Abstract
In HNSCC, protein- and mRNA-expression of the antileukoproteinase SLPI are significantly inverse correlated with HPV-infection suggesting that elevated expression of SLPI protects against HPV-infections. Moreover, SLPI-expression is up-regulated in HNSCC-patients reporting a smoking habit. Here, we investigate the described correlation in other HPV-driven cancers, namely vulvar squamous cell carcinoma (VSCC). FFPE samples of 99 VSCC were analyzed by PCR for HPV-DNA-expression and by RT-qPCR for SLPI-mRNA-expression. Of 99 VSCC 10 (10.1%) are HPV-positive; 9 were HPV16; 1 HPV18; all were E6/E7 mRNA-positive. 33 of the 99 patients (33.3%) reported a smoking habit; 7 (21.1%) of these were HPV-positive. Of 66 (66.7%) non-smokers 3 (4.5%) were HPV-positive. SLPI-expression was 4.0-fold lower in HPV-positive than HPV-negative patients. Smoking resulted in 2.3-fold higher SLPI expression. The data presented here indicate that SLPI plays a pivotal role in HPV-infection not only in HNSCC but also in VSCC and possibly also in other HPV-driven cancers. This however, needs to be analyzed in future studies. Furthermore these data lead to the hypothesis that the smoking induced SLPI-increase is systemic rather than local, as assumed based on the HNSCC data.
Introduction
Infection of the mucosa of the anogenital and upper aerodigestive tract with human papillomaviruses (HPV) induces carcinogenesis [1]. In the upper aerodigestive tract, a significant proportion of squamous cell carcinomas (SCC) of the oropharynx, specifically of the tonsils [2], [3], [4], and in the anogenital region SCC of the cervix uteri, vulva, penis, and specifically in HIV-infected men of the anus are caused by HPV-interaction [5]. While SCC of the cervix uteri show HPV-prevalence rates of nearly 100%, in SCC of the tonsils (TSCC) and the vulva (VSCC) the prevalence rate shows a higher divergence namely 20% to 60% HPV-positive cases. This might in part be due to differences depending on the geographical region the study population lives in [2], [6]. Moreover, for VSCC it is well established that there are two distinguishable entities: HPV-associated carcinogenesis in rather younger patients and carcinogenesis in elderly patients with other causative agents than HPV-infection and consecutively low HPV-prevalence [7]. In the upper aerodigestive tract, HPV-negative SCC can most likely be attributed to the carcinogenic ingredients of alcohol and tobacco smoke [8]. Recent data support the notion that a majority of smokers suffers from HPV-negative and a majority of non-smokers from HPV-positive cancers. Smoking does, however, not only influence carcinogenic pathways, but impacts patients' survival negatively, jeopardizing for instance the established positive effect of HPV-infections on overall (OS) and progression free survival (PFS) in cancers of the upper aerodigestive tract [9]. Until only recently, there have been no suggestions how smoking might interfere with the susceptibility of HPV infections in SCC of the head and neck (HNSCC). Intriguingly, significant data from own studies of various study populations [10], [11], [12], [13] were supported by in vitro studies using cervix SCC cell lines [14] and taken together led to the following hypothesis: Smoking induces elevated protein- and mRNA-expression of a protective protein in mucosal surfaces, namely the antileukoproteinase secretory leucocyte protease inhibitor (SLPI). SLPI binds to the membrane bound receptor Annexin A2 (AnxA2). HPV itself, however, can also bind to AnxA2. Thus, it can be assumed that smoking induced elevated SLPI expression leads to quantitatively proportional more binding of SLPI to AnxA2, consequently blocking the latter for HPV-binding. Since even in smokers the cellular milieu most likely is not fully saturated by SLPI, this model does allow for the explanation why smokers develop HPV-negative and non-smokers HPV-positive SCC, yet, does not exclude the coincidence of HPV-infection and a positive smoking history and vice versa. The proportion of HPV-positive smokers might be more pronounced in populations with higher proportions of smokers as is seen in some European countries when compared to US-American populations. Similar observations as described for HNSCC have been made in anal SCC in HIV-infected men [15], supporting the notion of the above mentioned hypothesis. To extend the knowledge of the interaction of SLPI, AnxA2, smoking habit of patients, and its impact on HPV susceptibility, we here investigate these parameters in tissue specimens derived from VSCC. This tumor entity is characterized by a distinct proportion of HPV-negative cases [7], similar as HNSCC, hence allowing for the here performed analysis.
