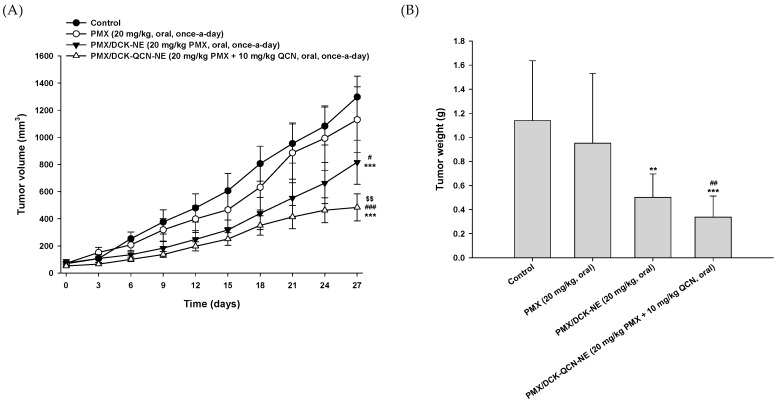
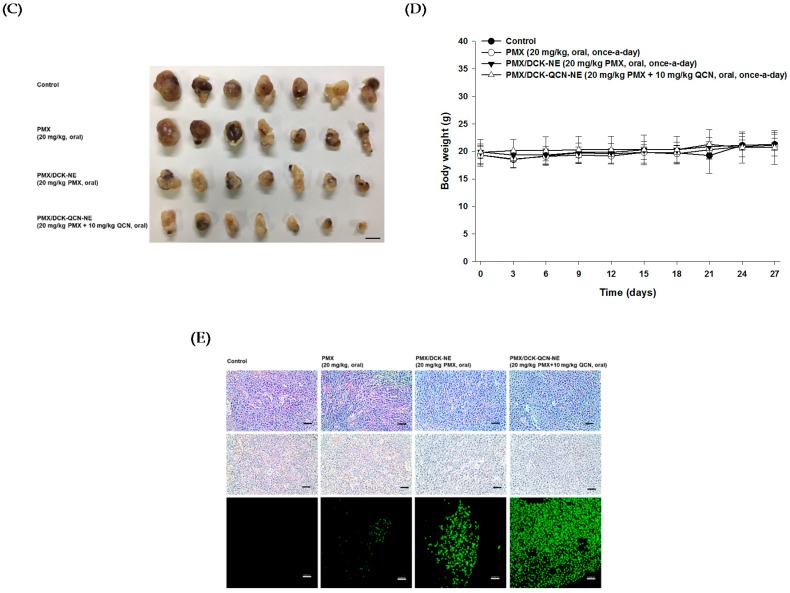
Figure 6.
In vivo tumor growth inhibition efficacy in A549 tumor-bearing mice after treatment with once-daily oral administration of PMX (20 mg/kg), PMX/DCK-NE (equivalent to 20 mg/kg PMX), or PMX/DCK-QCN-NE (equivalent to 20 mg/kg PMX and 10 mg/kg QCN) for 27 days. (A) Tumor volume in mice (n = 10; *** p < 0.001 compared with the control group; # p < 0.05, ### p < 0.001 compared with the oral PMX group; $$ p < 0.01 compared with the oral PMX/DCK-NE group); (B) Variation in body weight in mice during treatment (n = 10); (C) Isolated tumor weight in A549 tumor-bearing mice (n = 10; ** p < 0.01, *** p < 0.001 compared with the control group; ## p < 0.01 compared with the oral PMX group); (D) Photographs of isolated tumors from each group on day 21. Scale bar represents 10 mm; (E) Representative cross-sectional images of isolated tumor tissues stained with anti-CD31 antibody for microvessels (brown), PCNA for proliferating cells (brown), and TUNEL for apoptosis (green fluorescence) at 27 days after treatment. Scale bar represents 50 µm.