Abstract
Purpose
Severing of corneal nerves in preparation of corneal transplantation abolishes immune privilege of subsequent corneal transplants placed into either eye: a phenomenon termed sympathetic loss of immune privilege (SLIP). SLIP is due to the disabling of T regulatory cells (Tregs) by CD11c+ contrasuppressor (CS) cells. This study characterized the induction, function, and manipulation of CS cell activity and the effect of these cells on Tregs induced by anterior chamber-associated immune deviation (ACAID).
Methods
CS cells were induced using a 2.0-mm trephine to score the corneal epithelium. CD11c+ CS cells were evaluated by adoptive transfer and by their capacity to disable CD8+ ACAID Tregs in local adoptive transfer (LAT) of suppression assays. CD11c+ cells were deleted from the ocular surface by subconjunctival injection of clodronate-containing liposomes.
Results
CD11c+ CS cell were radiosenstive and long lived. As few as 1000 CS cells blocked the suppressive activity of previously generated CD8+ ACAID Tregs, indicating that CS cells act at the efferent arm of the immune response. Depletion of resident CD11c+ cells at the ocular surface prevented the generation of CS cells.
Conclusions
Corneal nerve injury that occurs during keratoplasty converts ocular surface CD11c+ cells into CS cells that block CD8+ Tregs, which are induced by introducing antigens into the anterior chamber (i.e., ACAID Tregs). Depletion of CD11c+ cells at the ocular surface prevents the generation of CS cells and may be a useful strategy for preventing SLIP and enhancing the survival of second corneal transplants.
Keywords: anterior chamber, contrasuppressor cells, cornea, immune privilege, transplantation, Tregs
The eye is exempt from many of the immunologic ground rules that are enforced in other parts of the body. This was recognized more than 145 years ago by the Dutch ophthalmologist van Dooremaal, who reported the prolonged survival of mouse skin grafts placed into the anterior chamber (AC) of the dog eye.1 Seventy-five years later, Medawar performed similar experiments and noted the extended survival of skin allografts placed into the eyes and brains of rabbits and coined the term “immune privilege” to describe this phenomenon.2 For more than a half century, immune privilege was viewed as an anatomical anomaly in which the interior of the eye lacked lymph vessels to shuttle antigens and antigen presenting cells to regional lymph nodes. The apparent sequestration of antigens resulted in what contemporary immunologist would describe as “immunological ignorance”.3 The last 50 years of research on immune privilege have revealed that the eye does in fact possesses lymphatic drainage, albeit greatly reduced compared with other organs, and the peripheral immune apparatus is keenly aware of antigens that are introduced into the AC.4,5 This was established by the seminal studies of Kaplan and Streilein, who demonstrated that antigenic cells introduced into the AC were perceived by the peripheral immune apparatus resulting the generation of a deviated immune response that was manifested as an antigen-specific downregulation of cell-mediated immunity.6 This dynamic modulation of the systemic immune response was subsequently characterized and termed AC-associated immune deviation (ACAID).7 ACAID is now recognized as an important component of immune privilege in the eye and is intimately involved in promoting the survival of orthotopic corneal allografts.8,9
Corneal allografts enjoy a remarkable success, with less than 10% undergoing immune rejection, even though HLA matching is not routinely used or needed, and topically applied steroids are the only antirejection drugs used.10 However, immune privilege is not permanent, as corneal graft rejection rises threefold in patients receiving as second corneal transplant.11 This heightened incidence of immune rejection is probably not the result of prior immune sensitization because HLA typing is not normally used, and the likelihood that hosts receiving a second transplant expressing the same histocompatibility antigens of the first transplant is remote. With this in mind, we recently used a mouse model of penetrating keratoplasty to test the hypothesis that the corneal transplantation procedure itself robs the opposite eye of its immune privilege. Our results showed that orthotopic corneal transplantation and, more specifically, the cutting of corneal nerves that occurs during the transplantation procedure in one eye abolish immune privilege in the second eye, leading to >90% incidence of graft rejection in mice receiving a second transplant.12 We subsequently found that in addition to exacerbating corneal allograft rejection in the opposite eye, severing corneal nerves also abolishes ACAID in the opposite eye.13 That is, simply severing corneal nerves with shallow circular incisions with a trephine in one eye prevents the induction of ACAID in the opposite, nonmanipulated eye. Thus, severing corneal nerves in one eye elicits a sympathetic loss of immune privilege (SLIP) for corneal allografts and for the induction of ACAID in the opposite eye.12,13 In both cases, the termination of immune privilege is closely associated with increased production of the neuropeptide substance P (SP) in both eyes and the loss of T regulatory cells (Tregs). The loss of Treg activity was found to be due to the generation of CD11c+ contrasuppressor (CS) cells that disable both CD8+ ACAID Tregs and CD4+CD25+ Tregs induced by orthotopic corneal allografts.13 The present study sought to characterize CD11c+ CS cells induced by corneal nerve ablation, determine how they are induced, and determine whether their function can be circumvented as a means of restoring ocular immune privilege.
Materials and Methods
Animals
BALB/c (H-2d) and C57BL/6 (H-2b) mice were purchased from the UT Southwestern Mouse Breeding Facility (Dallas, TX, USA). Congenic C57BL/6 mouse strains CD45.1+ (C57BL/6j) and CD45.2+ (B6Ly5.2/Cr) were purchased from The Jackson Laboratories (Bar Harbor, ME, USA). All mice were maintained in a pathogen-free environment and were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of ACAID
ACAID was induced as described previously using microinjection of antigen into the AC of the eye.13 A Hamilton automatic dispensing apparatus was used to inject 6 μL 16.67 mg/mL ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO, USA) in PBS (100 μg OVA) into the AC. Seven days after the AC injection, animals were subcutaneous (SC) immunized with 200 μg OVA in an equal volume of Complete Freud's Adjuvant (CFA; Sigma-Aldrich). Ears were challenged 7 days after SC immunization by injecting OVA (400 μg in 20 μL PBS). The opposite ear was injected with 20 μL PBS as a negative control. Ear swelling was measured 24 hours later to measure delayed-type hypersensitivity (DTH).
Subcutaneous Immunization and SP Injections
Positive control mice were sensitized by one SC injection of 250 μg OVA (Sigma-Aldrich) emulsified 1:1 in CFA in a total volume of 200 μL. In some experiments, a single intravenous (IV) injection of 1 pg SP (Sigma-Aldrich) was administered to BALB/c mice prior to their use in either ACAID investigation.