Patients and Methods
Patients
Patient characteristics were as follows: age at diagnosis: 26.3 to 93.2 years, median age: 60.1 years. After surgery patients were followed until death or until August 2017, median follow-up: 7.59 years (range 0.08 to 30.85 years).
Smoking habit was analyzed by separating the patients into smokers and non-smokers. Tissue samples were obtained between 1982 and 2011 during surgery at the Department of Gynecology and Obstetrics, Christian-Albrechts-University Kiel, Germany. FFPE samples of these histopathologically confirmed VSCCs (n = 99) were retrieved from the Department of Pathology, Kiel, Germany. All samples were obtained following informed consent; the study was approved by the local Ethics Committee D 483/17.
Nucleic Acid Extraction, HPV-Detection, cDNA Synthesis and qPCR
DNA and RNA was simultaneously extracted from 4 to 6 consecutive 10 μm sections using the ExpressArt Mag FFPE RNA + DNA ready kit (AmpTec GmbH, Hamburg, Germany) according to the manufacturer's protocol. Nucleic acid quantity and quality was analyzed using the Nanodrop 1000 (PeqLab) and the Tapestation 2200 (Agilent, Böblingen, Germany), respectively. HPV-DNA detection was performed by PCR using the primers GP5+/GP6+, as described previously [16]. RNA (200 ng) was transcribed into cDNA using the TR cDNA synthesis kit (AmpTec GmbH). qPCR was performed as described previously [17]. Primers for SLPI and AnxA2 were designed and used as described elsewhere [11]. Primers for the housekeeping genes 18S rRNA, β-actin and b-2-microglobulin (B2M) were purchased from Promolgene (Berlin, Germany) and used according to the manufacturer's protocol. DNA integrity was analyzed using genomic B2M primers (Promolgene) and used according to the manufacturer's protocol.
Immunohistochemistry for SLPI
Paraffin embedded tissue specimens were cut in 4 μm sections. Immunohistochemical staining for SLPI expression was performed as described, previously [18]. In brief: to assess SLPI protein levels the entire biopsies were analyzed (magnification, x200). The percentage of positive epithelial cells of the tonsillar crypts was determined and cases were assigned to one of the categories: negative <5%, weak 5% to 30%, moderate 31% to 75% and strong >75% of the cells were stained.
Statistical Analysis
Immunohistochemical data were analyzed using two-sided Fisher's exact test (SPSS 20.0 software; IBM SPSS, Armonk, NY, USA). qPCR data were analyzed according to the ΔΔCt method [19] using the mean Ct value of the housekeeping genes. Fold changes of the expression levels were calculated as described previously [19] and the obtained values were used for statistical analysis (SPSS 20.0 software). Fisher's exact test was performed relating SLPI protein expression to HPV-positivity and smoking habit. For Kaplan–Meier survival analysis the primary statistical end points were PFS and OS; defined as time from diagnosis to date of disease progression or last follow-up or death, respectively. Factors tested for prognostic value included HPV-status, SLPI-protein-expression and smoking habit. P < .05 was considered statistically significant for all tests performed.
Results
Correlation of SLPI Protein Expression with HPV-Status and Smoking Habit of the Patients
HPV DNA PCR detected 10 (10.1%) HPV-positive cases; 9 were HPV-type 16; 1 was HPV-type 18 and all were E6/E7 mRNA positive, hence showed active HPV-infections. The results correlating HPV-status and SLPI protein expression are shown in Table 1 confirming the inverse correlation between HPV positivity and SLPI expression (P = .006).
Table 1.
Correlation of SLPI Expression and HPV Status
| SLPI | Negative | Weak | Moderate | Strong | Total |
|---|---|---|---|---|---|
| HPV-positive | 7 | 3 | 0 | 0 | 10 |
| HPV-negative | 20 | 26 | 18 | 25 | 89 |
| Total | 27 | 29 | 18 | 25 | 99 |
In Table 1 the correlation between SLPI expression and HPV status is shown. Demonstrating a significant inverse correlation between HPV positivity and SLPI expression (P = .006).
In Table 2 the correlation between smoking habit and SLPI expression is shown. Similar to the results obtained in HNSCC patients the results shown here demonstrate a significant correlation between smoking habit and SLPI expression (P < .0001).
Table 2.
