Abstract
Aim:
To investigate the role of SDH2 in Candida albicans filamentation and virulence.
Materials & methods:
Caenorhabditis elegans and mouse candidiasis models were used to assess the virulence of a sdh2Δ/Δ mutant. Various hypha-inducing media were used to evaluate the hyphal development of C. albicans. DCFH-DA was used to measure intracellular Reactive Oxygen Species (ROS) levels.
Results:
The sdh2Δ/Δ mutant was avirulent in the C. elegans model, hypovirulent in a murine candidiasis model, and defective to form filaments both in vitro and in vivo. Intracellular ROS level increased in the sdh2Δ/Δ mutant, and the filamentation defects of sdh2Δ/Δ were rescued by decreasing intracellular ROS.
Conclusion:
SDH2 plays an important role in C. albicans filamentation and virulence probably through affecting intracellular ROS.
Keywords: : Candida albicans, morphogenesis, succinate dehydrogenase, virulence
Candida albicans is a major fungal pathogen, causing diseases varying from superficial mucosal disorders to life-threatening bloodstream infections with a more than 40% mortality rate [1,2]. In contrast to the model yeast Saccharomyces cerevisiae which preferentially ferments, C. albicans is a Crabtree-negative species exclusively using respiration when oxygen is available [3]. Respiration is comprised of a set of metabolic reactions and processes including glycolysis, tricarboxylic acid (TCA) cycle, electron transport chain (ETC), and ATP synthesis [3] and appears to play a significant role in C. albicans biology [4–7]. Several respiration-related genes contribute to the morphological transition and pathogenicity of C. albicans [4–7]. For example, deletion of CaNDH51 [6], a gene functioning in utilizing NADH for ATP production, results in filamentation defects. Also, deletion of the mitochondrion-related genes GOA1 [4] and MCU1 [5] causes respiration defects and strikingly attenuated virulence in a murine candidiasis model. It is still unclear whether the contributions of CaNDH51, GOA1 and MCU1 to filamentation and virulence are based on their roles in ATP production.
We have developed a Caenorhabditis elegans-based infection model that offers a different approach for high-throughput screening work and yeast-to-hypha transition studies [8–10]. In this study, we utilized this model system in order to investigate the role of SDH2 that encodes a putative iron-sulfur subunit of succinate dehydrogenase (SDH, also named respiratory complex II), which is involved in both the TCA cycle and the ETC [11,12].
Materials & methods
Strains & growth conditions
Caenorhabditis elegans glp-4; sek-1 strain was propagated on nematode growth medium on lawns of Escherichia coli OP50 by using standard methods [8]. The C. albicans strains used in this study and the generation of the sdh2Δ/Δ mutant, the SDH2 reintegrated strain, the fum12Δ/Δ and adh1Δ/Δ mutant are described in Supplementary Table 1 and Text 1. Candida albicans strains were routinely propagated in YPD (1% yeast extract, 2% peptone and 2% dextrose) liquid medium at 30°C in a shaking incubator. To investigate the role of SDH2 in yeast-to-hypha morphological transition, C. albicans strains were grown in media known to induce the morphological transition, including YPD + serum, Lee, Spider, Spider + glucose and YPS. YPD + serum medium contains YPD and 10% (vol/vol) fetal calf serum. Lee medium is rich in amino acids and glucose. Spider medium contains 1% (wt/vol) nutrient broth, 1% (wt/vol) mannitol and 0.2% (wt/vol) K2HPO4. Spider + glucose medium is Spider medium with 100 mM glucose supplemented. YPS medium contains 1% yeast extract, 2% peptone, 2% sucrose and 1% agar [13].
Virulence assays using a Caenorhabditis elegans candidiasis model
As described previously [8], approximately 400 synchronized adult C. elegans glp-4; sek-1 nematodes were added to the center of the C. albicans lawns on brain heart infusion (BHI) media and incubated at 25°C for 4 h [14]. After a careful wash, worms were pipetted into wells of six-well tissue culture plates containing 2 ml of liquid medium (80% M9, 20% BHI) and kanamycin (45 μg/ml). Dead worms were scored and removed daily.
Virulence assay using a murine candidiasis model
Mice were infected with C. albicans according to an established protocol [15]. CD-1 female mice (6-week old, 18–22 g) were infected with 1.5 × 106 colony-forming units (CFUs) of C. albicans suspended in Phosphate-Buffered Saline (PBS) via a tail vein injection in a 100 μl volume. The murine protocol was approved by the Massachusetts General Hospital Committee on Research, Subcommittee on Research Animal Care (SRAC; OLAW Assurance # A3596-01). The protocol (2011N000175) conformed to the USDA Animal Welfare Act, Policy on Humane Care and Use of Laboratory Animals (PHS Policy), the ILAR Guide for the Care and Use of Laboratory Animals and other applicable laws and regulations. Special attention was given to minimize the suffering of the mice.
Kidney CFU assay
The kidney CFU assay was performed to assess the fungal burden in the kidneys. The kidney is the most frequent target of C. albicans, and the kidney CFU assay is the most commonly used method to investigate the infection with C. albicans [16,17]. Briefly, fungal cells were prepared and injected as described above for the disseminated murine candidiasis model. 2 days after inoculation, kidneys were removed aseptically, weighed and homogenized in 1 ml sterile PBS. Serial dilutions were plated on YPD agar with antibiotics to determine the CFU/g kidney.
Fungal cells staining with periodic acid Schiff
Histopathological analysis was performed to assess kidney damage. Kidney tissue was fixed in 10% neutral buffered formalin and embedded in paraffin. Thin sections were stained using periodic acid Schiff (PAS) to reveal the hyphal structure of the fungal pathogens [17].
Measurement of intracellular ethanol content
Candida albicans cells were grown on Spider + glucose (100 mM) at 30°C for 5 day or YPD + serum medium at 37°C for 3 day. Candida albicans cells (0.1–0.3 g) were picked up, accurately weighted, and suspended with 1 ml sterilized water. Glass beads (0.5 mm, 700 μl) were added to each suspension, and the C. albicans cells were lysed by vigorous vortexing, interrupted by cooling on ice. Glass beads were removed by centrifugation and the ethanol concentrations in the supernatants were determined by Enzytec™ fluid ethanol kit (R-Biopharm AG, Darmstadt, Germany).