Delayed-Type Hypersensitivity Assay
DTH was measured using a conventional ear-swelling assay. An eliciting dose of 400 μg OVA in 20 μL Hank's balanced salt solution (HBSS) was injected into the SC tissue of the right ear. The left ear served as a negative control and was injected with 20 μL HBSS without cells. Results were expressed as antigen-specific ear-swelling response (experimental ear 24-hour measurement − experimental ear 0-hour measurement) − (negative control ear 24-hour measurement − negative control ear 0-hour measurement).
Antibodies and Flow Cytometry
CD8+ ACAID Tregs (see below) were interrogated for cytoplasmic expression of Foxp3 and surface expression of GITR and CTLA4 as previously described.14 The antibodies used for these flow cytometric analyses were allophycocyanin (APC)-conjugated Armenian hamster anti-mouse CTLA-4 (eBioscience, Grand Island, NY, USA), APC-conjugated rat anti-mouse GITR (eBioscience), and APC-conjugated rat anti-mouse Foxp3 (eBioscience). All flow cytometric analyses were performed on a FACSCalibur with CellQuest software (BD Biosciences, San Jose, CA, USA).
Isolation of ACAID CD8+Tregs
Spleen cells were isolated from BALB/c mice 10 days after AC injection of OVA for the isolation of CD8+ ACAID Tregs. Spleen cells from were isolated using magnetically labeled CD8 (Ly-2) microbeads. The cell suspension was then loaded onto a magnetic activated cell sorting (MACS) column, which was placed in a magnetic field of the MACS separator. The magnetically labeled CD8+ Tregs were retained within the column, and the unlabeled cells were run through. The column was removed from the magnetic field, and the retained CD8+ cells were eluted as the positively selected cell fraction. We previously found that this enrichment technique yields >95% CD8+ T cells. Flow cytometric analysis revealed that the expression of Foxp3, CTLA4, and GITR on ACAID CD8+ Tregs was not significantly different from normal CD8+ non-Tregs (data not shown).
Isolation of CS Cells
We previously reported that 2.0-mm circular incisions of the corneal epithelium induce the generation of CS cells that express the CD11c surface marker.13 Corneas of BALB/c mice were trephined as described previously, and CD11c+ spleen cells were isolated 14 days later using a Miltenyl Biotec pan dendritic cell isolation kit (Auburn, CA, USA).13 The CD11c+ cells from trephined mice and untreated mice were used in local adoptive transfer assays for detecting regulatory cell activity in vivo.13
Local Adoptive Transfer Assay
The local adoptive transfer (LAT) assay was used as an in vivo test for Treg activity.13 ACAID CD8+ Tregs (1 × 106) were incubated with BALB/c APC pulsed with OVA and immune CD4+ T cells from SC immunized BALB/c mice. Cells were mixed in a 1:1:1 ratio. The right ears of naive BALB/c mice were injected with 20 μL of the mixed-cell population. The opposite ear was injected with 20 μL HBSS as a negative control. Ear swelling was measured 24 hours later to measure DTH. In some experiments, CS cell activity was assessed by mixing CD11c+ CS cells with immune cells, CD8+ Tregs, and OVA-pulsed APC at a 1:1:1:1 ratio.
CS Cell Cytotoxicity Assay
Cytotoxicity assays to test whether the CS cells can inhibit Tregs were performed using CFSE (carboxyfluorescein succinimidyl ester; Invitrogen, Waltham, MA, USA). OVA was injected into the AC of mice, and 10 days later, CD8+ Tregs were isolated from the spleens using the Treg isolation kit (Miltenyi Biotec). CS cells were induced by trephining the corneas of both eyes and isolating CD11c+ cells 14 days later using a pan dendritic cell isolation kit (Miltenyi Biotec). Tregs were labeled with 5 μM CFSE/mL at room temperature for 10 minutes. After 10 minutes, three times the volume of cold PBS + BSA was added and incubated on ice for 2 minutes. The cells were washed twice and centrifuged at 300g for 10 minutes with 10 mL culture medium (RPMI + 2 mercaptoethanol). Tregs were suspended at 2.5 × 105 cells/mL. A total of 5 × 105 CS cells were incubated with 2.5 × 105 CFSE-labeled CD8+ Tregs. After overnight incubation at 37°C, the CFSE-labeled cells were analyzed by flow cytometry using the Attune NxT acoustic focusing cytometer (Applied Biosystems; Life Technologies, Grand Island, NY, USA). The data from flow cytometer were analyzed using FlowJo v10 software (Tree Star, Ashland, OR, USA).
Depletion of Ocular Surface Dendritic Cells
We previously demonstrated that subconjunctival injection of liposomes containing dichloromethylene diphosphonate (clodronate) depletes CD11b+ DC, CD11c+ DC, and Iba+ macrophages at the ocular surface.15 Accordingly, clodronate-containing liposomes and PBS-containing liposomes were prepared as described elsewhere.16 Either PBS or clodronate liposomes were injected subconjunctivally (6 μL) on the same day that the eyes were trephined.
Bone Marrow Chimeras
The corneal nerves in the right eyes of congenic CD45.2 C57BL/6 mice were severed using a 2.0-mm trephine. Fourteen days later, the mice were given lethal whole-body radiation (13.5 Gy) split in two doses that were 3 hours apart. Six hours later, mice were reconstituted with 2 × 107 bone marrow (BM) cells from CD45.1+ congenic C57BL/6 donors. After 21 days, CD45.1/CD11c+ cells and CD45.2/CD11c+ cells were isolated using a cell sorter (Sony, San Jose, CA, USA) and used in a LAT assay with ACAID Tregs.
SP ELISA
An “in vitro LAT assay” was used to test whether the CD11c+ CS cells produced SP. The corneas of BALB/c mice were trephined, and CD11c+ CS cells were isolated 14 days later using the Miltenyl Biotec pan dendritic cell isolation kit. The CD11c+ cells (1 × 106) from the trephined mice were cocultured with ACAID CD8+ Tregs, BALB/c APC pulsed with OVA, and immune CD4+ T cells from SC-immunized BALB/c mice in the ratio of 1:1:1:1 in a total volume of 200 μL in a 96-well plate. The CD11c− cells from trephined mice were cocultured in a similar way with the CD8+ Tregs, OVA APCs, and immune cells in a 1:1:1:1: ratio. After a 24-hour incubation at 37°C, the 96-well plates were centrifuged, and 50 μL cell-free supernatant was used to measure SP levels by ELISA (Cayman Chemical, Ann Arbor, MI, USA).