Correlation of SLPI Expression and Smoking Habit of the Patients
| SLPI | Negative | Weak | Moderate | Strong | Total |
|---|---|---|---|---|---|
| Smoker | 4 | 5 | 6 | 18 | 33 |
| Nonsmoker | 23 | 24 | 12 | 7 | 66 |
| Total | 27 | 29 | 18 | 25 | 99 |
In Table 2 the correlation between SLPI expression and smoking habit is shown. Demonstrating a significant correlation between these two parameters (P < .0001).
Effect of HPV-Status on SLPI Gene Expression in Smoking and Non-Smoking Patients
Analyzing the effect of HPV-positivity in smoking and non-smoking patients separately showed that SLPI expression in HPV-positive non-smokers was 3.35 times higher than in HPV-negative non-smokers while this increase in HPV-positive smokers was only 1.85 fold, hence in smoking patients HPV-positivity had no significant effect on SLPI expression.
Analysis of SLPI and AnxA2 Gene Expression in Relation to HPV-Status and Smoking Habit
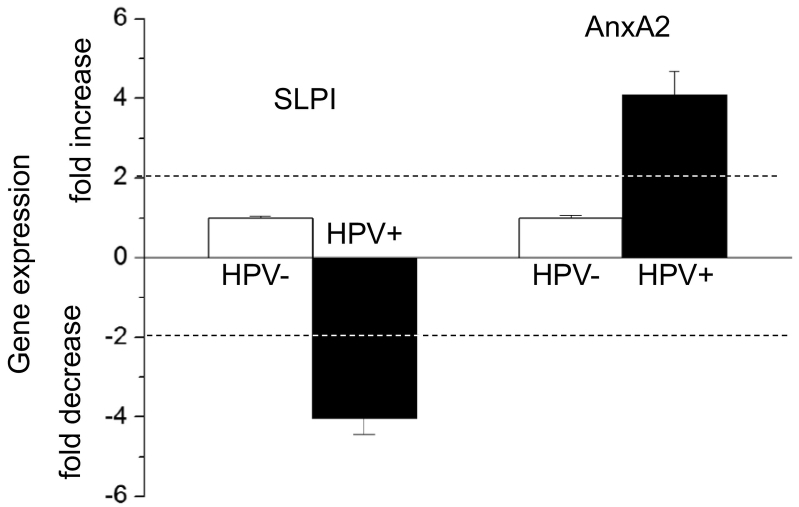
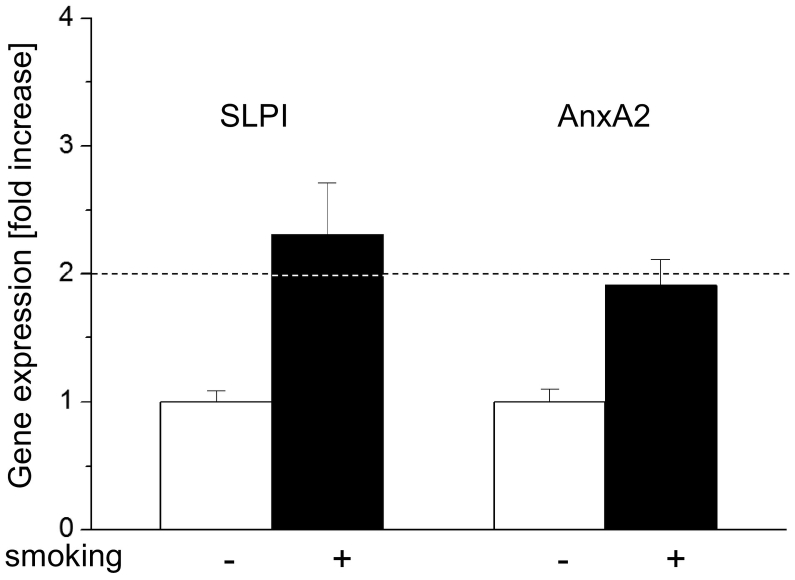
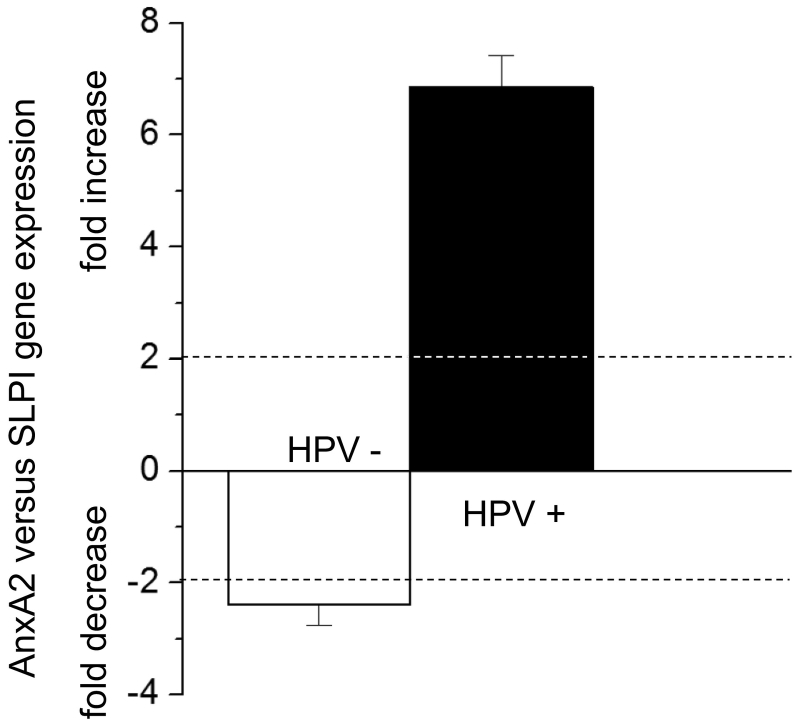
The effects of HPV-status and smoking on SLPI and AnxA2 gene expression are shown in Figures 1 and 2, respectively. As shown in Figure 1 HPV-positivity was, independent of the smoking history, associated with 4.03 times lower SLPI-and 4.07 times higher AnxA2 gene expression. Smoking, as shown in Figure 2, resulted, independent of the HPV-status, in a 2.34-fold increase in SLPI- and a 1.95 fold increase in AnxA2 gene expression. To determine the relation between SLPI- and AnxA2-gene expressions, the fold change of AnxA2 gene expression in relation to SLPI gene expression was calculated (for mathematical details see legend to Figure 3). HPV-positive tumors had 6.86 times more AnxA2 than SLPI while HPV negative tumors had 2.39 times less AnxA2 than SLPI (Figure 3).
Figure 1.
Effect of HPV-status on SLPI and AnxA2 gene expression.
