Abstract
Introduction: Manual analysis of two-dimensional (2D) scintigraphy to evaluate aerosol deposition is usually subjective and has reduced sensitivity to quantify regional differences between central and distal airways.
Aims: (1) To present a method to analyze 2D scans based on three-dimensional (3D)-linked anatomically consistent regions of interest (ROIs); (2) to evaluate peripheral-to-central counts ratio (P/C2D) and penetration indices (PIs) for a set of 16 subjects with moderate-to-severe asthma; and (3) to compare the reproducibility of this method against one with manually traced ROIs.
Methods: Two-dimensional scans were analyzed using custom software that scaled onto 2D-projections' 3D anatomical features, obtained from population-averaged computed tomography (CT) chest scans. ROIs for a rectangular box (bROI) and an anatomically shaped ROI (aROI) were defined by computer and by manually tracing the standard rectangular box (manual ROI [mROI]). These ROIs were defined five nonconsecutive times for each scan and average value and variability of the P/C2D were estimated. Based on CT estimates of lung and airways, volumes lying under the bROI and aROI, a 2D penetration index (PI2D) and a 3D penetration index (PI3D), were defined as volume-normalized ratios of aerosol deposition in central and peripheral ROIs and in central and distal airways, respectively.
Results: P/C2D values and their variability, were influenced by the shape and method to define the ROIs: The P/C2D was systematically greater and more variable for mROI versus bROI (p < 0.005). The P/C2D for aROI was higher and its variability lower than those for the bROI (p < 0.001). The PI2D was in average the same for aROI and bROI, and is substantially (∼30 × ) greater than PI3D (p < 0.001). Both PI2D and PI3D, obtained with our analysis, compared well with literature values obtained with two scans (deposition and volume).
Conclusion: Our results demonstrate that 2D scintigraphy can be analyzed using anatomically based ROIs from 3D CT data, allowing objective and enhanced reproducibility values describing the distribution pattern of radioaerosol deposition in the tracheobronchial tree.
Keywords: : aerosol deposition, regions of interest, scintigraphy, voxel influence matrix, 3D analysis, penetration analysis
Introduction
Planar Scintigraphy, a two-dimensional (2D) imaging technique in nuclear medicine, is widely used to assess aerosol deposition and mucociliary clearance in human and animal models. Compared with other three-dimensional (3D) imaging methods, the 2D technique is relatively simple, widely available, and exposes the subjects to acceptable doses of radiation.(1–4)
To quantify the distribution of the aerosol within the tracheobronchial tree, the 2D images are divided into regions of interest (ROIs) and the radioactive events detected within each 2D ROIs are counted.(1,5–7) Typically, central (C) and peripheral (P) ROIs are defined for one or both lungs but their shape and size may vary substantially between laboratories making it difficult to compare results from similar studies(2,8) Furthermore, due to the lack of 3D information, the activity recovered in the central ROIs includes activity from peripheral airways within the 2D ROIs, and the central airways may extend beyond the central region into the peripheral ROIs.
For example, in cystic fibrosis, patients' heavy airway deposition may extend to the lateral basal region of the lung, which is outside the typical square central ROI.(1,2,9) Also, depending on the depth of the lung sampled by each pixel,(10–13) and the attenuation of the emitted radioactivity, the peripheral-to-central counts ratio (P/C) obtained from 2D ROIs may overestimate or underestimate true values of the peripheral-to-central airways deposition ratio.
Normalization of the deposition data by counts from a second image representing the amount of lung volume sampled in each pixel, allows calculation of an aerosol concentration ratio, or penetration index (PI), defined as the P/C of aerosol counts divided by a P/C of counts from a radioactive gas (volume) scan that reduces the effect of the differences in lung depth and energy attenuation. Volume scans, although desirable are technically challenging, add cost, and are not always available in many institutions.(1,2,10,12,14,15)
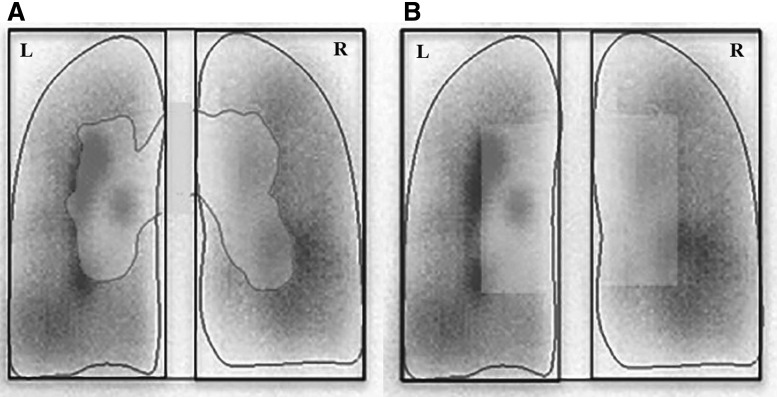
In addition to the limitations of quantifying in 2D, the distribution of a radionuclide within a 3D organ, analysis of the data is usually manual, time consuming, affected by intra and interoperator variability.(2,10) Ideally the anatomical location of the central airways within the central 2D ROIs and the contribution of peripheral airways should be known to estimate with some accuracy the regional distribution of aerosol deposition from 2D scintigraphy studies. Current methods use systematically defined, but arbitrarily shaped central ROIs, which may or may not be qualitatively inspired on anatomy. However, the lack of 3D information in scintigraphy prevents separation of activity coming from central airways versus that of distal airways sampled under standard 2D ROIs (Fig. 1).(3,10,16)
FIG. 1.
The conventional approach to drawing 2D ROIs for scintigraphy images involves drawing the boundary of the right lung by hand and then an algorithm is used to identify the central region.(5,6) 2D, two-dimensional; L, left lung; R, right lung; ROIs, regions of interest.
The objective of this study was threefold: (1) to implement a novel method to analyze scintigraphic images of aerosol deposition based on anatomically consistent ROIs that allow systematic separation of aerosol deposition between central airways and the rest of the lung, (2) to compare the reproducibility of results obtained with that method against those obtained with the conventional manual method, and (3) to evaluate and compare values of P/C and PI obtained for 2D and 3D derivations.
Methods
Defining anatomically consistent ROIs
In radionuclide imaging, blurring, uncertainty, and discretization can cause activity originating within an anatomical region (AR) to be sampled in regions outside of it. In a previous report we showed how careful consideration of each of these effects permits the estimation of the contribution of activity within each AR to the counts sampled within any voxel of the 3D imaging space. Using the 3D information from the two computed tomography (CT) scans of the lung, taken one at mean breathing volume and the other at maximal inhalation, and knowing the spatial resolution properties of the imaging instrument, a voxel influence matrix (VIM) was defined to estimate the local contributions of each of the ARs to each voxel.(17)
The sum of any ARs VIM is equal to its volume (number of voxels within the CT scan times' voxel volume), so that a VIM essentially describes the redistribution of that volume according to the expected sampled representation in each voxel of the aerosol distribution 3D image. VIMs are therefore a model of the fraction of each voxel that samples a specific AR as seen by the 3D imaging scanner, and its units are dimensionless.(17)
In our previous work,(18) we used the concept of VIMs to quantify the anatomical location of the aerosol deposition and the alveolar ventilation within positron emission tomography (PET) images. Using Apollo™ software (Vida Diagnostics, Mountain View, CA) lung scans of 14 subjects were segmented into 14 ARs that included 5 peripheral lobar regions and 9 segments of the airway tree. The lung periphery included the five lobes; central airways included the bronchus intermedius, the right and left main bronchus, and the trachea, as well as five airway groups feeding each lobe that included the lobar, segmental, and subsegmental airways.(17) The blurring caused by the motion of the lung and by the limited spatial resolution of the imaging method, was modeled to generate VIMs for each of the 14 ARs.