Measurement of intracellular ATP levels
The experiment was carried out according to the protocol described previously [18]. Candida albicans cells were adjusted to 1 × 106 cells/ml with culture medium and incubated at 30°C with shaking for 30 min. Intracellular ATP was detected using BacTiter-Glo reagent (Promega, WI, USA). Luminescent signals were determined on a Tecan Infinite® 200 Pro Multilabel Counter. A standard curve for ATP increments (from 100 nM to 10 pM) was constructed, and the ATP contents were calculated from the standard curve.
Measurement of intracellular ROS levels
Intracellular ROS levels were assessed as reported before [18–20]. In each group, C. albicans cells (1 × 107 cells/ml) were incubated with 20 μg/ml of 2,7-dichlorofluorescin diacetate (DCFH-DA; Molecular Probes, Eugene, OR, USA) at 30°C for 3 h. After being washed and resuspended in PBS buffer, 100 μl C. albicans sample was used for intracellular ROS measurement. Fluorescence intensity (FI) values were detected on the Infinite 200 Pro (Tecan, Männedorf, Switzerland) with excitation wavelength at 480 nm and emission wavelength at 530 nm. The detected FI value can reflect the intracellular ROS level. ROS levels were assessed by subtracting the FI value of cells without DCFH-DA from that of cells with DCFH-DA.
Statistical analysis
Statistical analyses were performed using analysis of variance (ANOVA) and post hoc (Bonferroni and Student–Newman–Keuls’) tests. Animal survival was examined using the Kaplan–Meier method and differences were determined using the log-rank test (STATA 6; STATA, TX, USA). A p-value <0.05 was considered statistically significant.
Results
SDH2 is required for C. albicans virulence in vivo
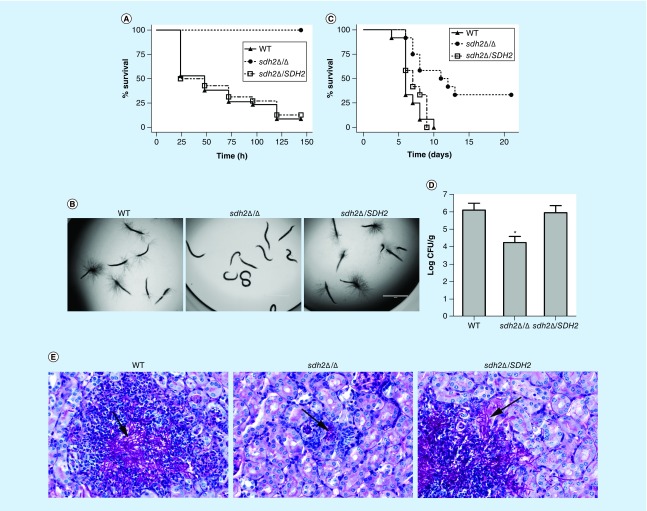
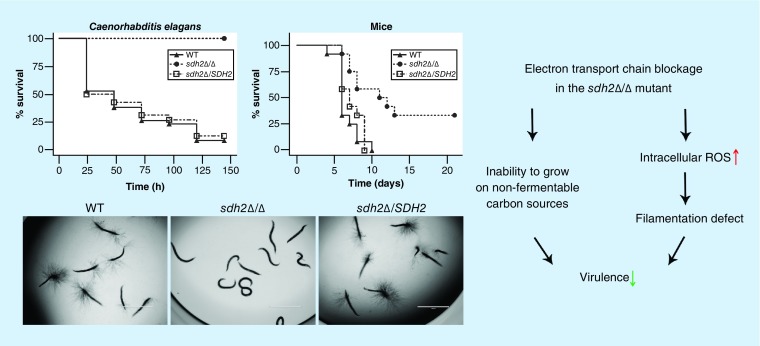
We used the C. elegans candidiasis model to screen libraries of homozygous mutants from the Fungal Genetics Stock Center (http://www.fgsc.net/candida/FGSCcandidaresources.htm). Of particular interest was the identification of a mutant lacking SDH2 that was avirulent in this model; the mutant lacked hypha piercing the worm cuticle (data not shown). To confirm the role of SDH2 in the virulence of C. albicans, we constructed a sdh2Δ/Δ mutant and the reintegrated strain sdh2Δ/SDH2 using the SAT1 flipping method [21] (Supplementary Table 1, Text 1). The growth of the wild-type, sdh2Δ/Δ and sdh2Δ/SDH2 strains in routine YPD culture medium and RPMI 1640 medium was monitored and there was no growth defect (Supplementary Table 2). In the C. elegans candidiasis model, more than 60% of the worms died within the first 48 h after infection with the wild-type or the reintegrated strains (Figure 1A), while at 120 h, more than 85% of the worms were dead, and every dead worm had visible hyphae piercing the cuticle (Figure 1B). In contrast, no dead worms and no hyphae were observed in the sdh2Δ/Δ group (Figure 1A & B).
Figure 1. . The sdh2Δ/Δ mutant is avirulent in the Caenorhabditis elegans candidiasis model and hypovirulent in the murine candidiasis model.
(A) Caenorhabditis elegans survival was followed for 144 h after Candida albicans infection (n = 60–70 worms per strain of C. albicans). Compared with the C. albicans wild-type strain SC5314, the sdh2Δ/Δ mutant is avirulent to C. elegans (p < 0.001). The reintegration of SDH2 restored the virulence of C. albicans to wild-type levels. (B) The C. elegans glp-4; sek-1 nematodes were infected by C. albicans wild-type, sdh2Δ/Δ or sdh2Δ/SDH2 strain, respectively. On day 3, the worms were photographed. (C) Mice survival was followed over 21 days (n = 12 mice per strain of C. albicans). Mice were inoculated via the tail vein with 1.5 × 106 CFUs of the indicated strains, and observed twice daily. Compared with the wild-type strain, the sdh2Δ/Δ mutant is hypovirulent (p < 0.01). (D) Fungal burden of the kidneys of mice infected with the wild-type, sdh2Δ/Δ and sdh2Δ/SDH2 reintegrated strains 2 days after inoculation (n = 4 mice per strain of C. albicans). Statistical analyses were performed using ANOVA and post hoc (Bonferroni and Student–Newman–Keuls’) tests. p < 0.05 was considered significant. *p < 0.05 compared with mice infected with the wild-type strain. (E) PAS stained thin sections of kidneys 2 days after inoculation with the indicated strains of C. albicans demonstrate differences in filamentation within the organ. Tissues were examined microscopically. Arrows indicate C. albicans filaments in the tissues.