SLPI and AnxA2 gene expression in all 99 VSCC patients is shown. ΔΔct values obtained in HPV negative patients were set as “1” and fold changes of HPV-positive patients were calculated as described elsewhere (Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001). Dotted lines indicate 2-fold changes of gene expression (both decrease and increase) indicating, as described previously (Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001), significant changes in gene expression levels.
Figure 2.
Effect of smoking on SLPI and AnxA2 gene expression.
SLPI and AnxA2 gene expression is shown. The ΔΔct values obtained in non-smoking patients were set as “1” and fold changes of smoking patients were calculated as described elsewhere (Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001). Dotted lines indicate 2-fold changes of gene expression indicating, as described previously (Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001), significant changes in gene expression levels.
Figure 3.
Comparison of AnxA2 and SLPI gene expression.
The comparison of AnxA2 and SLPI gene expression is shown. To compare AnxA2 versus SLPI gene expression levels, SLPI gene expression for each patient group was set as “1”. Fold change expression levels of AnxA2 were then calculated as described elsewhere (Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001). Dotted lines indicate 2-fold changes of gene expression level (both decrease and increase) as described previously (Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001); ± 2-fold changes in gene expression levels are considered statistically significant.
OS and PFS Dependent on HPV-Status, p16INK4A-Protein-Expression, Treatment, and Smoking Habit
OS of the 99 patients was 77.6% after 3 years and 72.3% and 55.4% after 5 and 10 years, respectively. PFS was 74.0% after 3 years and 72.8% after 5 and 62.3% 10 years.
Data correlating OS and PFS with HPV-status, SLPI protein expression, T- and N category and smoking habit, are summarized in Table 3.
Table 3.