For the present work, we assembled the five lobar VIMs into a peripheral VIM and assembled the VIMs of the eight central airways (excluding the trachea) into a central VIM. These central and peripheral VIMs were bound with rectangular prisms for the right and left lungs and then scaled and mapped onto a standardized 2D rectangular shape, assuming that the scaling factor of the third (z) dimension equaled the average of the 2D (x and y) scaling factors. The standardized peripheral and central VIMs were averaged across the 24 subjects to create average anatomically derived VIMs (aVIMs) in Figure 2.(17)
FIG. 2.
After registration, 3D rendered lung external surface to a rectangular prism and scaling them to a standard size and shape, the scaled central and peripheral VIMs were averaged across the 24 subjects to define corresponding aVIMs. To define the peripheral aROIs, the peripheral aVIMs were trimmed to exclude pixels with values <1%. A third VIM that covered the trachea region (shown in green outline) was not used in the present analysis. 3D, three-dimensional; aROI, anatomically shaped region of interest; aVIM, anatomically derived VIM; VIM, voxel influence matrix.
Conventional rectangular central 2D ROIs were defined to cover the central half of the lung craniocaudal dimension and the medial half of each lung on the projected standardized lung (Fig. 3B). As the central and peripheral VIMs overlapped with each other, we defined a central averaged 2D anatomically shaped ROI (aROI) (Fig. 3A) by thresholding the central VIM with a threshold value selected to cover the same fraction of the lung pixels as that of the conventional rectangular ROI. Any pixels of the space with peripheral VIM >0.01 were designated as part of the peripheral 2D aROI.
FIG. 3.
Central and peripheral anatomically derived averaged 2D ROIs (left) and standard 2D bROI (right), after semimanual registration to the same scintigraphy image. bROI, rectangular box for region of interest.
The CT scans were obtained from a different population of subjects and we implemented a method to generate an averaged VIM that was used not only to generate an anatomically based ROI for the central region, but also to extract the fraction of volume corresponding to central airways and distal airways for any defined ROI.
Collection of scintigraphic images
The protocol to obtain scintigraphic images was approved by the Human Research Ethics Committee at Human at 437/2008 with all subjects giving written informed consent.
Included were 16 subjects with clinical diagnosis of persistent moderate-to-severe asthma (13 females and 3 males) with reversible obstruction >12% and a body mass index of 25.93 ± 5.04 (Table 1). Subjects with percent of predicted forced expiratory volume in 1st second (FEV1) from 60% to 80% for moderate (n = 7), and predicted FEV1 < 60% for more than 1 year in severe asthma (n = 9).(19) All patients were receiving standard combination therapy with bronchodilators and corticosteroids (Formoterol 12 μg and Budesonide 400 μg) and were instructed to suspend medication 24 hours before the study.
Table 1.
Characteristics of the Sample and Spirometric Values Baseline
| Variables | Mean ± SD |
|---|---|
| Age (years) | 46.52 ± 9.68 |
| Gender | 13 F/3 M |
| BMI (kg/m2) | 25.93 ± 5.04 |
| HR (bpm) | 81.44 ± 9.59 |
| RR (ipm) | 14.44 ± 2.90 |
| SpO2 (%) | 97.13 ± 1.02 |
| IC (L) | 2.15 ± 0.49 |
| FEV1 (% pred) | 59.00 ± 14.88 |
| FVC (% pred) | 74.69 ± 17.29 |
| PEF (% pred) | 41.13 ± 14.44 |
| FEF25%–75% (% pred) | 30.00 ± 16.82 |
| FEV1/FVC (% pred) | 79.31 ± 10.59 |
Values in mean ± SD.
BMI, body mass index; FEF25%–75% (% pred), percentage of predicted for forced expiratory flow between 25% and 75%; FEV1 (% pred), percentage of predicted for forced expiratory volume in 1 second; FEV1/FVC (% pred), percent predicted for the ratio of forced expiratory volume in 1 second and forced vital capacity; FVC (% pred), percentage of predicted forced vital capacity; HR, heart rate; IC, inspiratory capacity; PEF (% pred), percentage of predicted for peak expiratory flow; RR, respiratory rate; SD, standard deviation; SpO2, oxygen saturation.
Excluded from the study were patients: incapable to comprehend or perform the spirometric maneuver or who failed to maintain appropriate positioning during scintigraphic imaging. Also excluded were those with a history of smoking in the last 3 years with a consumption of more than 100 cigarettes per year, or had smoked for 10 years or more. Subjects with other pulmonary comorbidities such as chronic obstructive pulmonary disease (COPD), bronchiectasis and tuberculosis sequel, and pregnancy were also excluded.
Cardiopulmonary parameters
Initially, all patients were submitted to clinical evaluation—respiratory rate, peripheral oxygen saturation, heart rate, inspiratory capacity (IC), and spirometric parameters (portable MicroLoop spirometer, digital volume transducer; Cardinal Health).
The following measures were assessed: FEV1 and forced vital capacity, expressed both as absolute and predicted values for Brazilian subjects,(20) in accordance with the American Thoracic Society.(21) For IC measure patients were instructed to conduct inspiratory maneuvers from expiratory reserve volume to total lung capacity, at 2-minute intervals that agreed within 5% or 60 mL.(21) All parameters were reassessed following scintigraphy.
Lung inhalation scintigraphy
Diethylenetriaminepentaacetic acid was labeled with 925 Mbq (25 mCi) Technetium-99 m (DTPA-Tc99 m) and combined with 1 mg of fenoterol bromide and 2 mg of ipratropium using 0.9% saline solution to a total dose volume of 3 mL (Median Mass Aerodynamic Diameter—MMAD 0.9 μm). A delivery system was provided in a closed and orofacial mask (Vital Signs Ltd.) suited with unidirectional valves and with the inspiratory branch connected to a nebulizer for radio isotopes (Venticis® II Medical device, class II, CE 0459; Ventibox/CIS BioInternational).
Aerosol inhalation was conducted in the upright sitting position over 9 minutes. The subjects were previously instructed to breathe slowly and deeply through the mouth, executing an inspiratory pause for 3 seconds with every breath. After inhalation, subjects were asked to rinse their mouth and drink water to clear their throat and esophagus of radioaerosol.(1)
Image acquisition
Immediately following nebulization, deposition was imaged in the supine position from a posterior view with a single-head scintillation camera (STARCAM 3200 AC/T GE; Medical Systems) for 300 seconds, yielding a matrix of 256 × 256 pixels. Subjects were instructed to remain still during the imaging process.
Image analysis
Defining 2D ROIs
Central and peripheral ROIs were defined manually using the standard program Xeleris (STARCAM 3200 AC/T GE; Medical Systems). For the manual ROIs, a contour of each lung was drawn covering a region with activity higher than ∼15% of the highest pixel count of the image and a rectangular box was defined around it. A central manual ROI (mROIc) for the right lung was defined as a rectangular box extending from the medial edge of the lung to half of the width of the lung box, and a height equal to half of the height of the lung box and centered with respect to it. The peripheral manual ROI (mROIp) comprised the area of the lung region not covered by the mROIc(1,5,6) (Fig. 1).