CFU: Colony-forming unit; PAS: Periodic acid Schiff.
In a murine candidiasis model, the survival data indicated that the sdh2Δ/Δ mutant was significantly less virulent than the wild-type strain (p < 0.01; Figure 1C). The CFU data indicated that the fungal burden in the mouse kidneys was less in the sdh2Δ/Δ mutant infected group compared with the control group (p < 0.05; Figure 1D). PAS staining of the mouse kidneys indicated clusters of hyphae in the wild-type and reintegrated strain infection groups. In contrast, no obvious hyphal cluster was observed in the sdh2Δ/Δ group (Figure 1E). Collectively, the sdh2Δ/Δ mutant was avirulent in the C. elegans candidiasis model and hypovirulent in the murine candidiasis model.
The sdh2Δ/Δ mutant exhibits filamentation defects
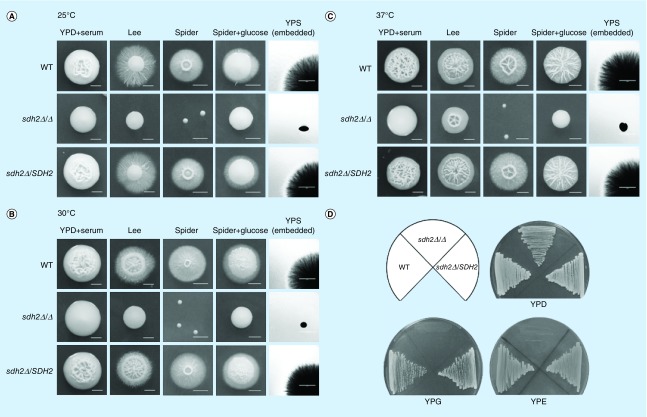
As described above, the sdh2Δ/Δ mutant did not result in the characteristic puncture of filaments through the C. elegans cuticle or in obvious hyphal clusters within the kidneys of mice. To study the invasive hyphal development, we used an agar system that mimics some of the features of tissue invasion [22,23]. The sdh2Δ/Δ mutant displayed severe filamentation defects on/within all the hypha-inducing agar media tested, including YPD + serum, Lee, Spider (with mannitol as carbon source), Spider + glucose and YPS. More specifically, the sdh2Δ/Δ mutant formed smooth-edged colonies, in contrast to wild-type and the reintegrated strains that developed wrinkled colonies and radial hyphae at all tested temperatures (25, 30 and 37°C) (Figure 2A–C), although the sdh2Δ/Δ colonies were still wrinkled at 37°C (Figure 2C).
Figure 2. . The sdh2Δ/Δ mutant exhibits filamentation defects.
(A) Filamentous growth of Candida albicans at 25°C. Times of incubation were as follows: YPD + serum, 9 days; Lee, 11 days; Spider, 11 days; Spider + glucose, 8 days; YPS, 3 days. (B) Filamentous growth of C. albicans at 30°C. Times of incubation were as follows: YPD + serum, 6 days; Lee, 9 days; Spider, 9 days; Spider + glucose, 5 days; YPS, 3 days. (C) Filamentous growth of C. albicans at 37°C. Times of incubation were as follows: YPD + serum, 3 days; Lee, 6 days; Spider, 6 days; Spider + glucose, 4 days; YPS, 3 days. (D) The sdh2Δ/Δ mutant cannot grow on nonfermentable carbon sources.
YPD: Contains fermentable glucose as carbon source; YPE: Contains nonfermentable ethanol as carbon source; YPG: Contains nonfermentable glycerol as carbon source.
In addition, the sdh2Δ/Δ colonies appeared to be small on Spider agar (with nonfermentable mannitol as carbon source), suggesting a specific growth defect on this medium. Moreover, after incubation at 30°C for 24 h, no obvious growth was observed of the sdh2Δ/Δ mutant on YPG (with nonfermentable glycerol as carbon source) and YPE (with nonfermentable ethanol as carbon source) agar (Figure 2D). In contrast, the sdh2Δ/Δ mutant grew on YPD (with fermentable glucose as carbon source), YPM (with fermentable maltose as carbon source) and YPS (with fermentable sucrose as carbon source) agar (Supplementary Figure 1), suggesting that the sdh2Δ/Δ mutant prefers fermentable carbon sources.
Ethanol accumulates in sdh2Δ/Δ cells grown on a fermentable carbon source, but this is not responsible for their defect in filamentation
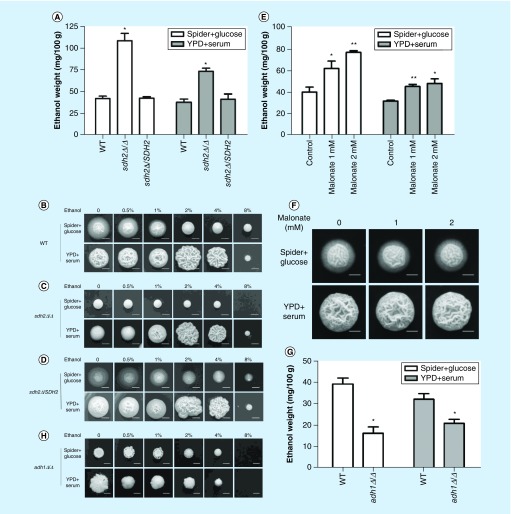
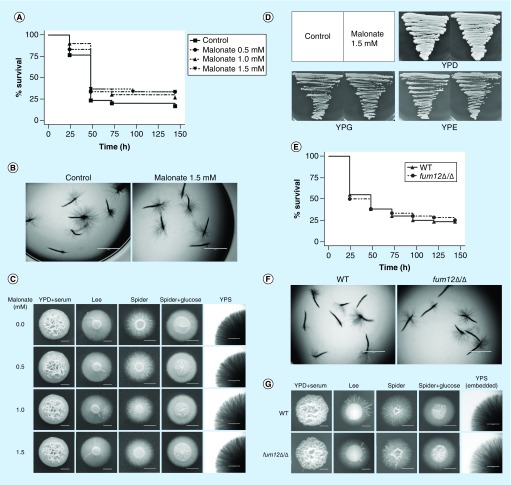
Based on the findings above, our initial hypothesis was that the sdh2Δ/Δ mutant depends on fermentation for growth. Since ethanol is the main product of fermentation in yeast [24], we measured the ethanol content within different C. albicans colonies. As expected, ethanol content significantly increased in the sdh2Δ/Δ mutant, compared with the wild-type strain grown on hypha-inducing media (Spider + glucose, p < 0.05; YPD + serum, p < 0.05; Figure 3A).