Statistical Analysis
Results for DTH assays and ELISAs were evaluated by Student t-test. Results are expressed as mean ± SEM. Differences in all experiments were considered statistically significant if P < 0.05.
Results
CD11c+ CS Cells Induced by Corneal Nerve Ablation Are Long-Lived, Radiosensitive, and Not Produced Continuously
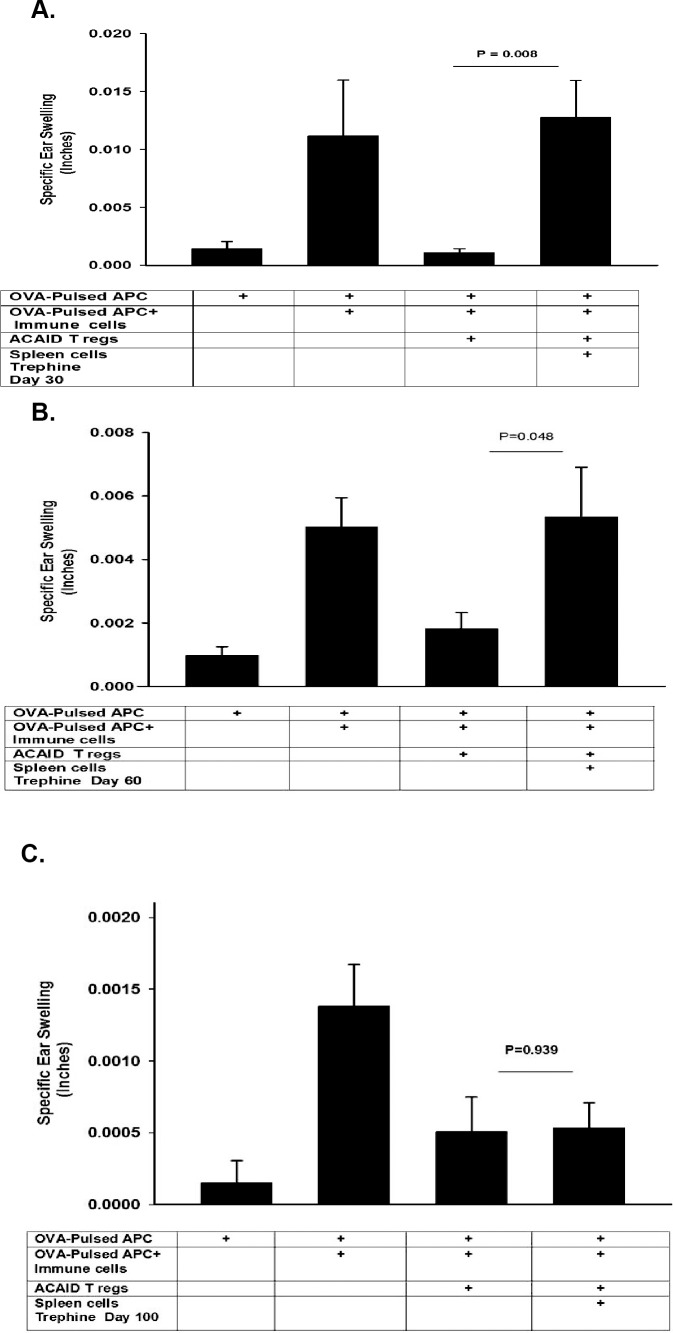
We previously reported that corneal nerve ablation abolishes immune privilege of corneal allografts that persists for at least 100 days.12 Corneal nerve ablation also induces CS cells that inhibit CD8+ ACAID Tregs and can be detected as early as 14 days after severing the corneal nerves.13 Accordingly, it was important to determine whether corneal nerve ablation produced a long-lived abrogation of ACAID similar to that which occurs with orthotopic corneal allografts. This was determined by trephining the right eyes of BALB/c mice and collecting CD11c+ CS cells from the spleen 30, 60, or 100 days later. Putative CS cells were examined for their capacity to inhibit OVA-specific CD8+ ACAID Tregs in a conventional LAT assay. As reported previously, spleen cells from trephined mice blocked CD8+ ACAID Treg suppression of DTH.13 The abrogation of CD8+ ACAID Treg activity was detected 30 and 60 days after trephining the cornea, but the CS cell activity was no longer present at day 100 (Figs. 1A–1C).
Figure 1.
Longevity of CS cells induced by corneal nerve ablation. The right eyes of BALB/c mice were trephined and CD11c+ DCs were collected from the spleens 30, 60, or 100 days later and tested for CS activity in a LAT assay. CD11c+ CS cells blocked ACAID Treg activity 30 days (A), 60 days (B), but not 100 days (C) after trephining the eye. Each experiment was performed at least twice with similar results. Results are expressed as mean ± SEM.
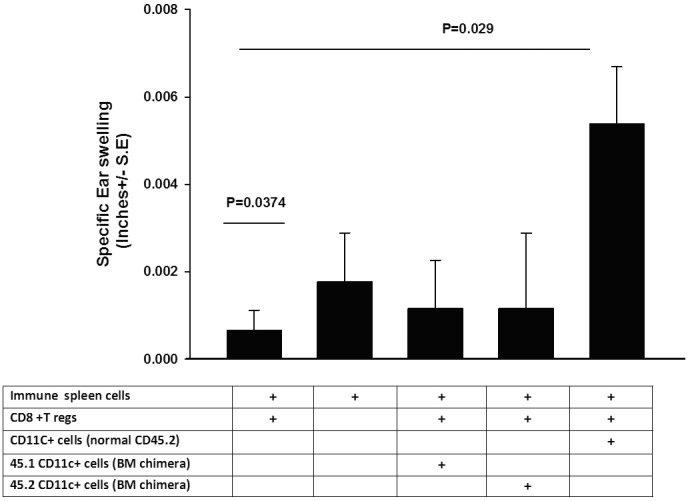
The presence of CS cell activity 60 days after corneal nerve ablation suggested that perhaps CD11c+ CS cells were continuously generated or that an initial wave of CS cells was long lived. To address this, the eyes of CD45.2 congenic C57BL/6 mice were trephined. Fourteen days later, mice were subjected to lethal whole-body ionizing radiation (13.5 Gy) and were infused with BM cells from CD45.1+ C57BL/6 congenic donor mice. CD11c+ spleen cells were collected 21 days after BM reconstitution and were sorted by flow cytometry into CD45.1+ and CD45.2+ cells and were tested for CS cell activity in a LAT assay. The results showed that neither CD45.1+ nor CD45.2+ cells from BM chimeric mice expressed CS activity (Fig. 2). However, CD11C+ cells that were collected from CD45.2+ congenic mice subjected to trephining but not exposed to ionizing irradiation and BM reconstitution displayed CS cell activity, indicating that the absence of CS cell activity in the CD45.2+ congenic mice subjected to irradiation and BM reconstitution was not due to an inability of the CD45.2+ congenic mouse to develop normal CS cell activity after trephining. The results indicate that CS cells were generated only during the first 14 days after trephining and that the initial population of CD11c+ CS cells was radiosensitive.
