, Azab AM, et al. Differentiation of tuberculous meningitis from acute bacterial meningitis using simple clinical and laboratory parameters. Diagn. Microbiol. Infect. Dis. 2006;55(4):275–278. doi: 10.1016/j.diagmicrobio.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Kalita J, Misra UK. Outcome of tuberculous meningitis at 6 and 12 months: a multiple regression analysis. Int. J. Tuberc. Lung Dis. 1999;3(3):261–265. [PubMed] [Google Scholar]
- 6.Ho J, Marais BJ, Gilbert GL, Ralph AP. Diagnosing tuberculous meningitis – have we made any progress? Trop. Med. Int. Health. 2013;18(6):783–793. doi: 10.1111/tmi.12099. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer J, Moreno S, Laguna F, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 1992;326(10):668–672. doi: 10.1056/NEJM199203053261004. [DOI] [PubMed] [Google Scholar]
- 8.Caws M, Thwaites GE, Duy PM, et al. Molecular analysis of Mycobacterium tuberculosis causing multidrug-resistant tuberculosis meningitis. Int. J. Tuberc. Lung Dis. 2007;11(2):202–208. [PubMed] [Google Scholar]
- 9.Bahr NC, Nuwagira E, Evans EE, et al. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect. Dis. 2018;18(1):68–75. doi: 10.1016/S1473-3099(17)30474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study to evaluate GeneXpert MTB/Rif Ultra for TB meningitis and while needing confirmation in other studies as of this writing may represent a major step forward in the diagnosis of TB meningitis.
- 10.Bahr NC, Tugume L, Rajasingham R, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert((R)) MTB/RIF of centrifuged CSF. Int. J. Tuberc. Lung Dis. 2015;19(10):1209–1215. doi: 10.5588/ijtld.15.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 12.Han W, Ye Q, Moore MA. A soluble form of human delta-like-1 inhibits differentiation of hematopoietic progenitor cells. Blood. 2000;95(5):1616–1625. [PubMed] [Google Scholar]
- 13.Abdallah BM, Ding M, Jensen CH, et al. Dlk1/FA1 is a novel endocrine regulator of bone and fat mass and its serum level is modulated by growth hormone. Endocrinology. 2007;148(7):3111–3121. doi: 10.1210/en.2007-0171. [DOI] [PubMed] [Google Scholar]
- 14.Bansal-Mutalik R, Nikaido H. Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacyl phosphatidylinositol dimannosides. Proc. Natl Acad. Sci. USA. 2014;111(13):4958–4963. doi: 10.1073/pnas.1403078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maekawa Y, Tsukumo S, Chiba S, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19(4):549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra S, Fakiola M, Mishra A, et al. Genetic and functional evaluation of the role of DLL1 in susceptibility to visceral leishmaniasis in India. Infect. Genet. Evol. 2012;12(6):1195–1201. doi: 10.1016/j.meegid.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matucci A, Maggi E, Vultaggio A. Cellular and humoral immune responses during tuberculosis infection: useful knowledge in the era of biological agents. J. Rheumatol. Suppl. 2014;91:17–23. doi: 10.3899/jrheum.140098. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, Wang C, Zhang G, Peng T. The clinical value of cerebrospinal fluid of notch1 of DLL1 detection of infectious diseases of the central nervous system. Life Sci. J. 2013;10(1):5. [Google Scholar]
- 19.Peng T, Zhou Y, Li J, Li J, Wan W, Jia Y. Detection of delta-like 1 ligand for the diagnosis of tuberculous meningitis: an effective and rapid diagnostic method. J. Int. Med. Res. 2014;42(3):728–736. doi: 10.1177/0300060513498669. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Li J, Jia Y. Levels of soluble delta-like ligand 1 in the serum and cerebrospinal fluid of tuberculosis meningitis patients. Neural Regen. Res. 2012;7(11):874–878. doi: 10.3969/j.issn.1673-5374.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li JY, Jia YJ, Jia YL, et al. [Significance of soluble DLL1 in diagnosis of intracranial infectious diseases in children] Zhongguo Dang Dai Er Ke Za Zhi. 2011;13(3):205–207. [PubMed] [Google Scholar]
- 22.Adams JS, Chen H, Chun R, et al. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J. Bone Miner Res. 2007;22(Suppl. 2):V20–V24. doi: 10.1359/jbmr.07s214. [DOI] [PubMed] [Google Scholar]
- 23.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol. Metab. 2000;11(8):320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 24.Lamont EA, Janagama HK, Ribeiro-Lima J, et al. Circulating Mycobacterium bovis peptides and host response proteins as biomarkers for unambiguous detection of subclinical infection. J. Clin. Microbiol. 2014;52(2):536–543. doi: 10.1128/JCM.02433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seth M, Lamont EA, Janagama HK, et al. Biomarker discovery in subclinical mycobacterial infections of cattle. PLoS ONE. 2009;4(5):e5478. doi: 10.1371/journal.pone.0005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Sama AE. Anti-inflammatory role of fetuin-A in injury and infection. Curr. Mol. Med. 2012;12(5):625–633. doi: 10.2174/156652412800620039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Zhu S, Li J, et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS ONE. 2011;6(2):e16945. doi: 10.1371/journal.pone.0016945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Sakurada S, Kano K, et al. Identification of tuberculosis-associated proteins in whole blood supernatant. BMC Infect. Dis. 2011;11:71. doi: 10.1186/1471-2334-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 2014;370(26):2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhein J, Morawski BM, Hullsiek KH, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect. Dis. 2016;16(7):809–818. doi: 10.1016/S1473-3099(16)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulware DR, Rolfes MA, Rajasingham R, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg. Infect. Dis. 2014;20(1):45–53. doi: 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect. Dis. 2010;10(11):803–812. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]; • Established consensus research case definitions for TB meningitis.
- 33.Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost–effective diagnostic checklists for meningitis in resource-limited settings. J. Acquir. Immune Defic. Syndr. 2013;63(3):e101–e108. doi: 10.1097/QAI.0b013e31828e1e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nhu NT, Heemskerk D, Thu do DA, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J. Clin. Microbiol. 2014;52(1):226–233. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Second major cohort study to evaluate GeneXpert MTB/Rif for diagnosis of TB meningitis.
- 35.Patel VB, Theron G, Lenders L, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med. 2013;10(10) doi: 10.1371/journal.pmed.1001536. e1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First major cohort study to evaluate GeneXpert MTB/Rif for diagnosis of TB meningitis.
- 36.Coussens AK, Wilkinson RJ, Nikolayevskyy V, et al. Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathog. 2013;9(7) doi: 10.1371/journal.ppat.1003468. e1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]