For defining anatomically consistent 2D ROIs, a custom program was developed using MATLAB (The Mathworks, Inc.). The program first read the scintigraphy image and used a threshold to exclude voxels with activity lower than 15% of the maximum voxel activity, and superimposed to rectangular prisms for the right and left lungs and the aROIs described above. The program allowed vertical and horizontal displacements and scaling of the peripheral lung boundary of the aROIs using the mouse to match the outline of the radioactive counts >15% of the maximum to define the anatomically based peripheral regions of both lungs while the central aROI was automatically assigned by the computer to the scaled peripheral aROI.
Two differently shaped ROIs for the central region, covering equal volumes of the lung, were defined for each lung: one with a shape based on the central average anatomy ROI (aROIc), and one with a rectangular box for central ROI (bROIc) using the same proportions and location defined in the manual analysis. The respective peripheral ROIs were automatically shaped with the average anatomy (aVIMs), excluding the central ROIs. There was no attempt to match the “hot spots” on the deposition images. For both the aROIc and bROIc definitions, the program estimated the total volume of the lung included under the corresponding central and peripheral ROIs, and the relative fraction of their volume occupied by central airways and by distal airways and parenchyma, consistent with the averaged anatomic data.
Estimation of deposition distribution parameters
Both manual ROIs (mROIs) and computer assisted (aROIs and rectangular box for ROIs [bROIs]) were defined for each subject and regional counts were extracted for each ROI. The process was repeated four additional times randomly selecting the order of the subjects' analyses. Since data for the left lung are not customarily evaluated using the manual methods, we only include analysis of data from the right lung. The manual analysis was carried out by an experienced operator and the computer-aided analysis was conducted by a different person with computer experience, but with no previous experience in the analysis of these images.
For this analysis, it is important to consider that the scintigraphy counts from central and peripheral areas are attenuated by the thorax to different extents.(22) Thus, to estimate the true number of counts originating from central and peripheral ROIs, the measured counts have to be adjusted by an attenuation correction factor (ACF), k:
 |
Based on a previous theoretical estimate,(22) we assigned the values of kc = 4.7 and kp = 3.0 for the ACF of the central and peripheral ROIs, and initially assumed that they were equal for all subjects and independent of the geometrical details of the ROIs. In three of the subjects, this assumption was reexamined as presented in the Discussion section.
Using attenuation-corrected counts, we estimated the following parameters.
-
(1) True Peripheral-to-Central count ratio (P/C)* was estimated for each set of ROIs as:

and the average and coefficient of variation (COV) among the five estimations of (P/C)* values for each subject were calculated and compared among the three different sets of ROIs: (P/C)*mROI, (P/C)*bROI, and (P/C)*aROI, with a paired t test.
-
(2) A 2D penetration index (PI2D) was calculated as the volume-normalized ratio of the (P/C)* using the estimates of central and peripheral lung volumes subtended by the central and peripheral ROIs for both aROIs and bROIs,

where VP/VC is the ratio of volumes subtended by the peripheral and central ROIs derived from the averaged CT data for each of the three ROI sets (mROI, bROI, and aROI). Thus, PI2D is equivalent to a standard PI estimated when the P/C aerosol deposition counts are normalized by the P/C counts obtained from a volume scan. Note that to estimate PI with the standard (two scans) method there is no need to include attenuation factors because the deposition and volume scans are attenuated by the same factor.
-
(3) Similarly, a 3D penetration index, PI3D, was defined as the ratio of counts assigned to distal airways (CD) over those assigned to central airways (CA) normalized by their respective volumes obtained from the averaged anatomically consistent data.

Here, the count per unit volume (specific counts, sC) originating from the central airways is sCA, or from the distal airways is sCD. Note that this is equivalent to the PI2D, but with the counts from the ARs and normalized by the respective volumes in 3D instead of the volumes of lung lying under the 2D ROIs.
To estimate the value of sCA (or sCD,) we assume that activity per unit volume within central airways (or distal airways) is the same and independent of whether they were under the central or peripheral ROIs.
Thus, the true counts originating from a central ROI are equal to Cc·kc and also equal to the sum of the products of the respective specific counts (sCA and sCD) times their corresponding volumes (VDC and VAC): A similar equation can be formulated for the true counts originating from the peripheral ROI:
A similar equation can be formulated for the true counts originating from the peripheral ROI: where VAP refers to volume occupied by central airways under the peripheral ROI and VDP refers to volume occupied by distal airways on the peripheral ROI. Thus, the values, sCA and sCD, could be estimated by simultaneously solving the above set of two equations with two unknowns yielding:
where VAP refers to volume occupied by central airways under the peripheral ROI and VDP refers to volume occupied by distal airways on the peripheral ROI. Thus, the values, sCA and sCD, could be estimated by simultaneously solving the above set of two equations with two unknowns yielding:

Statistical analysis
Statistical analysis was carried out using SPSS 18.0 software (Statistical Package for the Social Sciences). Data in each ROI are represented by their means and standard deviation (SD). Differences between the results using the two sets of computer-derived ROIs were evaluated using a paired t-test and the degree of association between methods was estimated with the Pearson correlation coefficient. For comparisons between mean obtained with manual or anatomically derived sets of ROIs, unpaired t-test statistics were used. Differences were assumed significant if p < 0.05.
Results
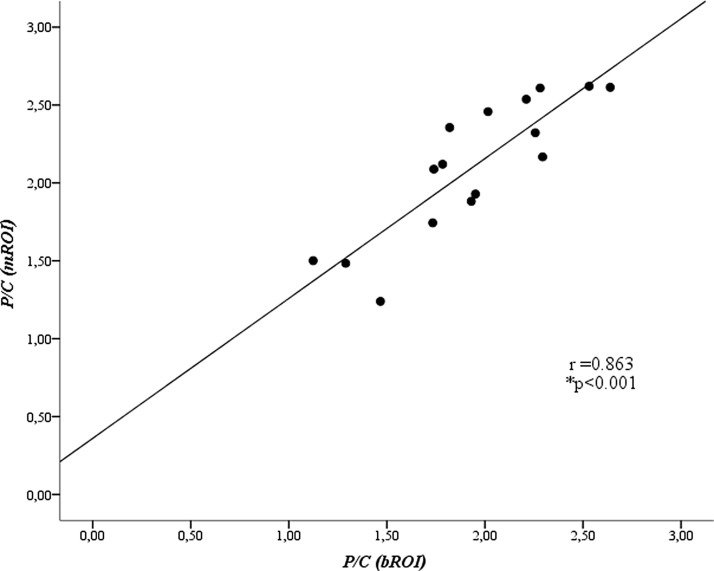
P/C: for manual versus computer-defined box ROIs
Although well correlated (R = 0.863), there were systematic differences in the median (p = 0.011) and variability (COV; p = 0.0008) between the P/C values measured using the manual versus those measured with the computer-defined box ROIs. (Fig. 4 and Table 2). Average P/C for the computer-derived bROI (1.94 ± 0.423 and its variability (COV = 0.064 ± 0.029) was lower than those for mROI (Average P/C = 2.104 ± 0.440 and COV 0.101 ± 0.04). The mean intersubject variability of the P/C for the computer-defined bROI (COV between five measures in each subject) was in average 63% lower than that for the mROIs (p < 0.0005).