Figure 2.
CD11c+ CS cells induced by corneal nerve ablation are short lived and radiosensitive. Both eyes of CD45.2+ congenic C57BL/6 mice were trephined and subjected to lethal whole-body irradiation (13.5 Gy) 14 days later. Mice were reconstituted with CD45.1+ C57BL/6 BM cells immediately after whole-body irradiation. CD11c+ spleen cells were collected 21 days later, sorted by flow cytometry into either CD45.1+ or CD45.2+ cells, and used in LAT assays. CD11c+ spleen cells were collected from nonirradiated CD45.2+ congenic C57BL/6 mice 21 days after trephining and were also tested for CS cell activity in a conventional ACAID LAT assay. Results are expressed as mean ± SEM. This experiment was performed twice with similar results. There were five mice in each group.
Minimum Number of CS Cells That Block CD8+ Tregs
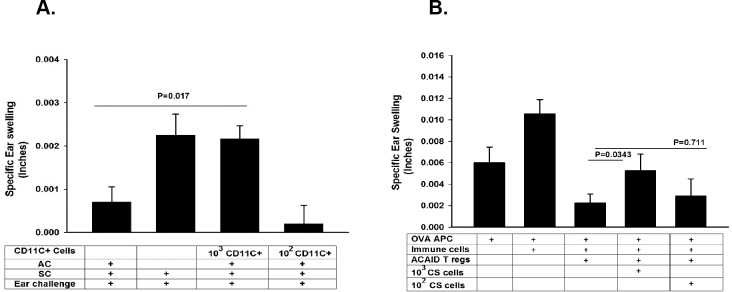
CD11c is expressed on various cells including dendritic cells (DCs), as well as CS cells that are induced by corneal nerve ablation.13 One of the remarkable features of DCs is their potency in stimulating T-cell responses. As few as 10 allogeneic dendritic Langerhans cells can induce allospecific cytotoxic T-lymphocyte (CTL) responses and elicit accelerated rejection of allogeneic skin grafts.17 With this in mind, we sought to determine the minimum number of CD11c+ CS cells that would block the action of CD8+ ACAID Tregs. Two approaches were used. This first strategy sought to determine the minimum number of CD11c+ CS cells that, when adoptively transferred to naïve recipients, would prevent the induction of ACAID. The right eyes of naïve BALB/c mice were trephined, and CD11c+ cells were isolated from the spleens 14 days later using a Miltenyl Biotec pan dendritic cell isolation kit.13 Either 1 × 102 or 1 × 103 CD11c+ cells were injected IV into naïve BALB/c mice. One day later, mice were primed in the AC with OVA to induce ACAID. The results showed that ACAID was blocked in recipients of as few as 1 × 103 CD11c+ cells from trephined donors. The OVA-specific DTH responses in these mice were the same as the positive control mice but significantly different from the ACAID controls, indicating that Tregs either had not been induced or they were silenced by the adoptively transferred CD11c+ CS cells (Fig. 3A). A LAT assay was used to determine whether the CS cells blocked the suppressive activity of ACAID Tregs after they had been induced. In the LAT assay, CD8+ Tregs were collected from mice primed in the AC with OVA and were mixed with immune spleen cells from mice immunized SC with OVA, along with OVA-pulsed APC and either 1 × 102 or 1 × 103 CD11c+ CS cells. The results revealed that ACAID Treg activity was inhibited by 1 × 103 but not by 1 × 102 CD11c+ CS cells (Fig. 3B). Collectively the results indicated that as few as 1000 CD11c+ CS cells can block the suppressive activity of CD8+ Tregs in situ, indicating that the CS cell activity acts at the efferent arm of the immune response and disables Treg activity even after the Tregs have been induced.
Figure 3.
Minimal number of CD11c+ CS cells that disable ACAID Treg activity. (A) Minimal number of CD11c+ spleen cells required for the adoptive transfer of contrasuppression was determined in BALB/c mice. The right eyes of BALB/c mice were trephined, and CD11c+ cells were collected from the spleens 14 days later and adoptively transferred to naïve BALB/c mice. One day later, ACAID was induced by AC injection of OVA followed by SC immunization with OVA plus CFA. (B) Minimum number of CD11c+ cells that ablate Tregs in situ was determined by isolating CD11c+ spleen cells from BALB/c mice 14 days after trephining the eyes and testing in a LAT assay containing CD8+ Tregs induced by AC priming with OVA. Results are expressed as mean ± SEM. This experiment was performed twice with similar results. There were five mice in each group.