Overall and Progression Free Survival After 3, 5 and 10 Years in Relation to Smoking Habit, SLPI Expression, HPV Status T- and N Category
| n | 3 yrs. [%] | 5 yrs. [%] | 10 yrs. [%] | Mean [yrs] | std [yrs] | Median [yrs] | Min [yrs] | Max [yrs] | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall survival | ||||||||||
| Smoking habit | ||||||||||
| Non-smoker | 66 | 78.5 | 70.5 | 51.7 | 7.13 | 4.85 | 7.17 | 0.34 | 30.85 | 0.217 |
| Smoker | 33 | 78.8 | 75.8 | 62.7 | 7.54 | 5.23 | 7.84 | 0.08 | 25.93 | |
| SLPI | ||||||||||
| Negative/weak | 56 | 78.6 | 71.2 | 52.0 | 7.32 | 4.75 | 7.30 | 0.58 | 30.85 | 0.502 |
| Moderate/strong | 43 | 78.7 | 73.7 | 60.2 | 7.19 | 5.27 | 7.84 | 0.08 | 25.93 | |
| HPV | ||||||||||
| Negative | 89 | 78.4 | 71.4 | 55.8 | 7.31 | 5.13 | 7.50 | 0.08 | 30.85 | 0.972 |
| Positive | 10 | 80.0 | 80.0 | 6.90 | 3.16 | 7.75 | 0.58 | 9.93 | ||
| T category | ||||||||||
| Cis | 8 | 87.5 | 87.5 | 87.5 | 11.75 | 8.74 | 9.93 | 2.83 | 30.85 | 0.027 |
| T1a/1b | 40 | 85.0 | 77.3 | 58.2 | 7.77 | 4.53 | 7.72 | 1.08 | 25.93 | |
| T2/T3 | 51 | 70.1 | 66.0 | 47.4 | 6.16 | 4.09 | 7.09 | 0.08 | 14.09 | |
| N category | ||||||||||
| cN0/pN0 | 72 | 81.7 | 74.4 | 60.6 | 7.75 | 5.20 | 7.84 | 0.34 | 30.85 | 0.115 |
| pN1 | 12 | 75.0 | 75.0 | 43.8 | 6.50 | 3.97 | 6.17 | 0.50 | 13.09 | |
| pN2/N3 | 10 | 50.0 | 50.0 | 37.5 | 5.31 | 4.69 | 3.88 | 0.08 | 12.59 | |
| HPV /N category | ||||||||||
| HPV+ cN0/pN0 | 6 | 100 | 100 | 8.66 | 1.22 | 8.76 | 6.84 | 9.93 | 0.006 | |
| HPV+ pN1 | 1 | 100 | 100 | 6.68 | 6.68 | 6.68 | 6.68 | |||
| HPV+ pN2/3 | 2 | 1.33 | 1.08 | 1.33 | 0.58 | 2.08 | ||||
| HPV- cN0/pN0 | 66 | 80.0 | 72.0 | 59.0 | 7.66 | 5.41 | 7.71 | 0.34 | 30.85 | |
| HPV- pN1 | 11 | 72.7 | 72.7 | 40.9 | 6.48 | 4.16 | 5.84 | 0.50 | 13.09 | |
| HPV- pN2/3 | 8 | 62.5 | 62.5 | 46.9 | 6.31 | 4.73 | 7.51 | 0.08 | 12.59 | |
| Progression free survival | ||||||||||
| Smoking habit | ||||||||||
| Non-smoker | 66 | 67.7 | 65.9 | 53.8 | 5.55 | 3.84 | 5.55 | 0.34 | 12.59 | 0.031 |
| Smoker | 33 | 87.1 | 87.1 | 79.2 | 6.56 | 3.88 | 7.59 | 0.08 | 12.92 | |
| SLPI | ||||||||||
| Negative/weak | 56 | 78.0 | 78.0 | 68.9 | 6.04 | 3.60 | 6.76 | 0.50 | 12.59 | 0.044 |
| Moderate/strong | 43 | 69.0 | 66.5 | 52.5 | 5.68 | 4.21 | 6.00 | 0.08 | 12.92 | |
| HPV | ||||||||||
| Negative | 89 | 72.4 | 71.1 | 59.3 | 5.79 | 3.91 | 5.84 | 0.08 | 12.92 | 0.023 |