FIG. 4.
Comparison between average P/C values, measured five times in each subject, using the computer-defined bROI versus those using the mROI. P/C was not corrected for attenuation differences between central and peripheral regions to allow comparison with existing published data (*Pearson correlation). mROI, manual region of interest; P/C, peripheral-to-central counts ratio.
Table 2.
Mean and Coefficient of Variation of the Peripheral-To-Central Count Ratio Values Without and With Attenuation Correction for Manual Region of Interest and Computer-Assisted Box Regions of Interest
| Variable | P/C(mROI) | P/C(bROI) | pa |
|---|---|---|---|
| Mean ± SD (without attenuation correction) | 2.1044 ± 0.4409 | 1.238 ± 0.29 | <0.001 |
| Mean ± SD (with attenuation correction) | 1.2333 ± 0.2901 | 1.1280 ± 0.2265 | <0.001 |
| COV | 0.10 ± 0.04 | 0.064 ± 0.029 | <0.001 |
Test t/values are mean ± SD.
bROI, rectangular box for central ROI; COV, coefficient of variation; mROI, manual region of interest; P/C, peripheral-to-central counts ratio.
Although the correction for attenuation affected the values of P/C as expected, the differences for the two sets of ROIs were similar, whether data were corrected by attenuation or not (Table 2).
P/C: for computer-defined aROI versus bROI
The values of P/C were affected by the shape of the ROI used. The average of five measures of P/C for each subject obtained using aROI, was highly correlated (r = 0.98, p < 0.001) with using bROI (Fig. 5). However, P/C measured with aROIs (1.646 ± 0.02) was systematically and significantly higher (p < 0.001) than that measured for bROI (mean 1.128 ± 0.22).
FIG. 5.
Plot showing average of five measures of P/C for each subject calculated using the computer-assisted aROI versus bROI (*Pearson correlation). The lines correspond to identity and correlation line (r = correlation coefficient).
In contrast, the intrasubject variability of P/C was not significantly affected by the shape of the computer-defined ROI: mean ± SD of COVP/C was 0.059 ± 0.024 and 0.064 ± 0.030 for aROI and bROI, respectively.
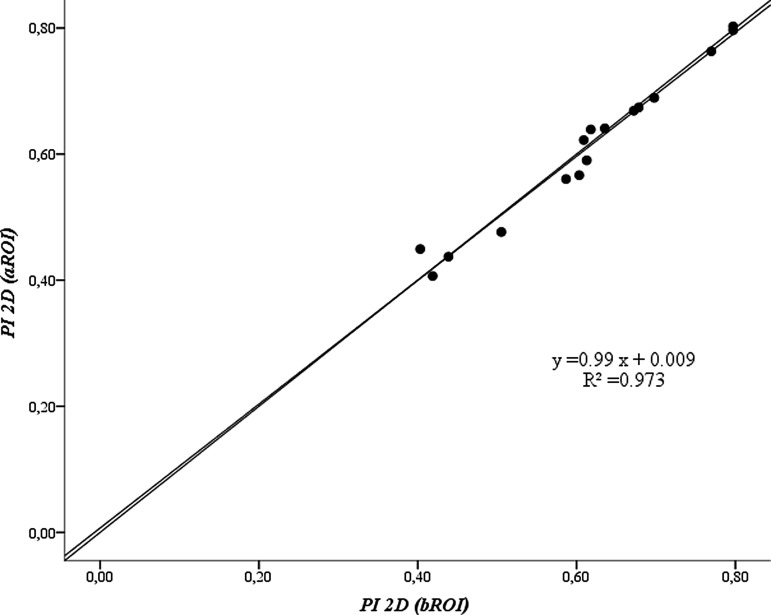
Two-dimensional penetration index
To account for differences in the subtended volume of lung under the different ROIs, the PI2D, which is equivalent to the standard PI,(1,2) was calculated by normalizing the activity measured for each ROI by their corresponding subtended volume of lung. With this normalization we found that PI2D over the 16 subjects, was virtually identical, and highly correlated (r = 0.986), when measured using bROI (0.615 ± 0.124) versus that using the aROI (0.611 ± 0.124) (Fig. 6). However, the intrasubject variability of PI2D (for five measures on each subject) was lower (p = 0.035) using the aROI (average COV = 0.059 ± 0.023) compared with that using the bROI (average COV = 0.064 ± 0.028) (Table 2).
FIG. 6.
Plot of average values of five estimates of PI2D for each subject using aROI versus bROI. The lines correspond to identity and best fit line. PI2D, two-dimensional penetration index.
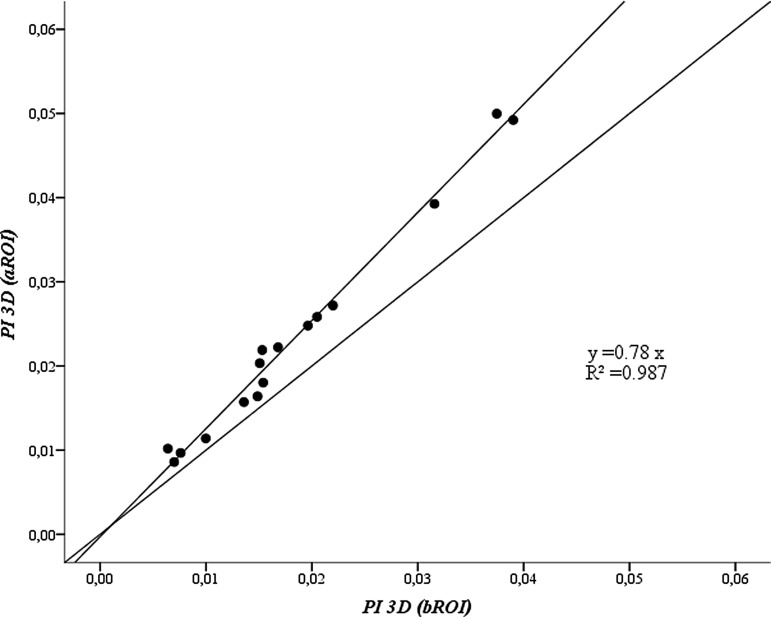
Three-dimensional penetration index
The average of the five values of PI3D for aROI was highly correlated to that estimated for bROI (r = 0.99, p < 0.01, Fig. 7). However, the values obtained for bROI systematically underestimated (87%) those obtained with the aROI. In average, for all subjects PI3D for aROI (0.023 ± 0.013) was significantly (p < 0.001) higher than that for bROI (0.018 ± 0.010). Also, the intrasubject variability of the PI3D for bROI (average COV = 0.171 ± 0.069) was higher (p = 0.015) than that for aROI (average COV = 0.156 ± 0.056) (Table 2).
FIG. 7.
Plot of average of five estimates per subject of PI3D using aROI versus bROI. The lines correspond to identity and best fit lines (R2 is the coefficient of determination). PI3D, three-dimensional penetration index.