SP Converts Naïve CD11c+ Cells Into CS Cells
Severing of corneal nerves is known to upregulate the expression of SP and the SP receptor, NK1-R, in the anterior segment of the mouse eye.12,13 The limbus of the mouse eye is richly endowed with CD11c+ DCs, which places them in close proximity to the area were the trephine incisions are made and SP accumulates. DCs stimulated through NK1-R display increased expression of costimulatory molecules CD80, CD86, and CD40 and elaborate elevated amounts of IL-12.18 Moreover, DCs exposed to an NK1-R agonist inhibit the production of IL-10,18 a molecule that is crucial for the induction of ACAID.19 We next tested the hypothesis that, in the trephined eye, locally produced SP converts DC into CD11c+ cells with CS cell activity. To determine whether SP could convert naïve CD11c+ DCs into CS cells, naive splenic CD11c+ cells were incubated in vitro with either 1.0 pg SP/mL or in PBS for 1 hour, washed in HBSS, and injected IV into naïve BALB/c mice. Ten days later, ACAID was induced by AC injection of OVA, followed by SC immunization with OVA in CFA. The results show that CD11c+ cells conditioned with SP prior to IV injection prevented the development of ACAID, whereas CD11c+ cells exposed to PBS had no effect (Fig. 4A). Although adoptive transfer of SP-conditioned DC prevented ACAID, it was not clear if these DCs prevented the induction of ACAID or if they disabled ACAID Tregs once they were generated by AC injection of OVA. To test this, LAT assays were performed to determine whether SP-conditioned CD11c+ DCs were able to block the suppressive activity of previously induced CD8+ ACAID Tregs. CD11c+ DCs were incubated in SP (1 pg/mL) for 1 hour in vitro, washed in PBS, and mixed with ACAID CD8+ Tregs, immune T cells from SC-immunized donor mice, and OVA-pulsed APC. Cells were injected into the ears of naïve BALB/c mice, and ear swelling DTH responses were assessed 24 hours later. The results clearly demonstrated that SP-conditioned CD11c+ DCs expressed CS activity that blocked the suppression of DTH by CD8+ ACAID Tregs (Fig. 4B).
Figure 4.
SP converts naïve CD11c+ cells into CS cells. (A) Adoptive transfer of in vitro generated CS cells was tested by incubating naïve CD11c+ BALB/c spleen cells with either SP (1 pg/mL) or PBS for 1 hour prior to IV injection into naïve BALB/c mice. ACAID was induced by AC injection of OVA, and ear swelling was assessed 10 days after SC immunization with OVA plus CFA. (B) The capacity of in vitro generated CD11c+ CS cells to disable Treg activity in situ was determined using a LAT assay. Naïve CD1c+ cells were incubated with either SP (1.0 pg/mL) or PBS for 1 hourr, washed in PBS, and added to a cell suspension containing ACAID Tregs, immune cells, and OVA-pulsed APC, which were injected into the ears of naïve mice and tested in a conventional LAT assay. Results are expressed as mean ± SEM. This experiment was performed twice with similar results. There were five mice in each group.
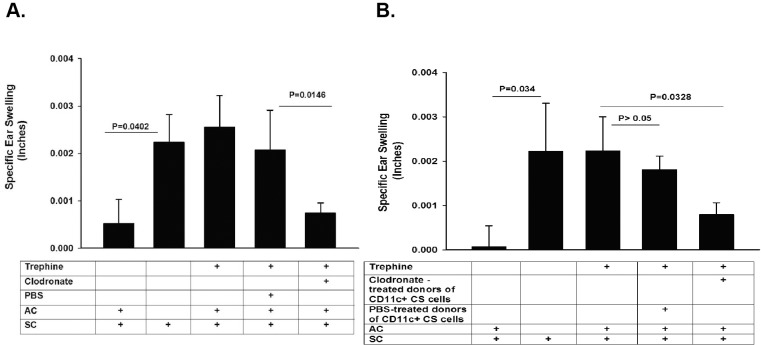
Depletion of Ocular Surface DCs Prevents the Generation of CS Cells
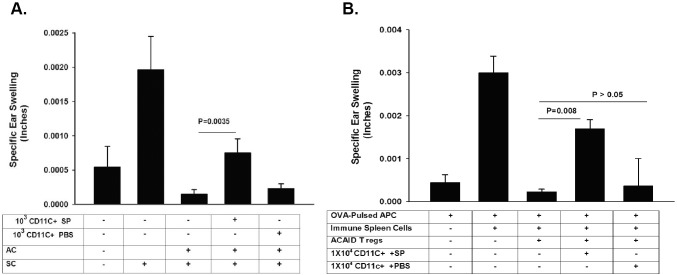
The results to this point supported the notion that corneal nerve ablation induces the local release of SP and the upregulation of NK1-R on cells in the anterior segment of the eye, which in turn converts ocular surface DCs into CS cells. To test this hypothesis, ocular surface DCs were depleted by subconjunctival injection of liposomes containing clodronate into both eyes. For comparison, PBS-containing liposomes were injected subconjunctivally. The right eye of each mouse was subjected to trephining the same day as the liposome injections, and ACAID was induced by AC injection of OVA 10 days after trephining. Mice were immunized SC with OVA emulsified in CFA 7 days after AC injections, and DTH was assessed 10 days after SC immunization. Depletion of ocular surface DC prior to corneal nerve ablation prevented the development of CS activity: these mice developed ACAID, as demonstrated by the suppression of DTH responses to OVA immunization (Fig. 5A). Additional experiments were performed to confirm that the CS cells detected in the spleens of trephined mice arose from the ocular surface. Ocular surface DCs were depleted by subconjunctival injection of clodronate-containing liposomes in both eyes immediately prior to trephining the right eye. Ten days later, CD11c+ cells were isolated from the spleens, and 1 × 104 CD11c+ cells were adoptively transferred to naïve recipients. ACAID was induced by AC injection of OVA 1 day after adoptive transfer of CD11c+ cells. Mice were immunized SC 7 days later with OVA emulsified in CFA, and DTH was measured 10 days after SC immunization. The results of these adoptive transfer experiments confirmed the role of ocular surface DCs in the generation of CS cells in trephined mice, as recipients of CD11c+ cells from mice subjected to trephining of the cornea and treated with PBS-containing liposomes failed to develop ACAID, indicating that CS cells were indeed present in the spleens after trephining (Fig. 5B). By contrast, depletion of ocular surface DCs by subconjunctival injection of clodronate-containing liposomes prevented the generation of CS cells in the spleen, and these hosts expressed suppression of DTH that was indistinguishable from that occurring in control price primed in the AC with OVA (Fig. 5B).
Figure 5.
Depletion of DCs at the ocular surface prevents the generation of CS cells. (A) Ocular surface DC were depleted by subconjunctival injection of liposomes containing clodronate into both eyes 1 day prior to trephining the right eyes of BALB/c mice. ACAID was induced by AC injection of OVA into the right eyes of BALB/c mice 10 days after trephining both eyes. Ear swelling responses were measured 10 days after SC immunization with OVA and CFA. (B) Effect of DC depletion on the generation of splenic CD11c+ CS cells was determined by isolating splenic CD11c+ cells from mice prepared as in A and evaluating their CS cell activity in a LAT assay. Results are expressed as mean ± SEM. This experiment was performed twice with similar results. There were five mice in each group.