Figure 3. . Ethanol accumulates in the sdh2Δ/Δ mutant with fermentable carbon source, but this is not related to the filamentation defects of the sdh2Δ/Δ mutant.
(A) The deletion of SDH2 results in ethanol accumulation in Candida albicans. The ethanol content per 100 g C. albicans is shown. *p < 0.05 compared with the wild-type control group. (B–D) Ethanol did not inhibit the filamentation of C. albicans in a dose-dependent manner. Ethanol at a range of concentrations was added to the media. WT, sdh2Δ/Δ, sdh2Δ/SDH2 cells plated on Spider + glucose medium were incubated at 30°C for 3 days; C. albicans on YPD: + serum medium was incubated at 37°C for 3 days. **p < 0.01 compared with the control group without treatment. (E & F) Malonate treatment affects ethanol accumulation in C. albicans, but the treatment does not result in filamentation defect. (G & H) The adh1Δ/Δ mutant has less intracellular ethanol content, while this strain does not exhibit stronger filamentation phenotype.
Previous studies reported that ethanol could inhibit germ tube formation and the elongation of germ tubes [25]. To investigate whether the increased ethanol content is associated with the filamentation defects of C. albicans, we supplemented ethanol into hypha-inducing media. Unexpectedly, ethanol did not inhibit the filamentation of C. albicans in a dose-dependent manner, and 2 and 4% ethanol even enhanced the filamentous growth of C. albicans on YPD + serum medium (Figure 3B–D). To confirm that ethanol could not effectively suppress filamentation, we used malonate to disrupt the TCA cycle and thereby increase the intracellular ethanol content [26]. Although ethanol indeed accumulated in malonate-treated cells (Figure 3E), this was not accompanied by a defect in filamentation (Figure 3F). Moreover, we examined the adh1Δ/Δ mutant, which despite having a lower ethanol content (Figure 3G), actually displays a defect in filamentation rather than an enhancement (Figure 3H). Taken together, these findings suggested that ethanol accumulation is not associated with the filamentation defects of C. albicans. Thus, another mechanism must account for the filamentation defects of the sdh2Δ/Δ mutant.
A succinate dehydrogenase inhibitor specifically disrupting the electron transport chain mimics the impact of SDH2 deletion
As a succinate: ubiquinone oxidoreductase, SDH has two distinct inhibitors: malonate and carboxin [27,28]. Malonate is an analog of succinate that binds to the site where succinate normally binds, inhibiting the TCA cycle [12,26–27], while carboxin selectively binds to the site where quinone normally binds, disrupting respiration by interfering with the ETC [12].
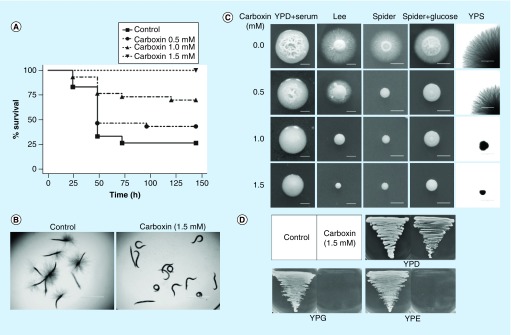
Since SDH2 is predicted to encode an iron–sulfur subunit of SDH that is involved in both the TCA cycle and the ETC [11,12], we used carboxin, an SDH inhibitor targeting the ETC [12], to investigate if the virulence and filamentation defects of the sdh2Δ/Δ mutant is caused by the blockage of the ETC. Indeed, carboxin dose-dependently decreased virulence (Figure 4A) and abolished the filamentation of wild-type C. albicans in the C. elegans model (Figure 4B), as well as in vitro (Figure 4C). Moreover, carboxin (at 1.5 mM) treatment also inhibited the growth of wild-type C. albicans on media containing nonfermentable glycerol (YPG medium) or ethanol (YPE medium) as carbon sources (Figure 4D). These findings are in accordance with the phenotype observed in the sdh2Δ/Δ mutant, suggesting that the attenuated virulence, filamentation defects, and inability to grow on nonfermentable carbon sources of the sdh2Δ/Δ mutant may be caused by the blockage of the ETC.
Figure 4. . Carboxin attenuates the virulence and filamentous growth of the wild-type Candida albicans.
(A) Carboxin decreases the virulence of C. albicans and dose-dependently improves the survival of worms in the Caenorhabditis elegans candidiasis model. 1.5 mM carboxin protects the C. elegans completely. (B) 1.5 mM carboxin completely inhibits the filamentation of C. albicans in vivo in the C. elegans candidiasis model. (C) Carboxin dose-dependently inhibits the filamentous growth of C. albicans in vitro. (D) Carboxin at 1.5 mM completely abolishes the growth of wild-type C. albicans on nonfermentable carbon sources.
YPD: Contains fermentable glucose as carbon source; YPE: Contains nonfermentable ethanol as carbon source; YPS: With fermentable sucrose as carbon source.
To investigate if the phenotype of sdh2Δ/Δ is also associated with the blockage of the TCA cycle, we further used malonate to inhibit the TCA cycle [12,27]. In contrast to carboxin, malonate neither improved the survival of C. elegans with candidiasis nor abolished the filamentous growth of C. albicans (Figure 5A–C). Moreover, malonate did not affect the growth of the wild-type C. albicans on nonfermentable carbon sources (Figure 5D). In order to confirm that the virulence and filamentation defects are not associated with the blockage of the TCA cycle, we also investigated the role of a TCA cycle specific gene FUM12 which encodes the fumarate hydratase (http://www.candidagenome.org/). In accordance with the findings on malonate, the deletion of FUM12 did not affect the virulence of C. albicans in the C. elegans model (Figure 5E), and the fum12Δ/Δ mutant exhibited similar filamentous growth with the wild-type strain (Figure 5F & G).
Figure 5. . Malonate and FUM12 do not affect the virulence and filamentous growth of Candida albicans.