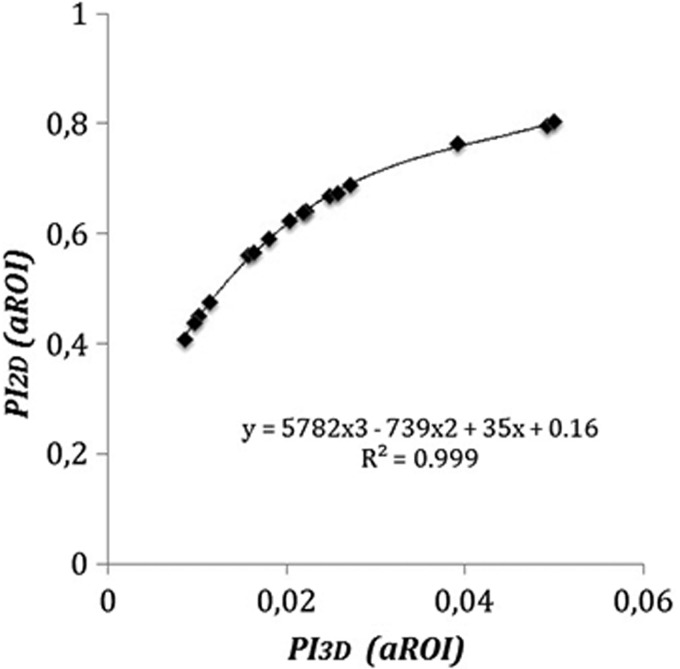
PI2D versus PI3D
There was a tight relationship between PI2D and PI3D that followed closely a cubic polynomial equation (R2 = 0.999). In average, PI2D obtained using aROI (0.611 ± 0.123) was 32 times higher than PI3D (0.023 ± 0.013; p < 0.001) (Fig. 8) and 40 times higher (p < 0.001) using the bROI: PI2D (0.615 ± 0.124) versus PI3D (0.018 ± 0.010; plot not shown). However, the intrasubject variability of PI3D, either for aROI or bROI, was higher than those for PI2D (p < 0.001) (Table 3).
FIG. 8.
Plot of the average of five estimates for each subject of the PI3D versus that of the PI2D for computer-defined aROI. The relationship follows tightly a cubic polynomial equation.
Table 3.
The Coefficient of Variation and Average of the Penetration Index Between Two-Dimensional Penetration Index and Three-Dimensional Penetration Index Between Anatomically Shaped Region Of Interest and Rectangular Box for Region Of Interest
| Variable | PI2D | PI3D | pa |
|---|---|---|---|
| COV (aROI) | 0.059 ± 0.023 | 0.159 ± 0.056 | <0.001 |
| Mean (aROI) | 0.611 ± 0.124 | 0.025 ± 0.013 | |
| COV (bROI) | 0.064 ± 0.028 | 0.174 ± 0.068 | <0.001 |
| Mean (bROI) | 0.615 ± 0.124 | 0.019 ± 0.010 |
Test t PI2D < PI3D/mean ± SD.
aROI, anatomically shaped region of interest; PI2D, two-dimensional penetration index; PI3D, three-dimensional penetration index.
Discussion
Proper evaluation of aerosol deposition distribution is important for estimation of local dosing of inhalation therapy, to bridge in vitro and animal model data to the human scale, and to understand the regional and global effectiveness of aerosol therapy. However, due to the limited resolution of existing imaging methods, accurate assessment of aerosol deposition along the airway tree cannot be properly evaluated.(17) With 2D scintigraphy, images correspond to planar projections of the lung and, without any further information, it is not possible to separate central from peripheral airways. With 3D tomographic techniques, the spatial/temporal resolution of the existing scanners limits the size of the smallest airways that can be evaluated to that of segmental airways (approximately >10 mm in diameter), for PET, and that of lobar airways (approximately >1.5) for single photon emission tomography (SPECT), after substantial computational effort.
Thus, methods of analysis of 3D scans based on the idealized geometry of the lung anatomy are presently the only viable method for evaluating deposition of aerosol beyond subsegmental generations.(12) Furthermore, the limited availability of 3D scanners and the cost and complexity of the imaging methods limits the clinical applicability of these methods.(3,4,11) Two-dimensional scans, in contrast, are widely used due to their relatively lower cost, acceptable subject radiation exposure, and predictive value regarding the total lung deposition of the aerosol. However, quantifying the regional distribution of deposition is limited to large regions of interest that, without additional knowledge, preclude a quantitative evaluation of the partition of aerosol deposition even between central and distal airways.(3,4,11)
Previous studies have proposed methods to use volumetric 3D information to evaluate the deposition of an aerosol obtained through 2D imaging methods. Schroeter et al.(23) described a method, where detailed airway dimensions and lung boundaries obtained for a standard lung model were used to interpret data obtained from scintigraphy deposition images. Using the 3D model they conducted simulations of 2D projections on a planar view to evaluate the deposition pattern from scintigraphy scans. However, the implementation of the method to analyze clinical data, where the size and shape of the lungs can vary between subjects, was not described making it difficult to compare their method with ours.
The manual central and peripheral ROIs were manually drawn based on the thresholded scintigraphy of each patient. The same thresholded scintigraphic image was used to guide the computer-aided coregistration of the scaled peripheral lung ROI to it. It is possible that using the recommended method of using a transmission scan to define the outer perimeter of the lung could reduce the variability of the method, particularly on subjects with large ventilation defects; it is not clear that the intraoperator variability would have been reduced in this set of subjects not showing obvious ventilation defects. However, in general, it is likely that the use of a transmission scan to delineate the outer contour of the lung may also reduce intraoperator variability to a similar extent.
In other studies, information of the space occupied by different airway generations in concentric shells in the lung was derived from idealized models of the lung(12) and, more recently, the method was combined with data from coimaged respiratory anatomy.(3) Simulation studies showed that planar imaging properly adjusted with attenuation and scatter corrections, provided reasonable accuracy and precision for global deposition compared with that obtained from 3D images obtained with single SPECT.(24) Such an approach provides a more granular characterization of the aerosol distribution than that given by the 2D P/C or PI parameters. Similarly, other studies(12,25,26) used lung models derived from magnetic resonance imaging to analyze planar images and radioaerosol distribution to evaluate the PI of inhaled aerosols.
However, those methods involve substantial computational effort and require patient-specific anatomic 3D data, adding a barrier for achieving their broad dissemination. This article describes an approach to derive from chest CT scans 3D anatomical information averaged for a population of human subjects. That information, combined with a computer-assisted procedure was used to generate anatomically shaped 2D ROIs that included estimates of lung volume covered by those ROIs and of the central and distal airways within them. Using this approach, we could characterize the central-to-distal airways deposition distribution of aerosol from clinical 2D scintigraphy scans, and could obtain objective and reproducible values of aerosol PIs.
Our method of analysis for 2D scintigraphy images has computerized features that simplify its implementation and makes it broadly applicable to clinical scintigraphy scans. For example, the 3D geometry and volume information was obtained using the 3D gray-scale analysis as previously described.(17) That analysis uses the concept of VIM to characterize 3D anatomical imaging data from central and distal airways from high-resolution computed tomography (HRCT) scans accounting for the blurring effects of breathing motion and the limited spatial resolution of the specific nuclear medicine technique.
Each subject's VIM was scaled to a standard lung shape and size and the resulting average VIM was used to define anatomically based central and peripheral 2D ROIs as well as to provide estimates of the volume of the central and the distal airways occupying each of them. For comparison with current methods, we defined a second central ROI with the standard rectangular shape.(1) Using a computer-aided algorithm, the lung shape, and its corresponding ROIs for the central airways were superimposed over the scintigraphy data of the subject and scaled to match the shape of the subject's lung. The program then estimated values for conventional parameters of pulmonary deposition distribution, such as P/C and PI, defined for the 2D and 3D frameworks.