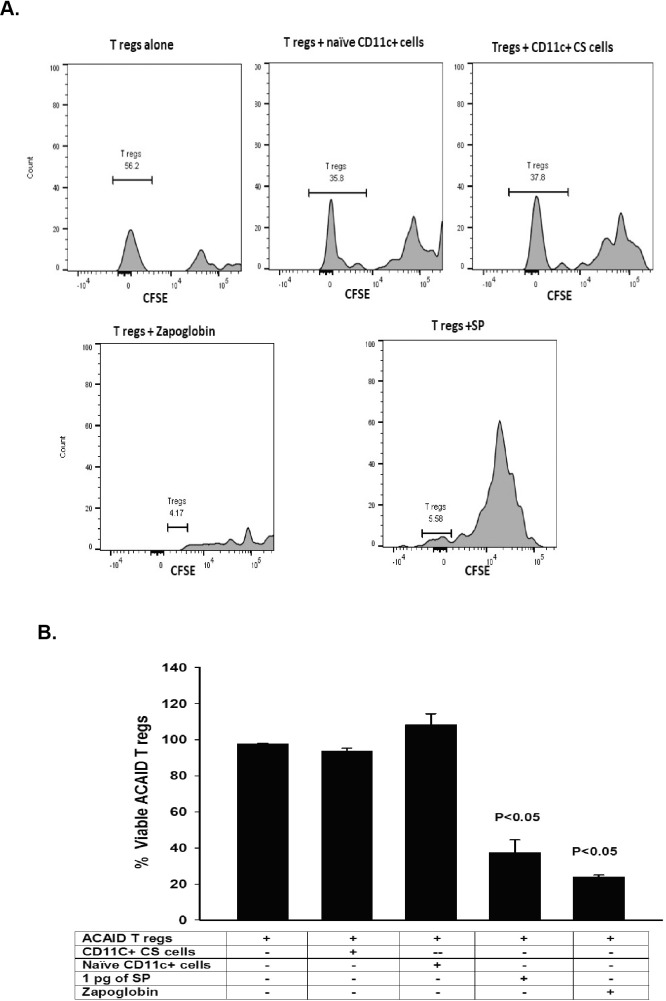
SP Disables but Does Not Kill CD8+ ACAID Tregs
We previously reported that SP was required for the generation of CS cells in trephined hosts,13 and the results shown here indicate that SP converts naïve CD11c+ cells into CS cells. It is noteworthy that DCs produce SP20 and also express NK1R, allowing them to respond to NK1R stimulation.21 Signaling through the NK1R on DCs blocks IL-10 secretion while stimulating Th1 immune responses.21 We next entertained the hypothesis that SP, ostensibly produced by CD11c+ CS cells, either killed CD8+ ACAID Tregs or disabled their suppressive functions. CD8+ ACAID Tregs were cocultured with 1 pg SP or with CD11c+ CS cells for 24 hours and assessed for viability using a CSFE-labeling assay.14 After 24 hours of incubation in SP, >60% of the CD8+ ACAID Tregs were dead (Fig. 6). However, the viability of CD8+ ACAID Tregs was not affected by coculture with the CD11c+ CS cells (Fig. 6), which were present at the same concentrations and ratios used in previous LAT assays in which CD11c+ CS cells blocked the suppressive activity of CD8+ ACAID Tregs (Figs. 1A–1C). This indicates that even though high concentrations of SP (i.e., 1.0 pg/mL) are toxic to ACAID Tregs, the amount of SP produced by the CD11c+ CS cells used in the LAT assays was not sufficient to kill the CD8+ ACAID Tregs and further suggests that the CS cells disable ACAID Tregs by a mechanism that does not lead to their death.
Figure 6.
Trephine-induced CS cells do not kill CD8+ ACAID Tregs. CD8+ ACAID Tregs were incubated for 24 hours with either CD11c+ CS cells isolated from the spleens of trephined or naïve donors, SP (1.0 pg/mL), or Zap-oglobin lytic reagent. Viability of CD8+ Tregs was determined using a CSFE labeling assay. Results are shown as flow cytometry profiles (A) or in histograms (B). This experiment was performed twice with similar results. There were five mice in each group.
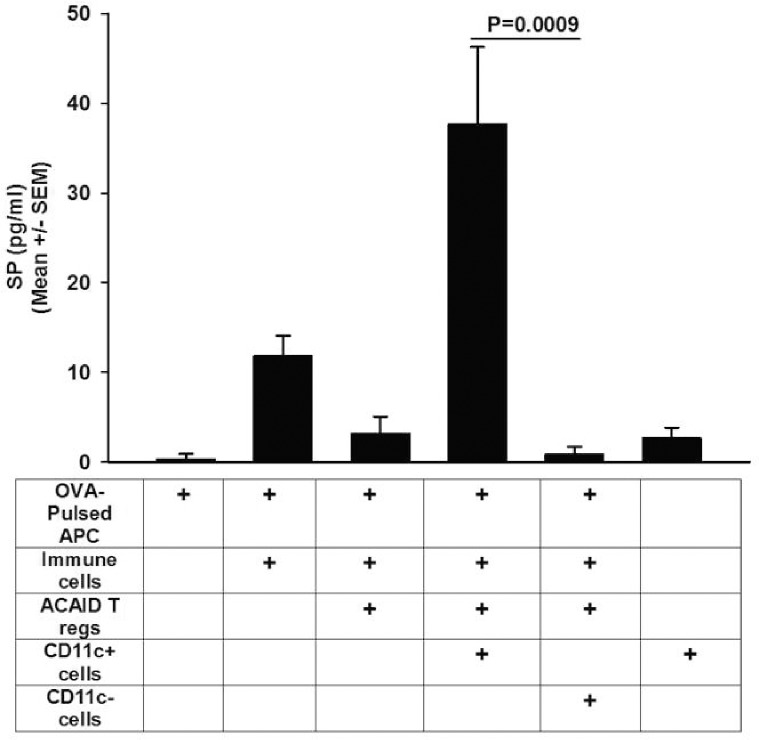
Trephine-Induced CS Cells Produce SP
We previously reported that addition of a SP receptor antagonist Spantide II to LAT assay cell suspensions blocked the CS activity of CD11c+ CS cells.13 Because LAT assays include CD8+ Tregs, antigen-pulsed APC, immune CD4+ T cells, and CD11c+ cells, each of which might be capable of producing SP, we were not able to conclude which cells produced the SP that disabled Treg activity. Accordingly, we used an “in vitro LAT assay” in which we cocultured the cell populations in the same proportions used in the aforementioned LAT assay and measured the SP in the supernatants 24 hours later. The results of a typical “in vitro LAT assay” are shown in Figure 7 and indicate that cell cultures containing CD11c+ CS cells from trephined donors contained significantly more SP than cultures not including CD11c+ cells or cultures containing CD11c− cells from trephined donors, suggesting that either the CD11c+ CS cells produced SP or stimulated one or more of the other cell populations to elaborate SP.