(A & B) Malonate does not attenuate the virulence and filamentation of wild-type C. albicans in the Caenorhabditis elegans candidiasis model. (C) Malonate does not affect the filamentous growth of wild-type C. albicans in vitro. (D) Malonate does not affect the growth of wild-type C. albicans on YPD, YPG and YPE agar plates. (E & F) The deletion of FUM12 does not attenuate the virulence and filamentation of C. albicans in the C. elegans candidiasis model. (G) The deletion of FUM12 does not affect the filamentous growth of C. albicans in vitro.
YPD: Contains fermentable glucose as carbon source; YPE: Contains nonfermentable ethanol as carbon source; YPG: Contains nonfermentable glycerol as carbon source; YPS: With fermentable sucrose as carbon source.
ATP content is normal, while ROS accumulates intracellularly in the sdh2Δ/Δ mutant, leading to the filamentation defects of the mutant
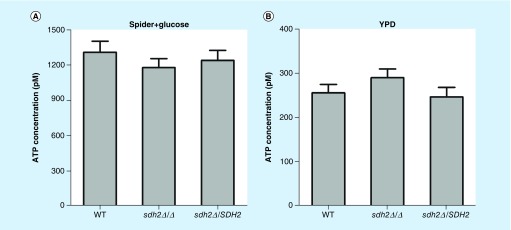
As detailed so far, we found that carboxin decreased the virulence of C. albicans in the C. elegans candidiasis model and inhibited the filamentation of C. albicans, which is in accordance with the phenotype observed in the sdh2Δ/Δ mutant, whereas malonate treatment neither attenuated virulence nor inhibited filamentation. Given that ETC is a critical process in respiration [29], a major energy provider to the cell [29], we next investigated the influence of SDH2 on the abundance of intracellular ATP. Interestingly, the ATP content in the sdh2Δ/Δ mutant was similar to that in the wild-type strain in the culture media tested in this study (Figure 6A & B).
Figure 6. . Intracellular ATP content in Candida albicans wild-type, sdh2Δ/Δ and sdh2Δ/SDH2 strains.
(A) ATP content of the strains in Spider + glucose medium. (B) ATP content of the strains in YPD medium. The ATP content in the sdh2Δ/Δ mutant was not less than that in the wild-type strain.
YPD: Contains fermentable glucose as carbon source.
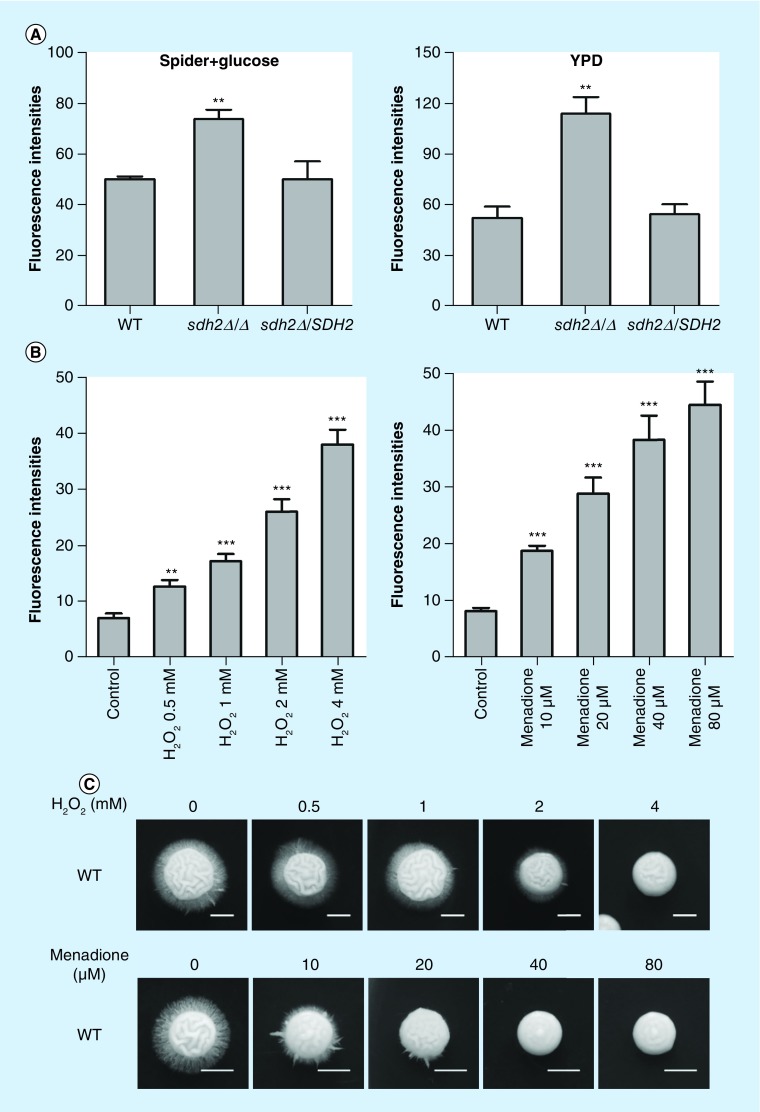
Previous studies suggest that SDH plays an important role in delivering electrons in the ETC, and suppressing the function of Sdh2p (SdhB) may increase ROS production in mammalian cells [30]. We thereby investigated the intracellular ROS abundance of C. albicans. Notably, the sdh2Δ/Δ mutant exhibited significantly increased intracellular ROS level compared with the wild-type or the reintegrated strains (Figure 7A). We further clarified the relationship between intracellular ROS abundance and filamentation using pharmacological tools and various culture conditions. To investigate whether the increased intracellular ROS is associated with the filamentation defects of C. albicans, we supplemented ROS-inducing agents, including H2O2, and menadione into the C. albicans culture media. As expected, the ROS inducing agents dose-dependently led to ROS accumulation in C. albicans (Figure 7B). Accordingly, the ROS-inducing agents inhibited the filamentous growth of C. albicans dose-dependently (Figure 7C).
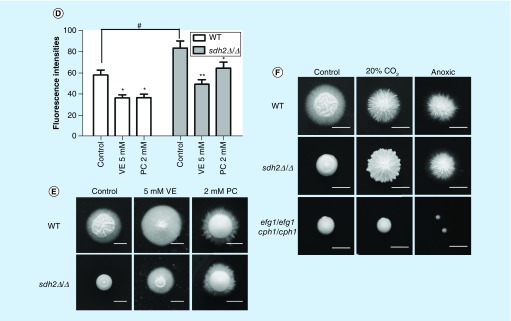
Figure 7. . Increased intracellular eactive oxygen species leads to filamentation defects of Candida albicans, and decreasing intracellular reactive oxygen species can rescue the filamentation of sdh2Δ/Δ.