The total time for analysis of a scintigraphy scan was less than 5 minutes. In the present study, despite the fact that we dimensioned a VIM for each subject, and in our sample did not have very thin individuals or obese subjects, or those with scoliosis, new studies will be necessary to consider the limitations of our method in those population of subjects.
Standard 2D P/C and PI analysis
The standard analysis of 2D scintigraphy scans yields parameters of central-to-peripheral or P/C. These parameters are highly dependent on the shape and size of the ROI used,(2) which tend to be variable between laboratories. Differences in P/C between studies can also be caused by differences in imaging methods, aerosol particle sizes, breathing patterns, and the diseases studied. In asthmatics, for example, the difference in inspiratory flow and the degree of obstruction cause a heterogeneous pattern of deposition,(27) concentrating on areas less compromised by the disease. In general, the distribution of the deposition in asthmatics tends to be elevated in the central airways,(28) but this effect is reduced for slow inhalations and for aerosols with smaller particle size.
Reproducibility of P/C values
We found that values of P/C obtained using our computer-assisted method, with either bROI or the aROI, were more reproducible (lower intrasubject COV of five independent measures) than those calculated with the manually defined lung ROI. This is expected given that the definition of the lung ROI is restricted to four degrees of freedom (x, y location, width, and height) and the software requires little training of the operator, whereas the manually defined lung ROI can vary substantially between measures, even when conducted by an experienced operator.
To define objectively the external shape of the lung ROI, an additional volume (Radioactive Xenon-133 [133Xe]), ventilation (Kripton [81mKr]), or transmission scan is recommended.(11) The 133Xe scan has the additional advantage of providing quantitative approximation of the lung volume being sampled by each 2D pixel, but as the 81mKr scan, it may provide erroneous lung shape and volume values in heterogeneously ventilated lungs (such as in severe asthma). The transmission scan provides a more robust delineation of the lung outline, but has no information about the depth of the lung being sampled by each voxel.
The PI2D is calculated using the 133Xe scan as the P/C deposition counts ratio, normalized by the P/C counts ratio of the 133Xe volume of lung subtended by the corresponding 2D ROIs. This definition is equivalent to the inverse of the normalized regional particle deposition (nC/P)−1.(29) Our method uses a population-averaged data scaled to the specific patient's lung size to define the outside shapes of the lungs and the central 2D ROIs and, as the 133Xe scan, provides estimates of regional lung volumes within the ROIs, and also the volumes within them that are occupied by central or peripheral airways without the need for a second scan.
The value of PI2D has been shown to be less sensitive to the shape used for defining the 2D ROIs and thus to better represent the distribution of radioaerosol within the ROIs.(2) Consistent with the findings of Biddiscombe et al.,(2) our results showed that the systematic differences in P/C caused by the shape of the central ROI (rectangular box vs. the aROI), were virtually eliminated when the PI2D was calculated using our method (Fig. 7). This indicates that the difference in P/C for the differently shaped central ROIs in our scans were fully accounted for by differences in the lung volumes subtended by the corresponding ROIs.
Considering that the regional lung volumes in our study were not measured for each subject, but instead estimated based on a population-averaged CT data scaled to the size of each subject, the insensitivity of the obtained values of PI2D to the central ROIs shape is an indirect validation of our method of estimating the subtended lung volumes. For physiological interpretation of the data, it is important to note that the apparently preferential deposition in peripheral airways with a P/C > 1 was actually found to be a preferential central deposition (PI2D < 1) in all of our subjects.
The average value of the PI2D for the bROI in our asthmatic subjects (mean = 0.615) compares well with the nC/P−1 = 0.62 reported for healthy individuals by Zeman et al.(29) In that study, the shape of the central ROI was the standard box, and the shape of the lung ROI, and the volumes of subtended lung under each ROI, were defined using Xe133 scans. This similarity in values is unexpected given that the PI2D from asthmatic subjects should have been substantially lower than those for healthy subjects. However, the very small aerosol particle size of the current study (∼0.9 μm MMAD) was much <5.4 m MMAD used by Zeman et al.,(29) possibly explaining the higher PI2D values in our study.
Biddiscombe et al.(2) also used the standard box ROI and Xe133 scans for analysis of deposition of monodisperse aerosols in mild asthmatic subjects. The authors reported values of PI2D raging from 0.81 to 0.50 for particle sizes of 1.5 and 3 μm MMAD, respectively. Our value of PI2D is within that range and we speculate that the deeper penetration expected for our smaller aerosol particles could have more than compensated by the greater severity of our moderate-to-severe subjects. Taken these results together, they suggest that the average values of PI2D obtained with our method are comparable with those reported in the literature after considering the differences in aerosol characteristics and disease severity.
PI3D analysis
The standard 2D ROIs divide the lung field into central and peripheral regions, each including different fractions of the central and distal airways of the bronchial tree. Our averaged CT imaging data allowed us to estimate the relative fraction of the volume occupied by central airways in the central aROI (1.28%) as well as in the bROI (0.98%).
Due to the manner in which the aROI was defined, the fraction of volume occupied by central airways was 30% greater than that under the bROI. Thus, due to its shape resembling that of the anteroposterior projection of the central airways, the aROI was more likely to include central airways than the bROI, even in the presence of random registration error between the ROIs and the anatomical structures. This feature could explain the lower average COV of the PI2D for the aROI (0.59) compared with that for the bROI (0.64).
Advantageously, knowledge of the volume occupied by central and distal airways within the 2D ROIs allowed us to estimate a PI3D, defined as the ratio of activity per unit volume between distal and proximal airways. This parameter has been previously calculated from PET/CT images of aerosol deposition and is thought to be a better estimator of the true penetration of the aerosol to distal airways.(17) The estimates of PI3D for our 2D scans ranged from 0.01 to 0.1 with an average of 0.0357 for the aROI and 0.031 for the bROI. These numbers are higher than those measured with PET(17) in a previous study in asthmatics bronchoconstricted with methacholine (mean 0.0086 ± 0.0039) and inhaling with tidal breathing an aerosol with 4.1 μm MMAD.
Thus, it is likely that the higher values of PI3D measured in the current study could be attributed to the smaller particle size of the aerosol (0.9 μm MMAD) and to differences in breathing pattern. In the PET studies the subjects were allowed to breath at spontaneous tidal volumes and frequencies (9–18 bpm), whereas in the present study, the subjects were instructed to take controlled deep breaths followed by 3 seconds of breatholding.
A comparison between the values of PI2D and PI3D for each subject showed that, regardless of the central 2D ROI shape, the values of PI2D always overestimated those of the PI3D by 32 times for the aROI and 40 times for bROI. This overestimation is expected as it reflects the high contribution of peripheral airways to the radioactivity sampled from the central 2D ROI reducing the denominator in the calculation of PI2D.
Tossici-Bolt et al.(12) compared results from planar lung image of healthy volunteers against those obtained with a 3D equivalent representation derived from SPECT images using a custom software assigning activity to the different generations of the bronchial tracheobronchial tree. These authors also concluded that approximate estimates of deposition per generation could be derived from planar imagery with similar accuracy for bronchial and conducting airways. However, the errors are higher than with SPECT.