Figure 7.
Trephine-induced CS cells produce SP. An “in vitro LAT assay” was used to determine whether CD11c+ cells from trephined donors produced SP. CD11c+ and CD11c− spleen cells were collected from BALB/c mice 14 days after both eyes had been trephined. CD11c+ and CD11c− cells were cocultured with CD8+ ACAID Tregs, OVA-immune T cells, and OVA antigen-pulsed APC in the ratios and concentrations used for in vivo LAT assays. Cells were cultured for 24 hours, and supernatants were evaluated for SP by ELISA. Results are expressed as mean ± SEM. This experiment was performed twice with similar results. There were five mice in each group.
Discussion
It is well known that injury to one eye can elicit a sympathetic proinflammatory response in the opposite eye. Sympathetic ophthalmia (SO), a condition that sometimes occurs after ocular trauma or surgery to one eye leads to inflammation in the opposite eye, was recognized by the ancient Greeks and discussed by Hippocrates.22 Both SLIP and SO lead to a loss of immune privilege in both eyes; however, that is where the similarity ends. Severing corneal nerves does not lead to inflammation unless the adaptive immune response is elicited either by alloantigens expressed on corneal allografts or by antigens injected into the AC. A condition very similar to what we describe as SLIP was reported by Lucas et al.,23 who observed that retinal laser burns (RLB) to one eye prevented the induction of ACAID in the opposite eye that was not subjected to RLB. Like SLIP, the loss of ACAID produced by RLB is closely associated with the elaboration of SP and the upregulation of the SP receptor NK1-R in the opposite, nonmanipulated eye.13,23 Moreover, blocking NK1-R with the receptor antagonist Spantide II restores ACAID in both models.13,23 A version of SLIP similar if not identical to our findings was reported by Streilein et al.24 more than two decades ago, when they discovered that circular incisions in the cornea prevented the induction of ACAID. However, unlike our investigations, they did not examine the induction of ACAID in the opposite eye or the possible role of CS cells in this loss of immune privilege.
The present findings extend our understanding of SLIP and the profound effect the corneal nerves in one eye can have on the ocular immune response in both eyes. We previously reported that severing corneal nerves results in the generation of CS cells that disable Tregs induced by either AC or mucosal administration of antigens (i.e., ACAID or oral tolerance) and Tregs present in hosts with long-standing corneal allografts.13 Although both CD8+ ACAID Tregs and corneal allograft-induced CD4+ Tregs promote corneal allograft survival and prevent immune rejection, they differ in several fundamental ways. Corneal allograft-induced CD4+ Tregs express Foxp3, GITR, and CTLA4,14 whereas CD8+ ACAID Tregs do not display enhanced expression of these markers (data not shown). Moreover, these two Treg populations display other differnces.25 For example, CD4+ corneal allograft-induced Tregs are disabled in hosts with allergic conjunctivitis and in IL-17–deficient hosts. By contrast, neither allergic conjunctivitis nor IL-17 deficiency adversely affects the induction and expression of CD8+ ACAID Tregs.25
We report here that severing corneal nerves leads to the appearance of CD11c+ CS cells that disable CD8+ Tregs induced by ACAID and that this effect persists for 60 days. The prolonged presence of CS cell activity suggested that perhaps the longevity of contrasuppression was either due to a continuous generation of CS cells in the trephined host or that the initial population of CS cells converted a population of naïve CD11c+ DCs to become second-generation CS cells by a mechanisms similar to “infectious tolerance” that is produced by CD4+CD25+ Tregs in other models.26,27 Results of experiments using BM chimeric mice indicated that whole-body irradiation 14 days after the initial trephining eliminated CS cell activity, indicating that the initial population of CD11c+ CS cells was radiosensitive. Moreover, lethally irradiated CD45.2+ congenic mice reconstituted with BM from CD45.1+ congenic donors failed to display CS cell activity in either CD45.1+ or CD45.2+ CD11c+ cell populations, thereby indicating that the naïve BM-derived cells introduced into trephined mice 14 days after corneal nerve ablation were not converted to CS cells. This in turn suggests that the initial induction of CS cells is complete on or before day 14 after trephining, and the signaling events involved in the induction of CS cells are not long lived. This is consistent with previous findings indicating that corneal nerves return to their normal density 4 days after trephining the cornea.13
DCs display a remarkable propensity to amplify immune responses. As few as 10 DCs can induce allospecific T-cell responses in mice.17 Our results indicated that as few as a 1000 CD11c+ CS cells can adoptively transfer contrasuppression that disables ACAID Tregs. Moreover, these CD11c+ CS cells block the expression of ACAID Treg suppression even after the Tregs have been generated.