(A) The sdh2Δ/Δ mutant exhibits significantly increased intracellular reactive oxygen species (ROS). **p < 0.01 compared to the wild-type control group. (B) ROS-inducing agents dose-dependently lead to ROS accumulation in wild-type C. albicans. ROS-inducing agents include H2O2 and menadione. **p < 0.01; ***p < 0.001 compared with the wild-type C. albicans cells without treatment. (C) The ROS-inducing agents inhibit the filamentous growth of C. albicans dose-dependently. (D) Antioxidative vitamin E and proanthocyanidins decreased intracellular ROS of C. albicans. *p < 0.05; **p < 0.01 compared with the group without treatment. #p < 0.05 compared with wild-type C. albicans. (E) The filamentation of sdh2Δ/Δ is rescued by the antioxidants (vitamin E and proanthocyanidins). (F) The filamentation of sdh2Δ/Δ is rescued under both the low oxygen and the anoxia conditions, while efg1Δ/Δ cph1Δ/Δ remains filamentation defective.
Moreover, we evaluated if decreasing intracellular ROS could rescue the filamentation of the sdh2Δ/Δ mutant. First, we used antioxidative vitamin E and proanthocyanidins. As expected, the antioxidants decreased intracellular ROS of C. albicans (Figure 7D). Meanwhile, the filamentation of the sdh2Δ/Δ mutant was actually rescued by these antioxidants, and radial hyphae were seen on the sdh2Δ/Δ colonies (Figure 7E). Given that respiration is the main resource of intracellular ROS [31,32], we further used low oxygen or anoxia culture condition to suppress respiration and thereby inhibit intracellular ROS production. Under both the low oxygen and the anoxia conditions, the sdh2Δ/Δ mutant exhibited similar filamentous growth to the wild-type strain (Figure 7E), as expected. In contrast, the filamentation defect of a efg1Δ/Δ cph1Δ/Δ strain could not be rectified by low oxygen or anoxia (Figure 7F), which served as a control in this experiment. Taken together, these series of experiments indicate that the SDH2 deletion results in ETC disruption and thereby ROS accumulation, and the increased intracellular ROS leads to filamentation defects of C. albicans.
Discussion
In this study, we found that the deletion of SDH2 significantly attenuates the virulence of C. albicans, as evidenced by the increased survival and decreased fungal burden of the sdh2Δ/Δ mutant infected hosts. A prominent reason for the attenuated virulence is the filamentation defects of the sdh2Δ/Δ mutant. The ability of C. albicans to form filaments is an important virulence attribute [33]. Mutants unable to form hyphae are reported to be avirulent [34]. In this study, the sdh2Δ/Δ mutant exhibited obvious filamentation defects: the sdh2Δ/Δ mutant could not develop filaments to puncture the cuticle of the C. elegans or form hyphal clusters in the kidneys of mice, and it exhibited obvious filamentation defects on all tested solid hypha-inducing media. The filamentation defects of the sdh2Δ/Δ mutant may well contribute to its attenuated virulence. Another contributing factor to the attenuated virulence may be the inability of the sdh2Δ/Δ mutant to grow on nonfermentable carbon sources. Recent studies have suggested that during systemic infections of C. albicans, few niches are rich in glucose and pathogenic microbes must assimilate a range of carbon sources to grow and colonize their hosts [35,36]. In particular, upon phagocytosis by macrophages C. albicans has to induce alternative carbon metabolism and form hyphae to escape from macrophages [37]. Thereby, the ability of C. albicans to assimilate a range of carbon sources is also important in virulence. The sdh2Δ/Δ mutant could not grow on nonfermentable carbon sources (Supplementary Figure 1) and this could have contributed in the virulence phenotype.
SDH2 encodes a subunit of SDH that is involved in both the TCA cycle and the ETC [11,12]. SDH catalyzes the oxidation of succinate to fumarate in the TCA cycle [11,12], which is coupled to the reduction of ubiquinone to ubiquinol as a part of the ETC [11,12]. Thereby, SDH is also known as mitochondrial complex II [12]. In the present study, we found that the role of SDH2 in virulence is specifically related to its function in ETC, but not TCA. Disruption of the SDH function in TCA using malonate had no obvious influence on virulence, filamentation, and growth on nonfermentable sources. In contrast, disruption of the SDH function in ETC in wild-type C. albicans using carboxin mimics the phenotype from deletion of SDH2, including the virulence in C. elegans candidiasis model, filamentation, and growth observation using nonfermentable carbon sources.
Since suppressing the function of Sdh2p (SdhB) disrupts its role in delivering electrons in the ETC and thereby leads to ROS accumulation [30], we analyzed the association between intracellular ROS content and filamentation. We used ROS-inducing agents including H2O2 and menadione to increase intracellular ROS abundance, and antioxidants, such as vitamin E and proanthocyanidins, to decrease intracellular ROS; furthermore, we used low oxygen or anoxia culture condition to suppress respiration and thereby inhibit the intracellular ROS production. With the increase of intracellular ROS, filamentation is inhibited, while with the decrease of intracellular ROS, filamentation is enhanced. Importantly, the filamentation defects of the sdh2Δ/Δ mutant were rescued by decreasing intracellular ROS under various conditions, including supplementation with antioxidants, and low oxygen or anoxia culture conditions. Thus, it can be inferred that intracellular ROS accumulation results in the filamentation defects of the sdh2Δ/Δ mutant.
Interestingly, although SDH2 deletion disrupts ETC in aerobic respiration which plays a leading role in supplying ATP [38], the deletion of SDH2 did not result in reduced ATP levels in this study. A possible explanation is that ATP can be produced through fermentation [39]. In our experiment setting that enough glucose (at least 100 mM) was supplied, sufficient ATP could be generated through fermentation.