Similar to the findings of Tossici-Bolt et al.,(12) in our study, we found a close relationship between PI2D and PI3D values, but the values of PI2D were 32 times higher than those of PI3D using aROI (p < 0.001) and 40 times higher (p < 0.001) using the bROI.
Limitations
As described above, in our method we scale averaged anatomical and volumetric data to the specific patient size and lung aspect ratio (height/width). Thus, these data are not actually measured from the individual subject. In spite of this limitation, the normalization of the regional activity by the predicted volumes in the PI2D eliminates the difference in P/C caused by the 2D ROI shape, a finding consistent with that observed when volumes are measured for each patient with a second Xe133 scan.(2) It is worth emphasizing that our method yields a PI2D parameter without the need of the second scan, broadening its applicability to laboratories without such capability.
However, one needs to be aware that anatomical difference in the shape of the lungs, or the location of the airway tree within the chest in certain subjects, could result in erroneous estimates of lung and airway volumes. For example, lung hyperinflation with barrel chest, or localized areas of tissue destruction, could introduce a systematic bias in the calculations of PI2D or PI3D in patients with COPD. This bias may, or may not, be larger than that caused by the error induced by the heterogeneous distribution of ventilation and lung parenchyma in that disease.
In this study, we implemented our method to retrospectively analyze a set of existing deposition scintigraphy scans and demonstrated that it could provide objective distribution deposition parameters that were more reproducible than those estimated by defining the lung shape with the manual technique and using the standard rectangular shape for the central ROI. However, in our study it was not possible to carry out comparative analysis of the volumes and distribution parameters obtained using 2D or 3D scans because the institution where the scintigraphy data were collected, as many laboratories around the world, lacks the 3D imaging capability. Thus, the method awaits further validation comparing its predictions against those using a second Xe133, or 3D PET/CT scans.
Similarly, the method awaits validation using 3D anatomical data from healthy volunteers and other populations, since the anatomical and aerosol deposition data from this study were from patients with asthma who could have had specific characteristics.
It must also be pointed out that in the standard calculation of PI2D using a second Xe133 scan, there is no need to correct for attenuation of the radioactivity by the tissues, since both the deposition and the lung volume scan are regionally affected by attenuation in the same proportion. With our method, since the subtended volumes were estimated from the HRCT scan, and thus their values were not affected by attenuation, to estimate the PI2D or PI3D, it was necessary to account for the differences in attenuation between the two ROIs in the deposition scans. Regional attenuation in this article was estimated from a previous theoretical work(22) that, using CT scans, assigned attenuation coefficient factors of kc = 4.7 and kp = 3.0 for the central and peripheral ROIs. Initially we assumed that the ratio of these factors was the same for all subjects and independent of the geometrical details of the ROIs.
Although the magnitude of the differences has not been evaluated, it is expected that attenuation factors could be different for different subjects, particularly if they are obese. Nonetheless, the regional attenuation approximation could be avoided if attenuation factors are estimated directly for each subject from existing CT scans. In spite of this limitation, our assumption gave reasonably robust values of PI2D that were comparable to those reported in the literature, and had acceptable intraoperator variability of the PI2D among five samples, COV = 0.056 for the aROI, and 0.062 for the bROI.
However, for estimating values of PI3D, we noted that in two subjects their intrasubject variability was substantially elevated (COV ∼1.2 for bROI and ∼0.64 for aROI) compared with that for the rest (COV ∼0.17 for bROI and ∼0.16 for aROI). Further examination of the data revealed that the average PI3D values for those subjects were three to nine times greater than the average of the rest of the subjects. We speculated that in those subjects using an average value of the attenuation coefficients may have introduced substantial errors. Thus, in each of those subjects we adjusted iteratively the attenuation coefficients until values of PI3D were similar to those of the average subject. We found that adjusting the attenuation coefficients of the central regions by factors of 1.5 and 2 in these two subjects, the corresponding intrasubject variability was substantially reduced to values equal to those of the rest.
Although not a proof, this finding suggests that in those subjects the attenuation of central regions could have been particularly elevated. Clearly, future work will require the development of a method to estimate the attenuation coefficients in a subject-specific manner from CT scans or from transmission scans to evaluate the potential error generated by this assumption.
Conclusion
In conclusion, our study demonstrates that P/C is influenced by the shape of the 2D ROIs used overestimating values, when the central region was defined as a rectangular shape, bROI, compared with that using the aROI. Such difference was eliminated when the P/C values were normalized by the estimated regional volumes predicted by our averaged anatomical data.
Values of PI derived from our analysis defined for the 2D or 3D conditions, were of the same order of magnitude as those described in the literature accounting for differences in aerosol particle size, breathing pattern, and disease severity. PI2D values were comparable to those obtained by conventional methods estimating the regional lung from a second Xe133 scan, providing evidence for the validity for our quantitative method. PI3D values were higher than those reported for PET/CT in the literature, possibly because of differences in methodology and degree of obstruction of the subjects.
Thus, our results demonstrated the feasibility of using population-averaged data from a previous CT study(17) to generate realistic shapes of 2D ROIs and estimate the lung and airway's volume subtended by those ROIs. These shapes and volumes further allowed the calculation of realistic central-to-peripheral distribution parameters in 3D from single 2D scintigraphy scans. We hope that this approach may be incorporated to the clinical practice and allow more robust and representative estimates of aerosol deposition distribution in a normal and diseased lung.
Acknowledgments
Elliot Greenblatt, PhD generated the average VIM data from HRCT scans and developed an early version of the software used for the scintigraphic analysis. This study is sponsored by NIH grant R21EB020849, Santander—TOP USA Massachusetts Program (Brazil), CNPq-PVE 400801/13-2, and FACEPE-APQ-0154-408/15.
Author Disclosure Statement
The authors declare they have no conflicts of interest to report.