SP appears to be essential for both the induction and function of trephine-induced CS cells. Naïve CD11c+ DCs acquire CS activity after only a brief exposure to SP in vitro. Our ELISA data indicate that CD11c+ CS cells are also capable of elaborating SP, which is involved in the disabling of ACAID Tregs in the LAT assay. The weight of evidence presented here along with previous findings suggests that severing corneal nerves stimulates the production of SP in tissues in the anterior segment of the eye including corneal cells. The juxtaposition of ocular surface DCs, including CD11c+ DCs, to the tissues producing SP facilitates the conversion of resident CD11c+ DC to CS cells. This conclusion is supported by the present results indicating that depletion of CD11c+ cells by injection of clodronate-containing liposomes at the time when corneal nerves are severed prevents the generation of CS cells and restores ocular immune privilege. We previously reported that subconjunctival injection of clodronate-containing liposomes produces a steep reduction in multiple ocular surface APC populations including CD11b+ APC, CD11c+ DC, and Iba+ macrophages.15 Although macrophage populations that are associated with certain pathologic conditions can coexpress CD11c,28,29 we conclude that the results from the clodronate depletion experiments reported here indicate that CD11c+ DCs are the CS cells that disable ACAID Tregs. This conclusion is based on the results of negative selection experiments in which spleen cell populations collected from trephined donors and depleted of CD11c+cells but containing all of the other splenic lymphoid and myeloid cell populations (e.g., Iba+ CD11b+, and F4/80+ cells) did not display CS cell activity and had no effect of either the induction or expression of CD8+ ACAID Treg activity in either adoptive transfer experiments or in LAT assays.13
The finding that SLIP can be prevented by subconjunctival injection of clodronate-containing liposomes is noteworthy and may have a significant impact on the fate of both first and second corneal allografts. That is, subconjunctival injection of clodronate-containing liposomes is known to prevent the immune rejection of first-time corneal allografts in both rats and mice,30,31 and as shown here, the induction of SLIP is circumvented by depleting ocular surface CD11c+ cells. Thus, subconjunctival of clodronate-containing liposomes has a two-pronged benefit: it prevents the rejection of first-time corneal allografts and blocks the induction of SLIP, thereby sustaining immune privilege for second corneal allografts.
Acknowledgments
The authors thank Barbara Linzy for technical support.
Supported by National Institutes of Health Grants EY007641 and EY020799 and an unrestricted grant from Research to Prevent Blindness.
Disclosure: S. Neelam, None; J. Mellon, None; A. Wilkerson, None; J.Y. Niederkorn, None
References
- 1.van Dooremaal JC. Die Entwicklung der in fremden Grund versetzten lebenden Geweba [in German] Albrecht von Graefes Arch Ophthalmol. 1873;19:358–373. [Google Scholar]
- 2.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 4.Camelo S, Kezic J, Shanley A, Rigby P, McMenamin PG. Antigen from the anterior chamber of the eye travels in a soluble form to secondary lymphoid organs via lymphatic and vascular routes. Invest Ophthalmol Vis Sci. 2006;47:1039–1046. doi: 10.1167/iovs.05-1041. [DOI] [PubMed] [Google Scholar]
- 5.Egan RM, Yorkey C, Black R, et al. Peptide-specific T cell clonal expansion in vivo following immunization in the eye, an immune-privileged site. J Immunol. 1996;157:2262–2271. [PubMed] [Google Scholar]
- 6.Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- 7.Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–1067. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74:167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 9.Niederkorn JY. Immunology of corneal allografts: insights from animal models. J Clin Exp Ophthalmol. 2015;6:1–7. doi: 10.4172/2155-9570.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CCTSR Group. The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 11.Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140:1112–1122. doi: 10.1016/j.ajo.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Paunicka KJ, Mellon J, Robertson D, et al. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant. 2015;15:1490–1501. doi: 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mo J, Neelam S, Mellon J, Brown JR, Niederkorn JY. Effect of corneal nerve ablation on immune tolerance induced by corneal allografts, oral immunization, or anterior chamber injection of antigens. Invest Ophthalmol Vis Sci. 2017;58:137–148. doi: 10.1167/iovs.16-20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunnusamy K, Chen PW, Niederkorn JY. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune privilege of corneal allografts. J Immunol. 2011;186:6737–6745. doi: 10.4049/jimmunol.1100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaumburg CS, Siemasko KF, De Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 16.Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 17.McKinney EC, Streilein JW. On the extraordinary capacity of allogeneic epidermal Langerhans cells to prime cytotoxic T cells in vivo. J Immunol. 1989;143:1560–1564. [PubMed] [Google Scholar]
- 18.Janelsins BM, Sumpter TL, Tkacheva OA, et al. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12. Blood. 2013;121:2923–2933. doi: 10.1182/blood-2012-07-446054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Orazio TJ, Niederkorn JY. A novel role for TGF-beta and IL-10 in the induction of immune privilege. J Immunol. 1998;160:2089–2098. [PubMed] [Google Scholar]
- 20.Simeonidis S, Castagliuolo I, Pan A, et al. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci U S A. 2003;100:2957–2962. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janelsins BM, Mathers AR, Tkacheva OA, et al. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood. 2009;113:3017–3026. doi: 10.1182/blood-2008-06-163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert DM, Diaz-Rohena R. A historical review of sympathetic ophthalmia and its epidemiology. Surv Ophthalmol. 1989;34:1–14. doi: 10.1016/0039-6257(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 23.Lucas K, Karamichos D, Mathew R, Zieske JD, Stein-Streilein J. Retinal laser burn-induced neuropathy leads to substance P-dependent loss of ocular immune privilege. J Immunol. 2012;189:1237–1242. doi: 10.4049/jimmunol.1103264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streilein JW, Bradley D, Sano Y, Sonoda Y. Immunosuppressive properties of tissues obtained from eyes with experimentally manipulated corneas. Invest Ophthalmol Vis Sci. 1996;37:413–424. [PubMed] [Google Scholar]
- 25.Cunnusamy K, Paunicka K, Reyes N, et al. Two different regulatory T cell populations that promote corneal allograft survival. Invest Ophthalmol Vis Sci. 2010;51:6566–6574. doi: 10.1167/iovs.10-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobbold S, Waldmann H. Infectious tolerance. Curr Opin Immunol. 1998;10:518–524. doi: 10.1016/s0952-7915(98)80217-3. [DOI] [PubMed] [Google Scholar]
- 27.Jonuleit H, Schmitt E, Kakirman H, et al. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hegde S, Beauregard C, Mayhew E, Niederkorn JY. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation. 2005;79:23–31. doi: 10.1097/01.tp.0000147196.79546.69. [DOI] [PubMed] [Google Scholar]
- 31.Van der Veen G, Broersma L, Dijkstra CD, et al. Prevention of corneal allograft rejection in rats treated with subconjunctival injections of liposomes containing dichloromethylene diphosphonate. Invest Ophthalmol Vis Sci. 1994;35:3505–3515. [PubMed] [Google Scholar]