Another notable finding is that the ATP content of the cells in Spider + glucose medium is approximately four-times higher compared with that in YPD medium. Looking at this difference, the nutrients in the two media are different. YPD is nutrient-rich, while Spider is a hypha-inducing medium. In this series of experiments, the intracellular ATP content of cells in Spider + glucose medium was higher. This finding is consistent with previous reports indicating that high intracellular ATP content is associated with hypha-inducing signals [40]. Ras1, Cyr1 and Ira2 may be the major regulators of this reported association [40]. More specifically, Ras1, Cyr1 and Ira2 are upstream of a critical hypha-inducing signaling pathway, the cAMP-PKA pathway [23]. In hypha-inducing medium, Ras1, Cyr1 and Ira2 may form a master-regulatory complex, integrating different environmental and intracellular signals, including metabolic status [40]. Therefore, hypha-inducing signals are associated with high intracellular ATP levels, such as that provided by Spider + glucose medium in this study.
Notably, we observed that on YPD + 10% serum agar 1–4% ethanol could rescue the filamentation of the sdh2Δ/Δ mutant. Some previous studies have revealed that fusel alcohols and ethanol could induce a switch from vegetative to pseudohyphal growth [41]. More specifically, in nutrition-rich YPD medium, 2–6% ethanol could inhibit protein synthesis and promote the filamentation of C. albicans, even in the efg1Δ/Δ mutant and cph1Δ/Δ mutant, indicating a nonclassical filament-regulating pathway [41]. Our finding that ethanol could induce filamentous growth on YPD + 10% serum agar, even in the sdh2Δ/Δ mutant, may also be due to the translation-inhibitory effect of ethanol.
Taken in its totality, this study sheds light on the role of SDH in the pathogenicity of C. albicans and suggests that disrupting the ETC through blocking the quinone binding site could serve as a novel target for antifungal drug discovery.
Conclusion & future perspective
SDH plays an important role in C. albicans filamentation and virulence, mediated through its role of SDH in the ETC, probably through affecting intracellular ROS. The electron transport site of SDH may thus provide a target for antifungal drug discovery. Future studies on the association between the C. albicans redox system and filamentation may help identify additional C. albicans virulence factors that could serve as potential antifungal targets.
Summary points.
SDH2 is required for Candida albicans virulence in vivo
Based on C. albicans mutant libraries screening, the SDH2 null mutants was avirulent in a Caenorhabditis elegans candidiasis model.
The sdh2Δ/Δ mutant lacked hypha piercing the nematode worm cuticle in the C. elegans candidiasis model.
The sdh2Δ/Δ mutant was hypovirulent in mouse candidiasis model.
The sdh2Δ/Δ mutant exhibits filamentation defects.
The sdh2Δ/Δ mutant exhibits growth defects on nonfermentable carbon sources.
Ethanol accumulation in sdh2Δ/Δ cells grown on a fermentable carbon sources is not responsible for filamentation defects.
A succinate dehydrogenase inhibitor specifically disrupting the electron transport chain (ETC) mimics the impact of the SDH2 deletion.
Carboxin, an SDH inhibitor targeting the ETC, mimics the impact of the SDH2 deletion.
Malonate, an SDH inhibitor targeting the tricarboxylic acid cycle does not affect the virulence and filamentation of C. albicans.
The contribution of SDH2 to filamentation and virulence is specifically associated with the ETC, but not the tricarboxylic acid cycle.
The filamentation defects of the sdh2Δ/Δ mutant are not due to ATP insufficiency in cells grown on a fermentable carbon source.
ROS accumulated in the sdh2Δ/Δ mutant, leading to filamentation defects.
The sdh2Δ/Δ mutant exhibited significantly increased intracellular ROS.
ROS-inducing agents inhibited the filamentous growth of C. albicans dose-dependently.
The filamentation defects of the sdh2Δ/Δ mutant were rescued by antioxidative agents decreasing intracellular ROS.
The filamentation defects of the sdh2Δ/Δ mutant were rescued by decreasing intracellular ROS under low oxygen or anoxia culture conditions.
Conclusion
SDH2 is involved in proper hypha formation and virulence in C. albicans.
The impact of SDH2 on filamentation and virulence is mediated by its role in the ETC.
The role of SDH2 in filamentation is through affecting intracellular ROS.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/full/10.2217/fmb-2018-0033
Financial & competing interests disclosure
This work was supported by the National Natural Science Foundation of China (81273558 and 81772124), and the NIH through an R01 award (AI075286), and an R21 award (AI070569). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all animal experimental investigations. No human subjects were involved in this study.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin. Infect. Dis. 2015;60(6):892–899. doi: 10.1093/cid/ciu959. [DOI] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askew C, Sellam A, Epp E, et al. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans . PLoS Pathog. 2009;5(10):e1000612. doi: 10.1371/journal.ppat.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes that Candida albicans is a Crabtree-negative species exclusively using respiration when oxygen is available.
- 4.Bambach A, Fernandes MP, Ghosh A, et al. Goa1p of Candida albicans localizes to the mitochondria during stress and is required for mitochondrial function and virulence. Eukaryot. Cell. 2009;8(11):1706–1720. doi: 10.1128/EC.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan G, Wang H, Liang W, et al. The mitochondrial protein Mcu1 plays important roles in carbon source utilization, filamentation, and virulence in Candida albicans . Fungal Genet. Biol. 2015;81:150–159. doi: 10.1016/j.fgb.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Mcdonough JA, Bhattacherjee V, Sadlon T, Hostetter MK. Involvement of Candida albicans NADH dehydrogenase complex I in filamentation. Fungal Genet. Biol. 2002;36(2):117–127. doi: 10.1016/S1087-1845(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 7.Vellucci VF, Gygax SE, Hostetter MK. Involvement of Candida albicans pyruvate dehydrogenase complex protein X (Pdx1) in filamentation. Fungal Genet. Biol. 2007;44(10):979–990. doi: 10.1016/j.fgb.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell. 2009;8(11):1750–1758. doi: 10.1128/EC.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tampakakis E, Okoli I, Mylonakis E. A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat. Protoc. 2008;3(12):1925–1931. doi: 10.1038/nprot.2008.193. [DOI] [PubMed] [Google Scholar]
- 10.Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3(2):e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyedotun KS, Lemire BD. The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase. Homology modeling, cofactor docking, and molecular dynamics simulation studies. J. Biol. Chem. 2004;279(10):9424–9431. doi: 10.1074/jbc.M311876200. [DOI] [PubMed] [Google Scholar]
- 12.Sun F, Huo X, Zhai Y, et al. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121(7):1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]; •• Describes that carboxin selectively binds to the site where quinone normally binds, disrupting succinate dehydrogenase function by interfering with the electron transport chain.