Reviewed by:
Martyn Biddiscombe
Kirby Zeman
References
- 1.Newman S, Bennett WD, Biddiscombe M, Devadason SG, Dolovich MB, Fleming G, Haeussermann S, Kietzig C, Kuelh PJ, Laube BL, Sommerer K, Taylor G, Usmani OS, and Zeman KL: Standardization of techniques for using planar (2D) imaging for aerosol deposition assessment of orally inhaled products. J Aerosol Med Pulm Drug Deliv. 2012;25:510–528 [DOI] [PubMed] [Google Scholar]
- 2.Biddiscombe MF, Meah SN, Underwood SR, and Usmani OS: Comparing lung regions of interest in gamma scintigraphy for assessing inhaled therapeutic aerosol deposition. J Aerosol Med Pulm Drug Deliv. 2011;24:165–173 [DOI] [PubMed] [Google Scholar]
- 3.Conway J, Fleming J, Bennett M, and Havelock T: The co-imaging of gamma camera measurements of aerosol deposition and respiratory anatomy. J Aerosol Med Pulm Drug Deliv. 2013;26:123–130 [DOI] [PubMed] [Google Scholar]
- 4.Dolovich MB: Measuring total and regional lung deposition using inhaled radiotracers. J Aerosol Med. 2001;14:35–44 [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain MJ, Morgan WK, and Vinitski S: Factors influencing the regional deposition of inhaled particles in man. Clin Sci. 1983;64:69–78 [DOI] [PubMed] [Google Scholar]
- 6.Graham DR, Chamberlain MJ, Hutton L, King M, and Morgan WK: Inhaled particle deposition and body habitus. Br J Ind Med. 1990;47:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett WD, Xie M, Zeman K, Hurd H, and Donaldson S: Heterogeneity of particle deposition by pixel analysis of 2D gamma scintigraphy images. J Aerosol Med Pulm Drug Deliv. 2015;28:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheuch G, Bennett W, Borgstrom L, Clark A, Dalby R, Dolovich M, Fleming J, Gehr P, Gonda I, O'Callaghan C, Taylor G, and Newman S. Deposition, imaging, and clearance: What remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 2):S39–S57 [DOI] [PubMed] [Google Scholar]
- 9.Newman SP. and Wilding IR: Gamma scintigraphy: An in vivo technique for assessing the equivalence of inhaled products. Int J Pharm. 1998;170:1–9 [Google Scholar]
- 10.Conway J: Lung imaging—Two dimensional gamma scintigraphy, SPECT, CT and PET. Adv Drug Deliv Rev. 2012;64:357–368 [DOI] [PubMed] [Google Scholar]
- 11.Darquenne C, Fleming JS, Katz I, Martin AR, Schroeter J, Usmani OS, Venegas J, and Schmid O: Bridging the gap between science and clinical efficacy: Physiology, imaging, and modeling of aerosols in the lung. J Aerosol Med Pulm Drug Deliv. 2016;29:107–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tossici-Bolt L, Fleming JS, Conway JH, and Martonen TB: An analytical technique to recover the third dimension in planar imaging of inhaled aerosols—2 Estimation of the deposition per airway generation. J Aerosol Med. 2007;20:127–140 [DOI] [PubMed] [Google Scholar]
- 13.Fleming J, Conway J, Majoral C, Tossici-Bolt L, Katz I, Caillibotte G, Perchet D, Pichelin M, Muellinger B, Martonen T, Kroneberg P, and Apiou-Sbirlea G: The use of combined single photon emission computed tomography and X-ray computed tomography to assess the fate of inhaled aerosol. J Aerosol Med Pulm Drug Deliv. 2011;24:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett WD, Brown JS, Zeman KL, Hu SC, Scheuch G, and Sommerer K: Targeting delivery of aerosols to different lung regions. J Aerosol Med. 2002;15:179–188 [DOI] [PubMed] [Google Scholar]
- 15.Dolovich MB, Sanchis J, Rossman C, and Newhouse MT: Aerosol penetrance: A sensitive index of peripheral airways obstruction. J Appl Physiol. 1976;40:468–471 [DOI] [PubMed] [Google Scholar]
- 16.Newman S. and Fleming J: Challenges in assessing regional distribution of inhaled drug in the human lungs. Expert Opin Drug Deliv. 2011;8:841–855 [DOI] [PubMed] [Google Scholar]
- 17.Greenblatt EE, Winkler T, Harris RS, Kelly VJ, Kone M, and Venegas J: Analysis of three-dimensional aerosol deposition in pharmacologically relevant terms: Beyond black or white ROIs. J Aerosol Med Pulm Drug Deliv. 2015;28:116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenblatt EE, Winkler T, Harris RS, Kelly VJ, Kone M, Katz I, Martin A, Caillibotte G, Hess DR, and Venegas JG: Regional ventilation and aerosol deposition with helium-oxygen in bronchoconstricted asthmatic lungs. J Aerosol Med Pulm Drug Deliv. 2016;29:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global Initiative for Asthma: Global strategy for asthma management and prevention. Update 2015. Available from http://www.ginasthma.org/local/uploads/file/Gina—Report2015.pdf Last access: April5, 2018
- 20.Pereira CAdC, Barreto SdP, Simöes JG, Pereira FWL, Gerstler JG, and Nakatani J: Valores de referência para a espirometria em uma amostra da populaçäo brasileira adulta. Reference values for spirometry in Brazilian adults. J Pneumol. 1992;18:10–22 [Google Scholar]
- 21.Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agusti A, Criner GJ, MacNee W, Make BJ, Rennard SI, Stockley RA, Vogelmeier C, Anzueto A, Au DH, Barnes PJ, Burgel PR, Calverley PM, Casanova C, Clini EM, Cooper CB, Coxson HO, Dusser DJ, Fabbri LM, Fahy B, Ferguson GT, Fisher A, Fletcher MJ, Hayot M, Hurst JR, Jones PW, Mahler DA, Maltais F, Mannino DM, Martinez FJ, Miravitlles M, Meek PM, Papi A, Rabe KF, Roche N, Sciurba FC, Sethi S, Siafakas N, Sin DD, Soriano JB, Stoller JK, Tashkin DP, Troosters T, Verleden GM, Verschakelen J, Vestbo J, Walsh JW, Washko GR, Wise RA, Wouters EF, and ZuWallack RL; ATS/ERS Task Force for COPD Research: An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:e4–e27 [DOI] [PubMed] [Google Scholar]
- 22.Lee Z, Ljungberg M, Muzic RF, and Berridge MS: Usefulness and pitfalls of planar γ-scintigraphy for measuring aerosol deposition in the lungs: A Monte Carlo investigation. J Nucl Med. 2001;42:1077–1083 [PubMed] [Google Scholar]
- 23.Schroeter JD, Pritchard JN, Hwang D, and Martonen TB: Airway identification within planar gamma camera images using computer models of lung morphology. Pharm Res. 2005;22:1692–1699 [DOI] [PubMed] [Google Scholar]
- 24.Fleming JS, Conway JH, Bolt L, and Holgate ST. A comparison of planar scintigraphy and SPECT measurement of total lung deposition of inhaled aerosol. J Aerosol Med. 2003;16:9–19 [DOI] [PubMed] [Google Scholar]
- 25.Newman SP, Pitcairn GR, Hirst PH, and Rankin L: Radionuclide imaging technologies and their use in evaluating asthma drug deposition in the lungs. Adv Drug Deliv Rev. 2003;55:851–867 [DOI] [PubMed] [Google Scholar]
- 26.Fleming JS, Conway JH, Holgate ST, Moore EA, Hashish AH, Bailey AG, and Martonen TB: Evaluation of the accuracy and precision of lung aerosol deposition measurements from planar radionuclide imaging using simulation. Phys Med Biol. 1998;43:2423–2429 [DOI] [PubMed] [Google Scholar]
- 27.Greenblatt EE, Winkler T, Harris RS, Kelly VJ, Kone M, Katz I, Martin AR, Caillibotte G, and Venegas J: What causes uneven aerosol deposition in the bronchoconstricted lung? A quantitative imaging study. J Aerosol Med Pulm Drug Deliv. 2016;29:57–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galindo-Filho VC, Brandao DC, Ferreira Rde C, Menezes MJ, Almeida-Filho P, Parreira VF, Silva TN, Rodrigues-Machado Mda G, Dean E, and Dornelas de Andrade A: Noninvasive ventilation coupled with nebulization during asthma crises: A randomized controlled trial. Respir Care. 2013;58:241–249 [DOI] [PubMed] [Google Scholar]
- 29.Zeman KL, Wu J, Donaldson SH, and Bennett WD: Comparison of 133 xenon ventilation equilibrium scan (XV) and 99 m technetium transmission (TT) scan for use in regional lung analysis by 2D gamma scintigraphy in healthy and cystic fibrosis lungs. J Aerosol Med Pulm Drug Deliv. 2013;26:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]