- 13.Newport G, Kuo A, Flattery A, et al. Inactivation of Kex2p diminishes the virulence of Candida albicans . J. Biol. Chem. 2003;278(3):1713–1720. doi: 10.1074/jbc.M209713200. [DOI] [PubMed] [Google Scholar]
- 14.Tan X, Fuchs BB, Wang Y, et al. The role of Candida albicans SPT20 in filamentation, biofilm formation and pathogenesis. PLoS ONE. 2014;9(4):e94468. doi: 10.1371/journal.pone.0094468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs BB, Eby J, Nobile CJ, El Khoury JB, Mitchell AP, Mylonakis E. Role of filamentation in Galleria mellonella killing by Candida albicans . Microbes Infect. 2010;12(6):488–496. doi: 10.1016/j.micinf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walther A, Wendland J. An improved transformation protocol for the human fungal pathogen Candida albicans . Curr. Genet. 2003;42(6):339–343. doi: 10.1007/s00294-002-0349-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Jia XM, Jia JH, et al. Ascorbic acid decreases the antifungal effect of fluconazole in the treatment of candidiasis. Clin. Exp. Pharmacol. Physiol. 2009;36(10):e40–e46. doi: 10.1111/j.1440-1681.2009.05187.x. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Wang Y, Yan L, et al. Proteomic analysis reveals a synergistic mechanism of fluconazole and berberine against fluconazole-resistant Candida albicans: endogenous ROS augmentation. J. Proteome Res. 2009;8(11):5296–5304. doi: 10.1021/pr9005074. [DOI] [PubMed] [Google Scholar]
- 19.Rastogi RP, Singh SP, Hader DP, Sinha RP. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010;397(3):603–607. doi: 10.1016/j.bbrc.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu D, Zhang H, Gu W, Zhang M. Effects of exposure to high glucose on primary cultured hippocampal neurons: involvement of intracellular ROS accumulation. Neurol. sci. 2014;35(6):831–837. doi: 10.1007/s10072-013-1605-4. [DOI] [PubMed] [Google Scholar]
- 21.Reuss O, Vik A, Kolter R, Morschhauser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans . Gene. 2004;341:119–127. doi: 10.1016/j.gene.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu. Rev. Microbiol. 2005;59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 23.Sudbery PE. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011;9(10):737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]; • Discusses the importance of filamentous growth for C. albicans and describing the network of signal transduction pathways that regulate filamentation.
- 24.Albers E, Gustafsson L, Niklasson C, Liden G. Distribution of 14C-labelled carbon from glucose and glutamate during anaerobic growth of Saccharomyces cerevisiae . Microbiology. 1998;144(Pt 6):1683–1690. doi: 10.1099/00221287-144-6-1683. [DOI] [PubMed] [Google Scholar]
- 25.Chauhan NM, Raut JS, Karuppayil SM. A morphogenetic regulatory role for ethyl alcohol in Candida albicans . Mycoses. 2011;54(6):e697–e703. doi: 10.1111/j.1439-0507.2010.02002.x. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Ogasawara A, Mikami T, Matsumoto T. Hyphal formation of Candida albicans is controlled by electron transfer system. Biochem. Biophys. Res. Commun. 2006;348(1):206–211. doi: 10.1016/j.bbrc.2006.07.066. [DOI] [PubMed] [Google Scholar]
- 27.Potter VR, Dubois KP. Studies on the mechanism of hydrogen transport in animal tissues: VI. inhibitor studies with succinic dehydrogenase. J. Gen. Physiol. 1943;26(4):391–404. doi: 10.1085/jgp.26.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes that malonate is an analog of succinate that binds to the site where succinate normally binds, inhibiting the tricarboxylic acid cycle.
- 28.Matsson M, Hederstedt L. The carboxin-binding site on Paracoccus denitrificans succinate: quinone reductase identified by mutations. J. Bioenerg. Biomembr. 2001;33(2):99–105. doi: 10.1023/a:1010744330092. [DOI] [PubMed] [Google Scholar]
- 29.Kawamukai M. Biosynthesis of coenzyme Q in eukaryotes. Biosci. Biotechnol. Biochem. 2015;80(1):23–33. doi: 10.1080/09168451.2015.1065172. [DOI] [PubMed] [Google Scholar]
- 30.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell. Biol. 2008;28(2):718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes that loss of the SdhB (Sdh2p) may increase intracellular ROS production.
- 31.Kim HJ. Exploitation of reactive oxygen species by fungi: roles in host-fungus interaction and fungal development. J. Microbiol. Biotechnol. 2014;24(11):1455–1463. doi: 10.4014/jmb.1407.07072. [DOI] [PubMed] [Google Scholar]
- 32.Kowaltowski AJ, De Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47(4):333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell. 2003;2(5):1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo HJ, Kohler JR, Didomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90(5):939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 35.Rodaki A, Bohovych IM, Enjalbert B, et al. Glucose promotes stress resistance in the fungal pathogen Candida albicans . Mol. Biol. Cell. 2009;20(22):4845–4855. doi: 10.1091/mbc.E09-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandai D, Yin Z, Selway L, et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans . mBio. 2012;3(6):e00495-12. doi: 10.1128/mBio.00495-12. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes that few inches in the host are rich in glucose and concludes that C. albicans retains key metabolic functions, allowing it to continue to assimilate alternative carbon sources.
- 37.Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell. 2004;3(5):1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich PR. The molecular machinery of Keilin's respiratory chain. Biochem. Soc. Trans. 2003;31(Pt 6):1095–1105. doi: 10.1042/bst0311095. [DOI] [PubMed] [Google Scholar]
- 39.Hoogerheide JC. Studies on the energy metabolism during anaerobic fermentation of glucose by baker's yeast. Radiat. Environ. Biophys. 1975;11(4):295–307. doi: 10.1007/BF01326752. [DOI] [PubMed] [Google Scholar]
- 40.Grahl N, Demers EG, Lindsay AK, et al. Mitochondrial activity and Cyr1 are key regulators of Ras1 activation of C. albicans virulence pathways. PLoS Pathogens. 2015;11(8):e1005133. doi: 10.1371/journal.ppat.1005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egbe NE, Paget CM, Wang H, Ashe MP. Alcohols inhibit translation to regulate morphogenesis in C. albicans . Fungal Genet. Biol. 2015;77:50–60. doi: 10.1016/j.fgb.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.