| Positive | 10 | 88.9 | 88.9 | 6.76 | 3.42 | 7.75 | 0.58 | 9.93 | ||
| T category | ||||||||||
| Cis | 8 | 100 | 100 | 66.7 | 7.56 | 3.15 | 7.97 | 2.83 | 11.84 | 0.233 |
| T1a/1b | 40 | 79.6 | 76.8 | 69.6 | 6.54 | 3.69 | 7.05 | 0.58 | 12.59 | |
| T2/T3 | 51 | 65.3 | 65.3 | 56.1 | 5.11 | 3.98 | 5.42 | 0.08 | 12.92 | |
| N category | ||||||||||
| cN0/pN0 | 72 | 79.1 | 79.1 | 69.2 | 6.43 | 3.65 | 7.13 | 0.34 | 12.84 | 0.042 |
| pN1 | 12 | 50.0 | 41.7 | 41.7 | 4.34 | 4.03 | 3.55 | 0.42 | 12.92 | |
| pN2/N3 | 10 | 62.5 | 62.5 | 41.7 | 4.33 | 4.72 | 1.80 | 0.08 | 12.59 | |
| HPV /N category | ||||||||||
| HPV+ cN0/pN0 | 6 | 100 | 100 | 8.66 | 1.21 | 8.76 | 6.86 | 9.93 | 0.018 | |
| HPV+ pN1 | 1 | 100 | 100 | 6.68 | 6.68 | 6.68 | 6.68 | |||
| HPV+ pN2/3 | 2 | 0.62 | 0.56 | 0.62 | 0.58 | 0.66 | ||||
| HPV- cN0/pN0 | 66 | 77.2 | 77.2 | 66.0 | 6.23 | 3.73 | 7.01 | 0.34 | 12.84 | |
| HPV- pN1 | 11 | 45.5 | 36.4 | 36.4 | 4.12 | 4.16 | 2.75 | 0.42 | 12.92 | |
| HPV- pN2/3 | 8 | 71.4 | 71.4 | 47.6 | 5.25 | 4.74 | 3.76 | 0.08 | 12.58 | |
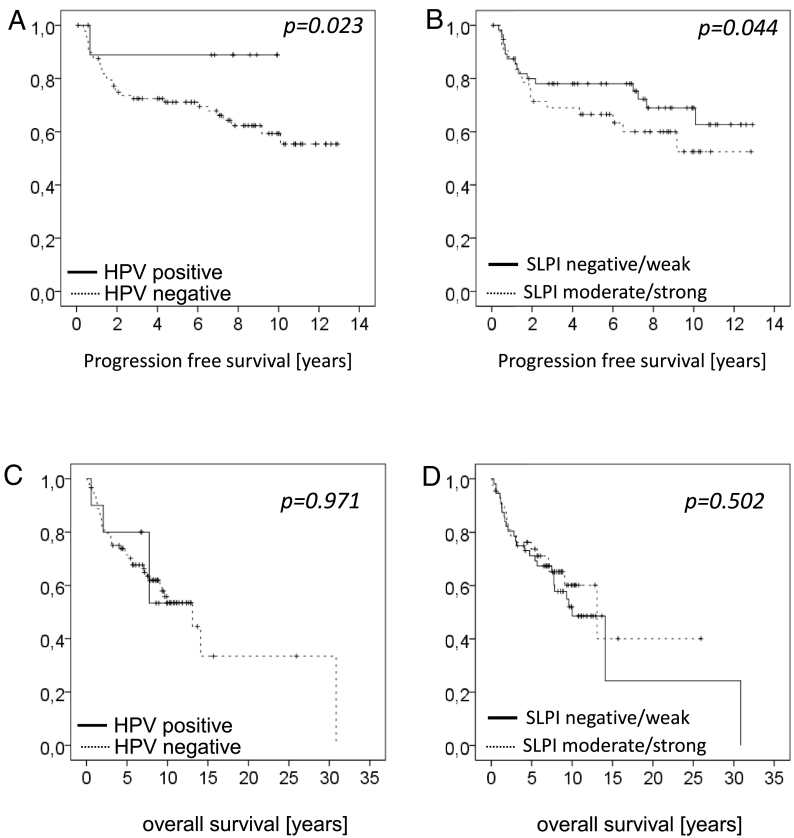
Patients with HPV-positive tumors show significantly better PSF (Figure 4A) than patients with tumors showing negative or weak SLPI expression (Figure 4B). These survival benefits can, however not be seen when analyzing OS (Figure 4, C and D). A similar loss of significance is seen when survival data are stratified for the lymph nodal status of the patients (c or pN0 vs. pN1 vs. pN1/N3) showing significance for PFS but not for OS (Table 3). In contrast significant differences in OS but not in PFS are seen when stratifying for the T-category of the tumors (Table 3). The only significant effect on both, OS and PFS, was detected when correlating the HPV status with the N-category of patients showing a clear survival advantage of HPV-positive patients among the different N-categories (Table 3).
Figure 4.
Overall and progression free survival of patients with VSCC in relation to HPV status and SLPI expression.
In Figure 4A and B, PFS and in Figure 4C and D OS is shown. PFS for HPV-negative patients (n = 89) was 72.4%, 71.1 and 59.3 after 3, 5, and 10 years respectively. HPV-positive patients (n = 10) showed 88.9% survival after 3 and 5 years. OS of the HPV-negative patients (n = 89) was 78.4%, 71.1% and 55.8% after 3, 5, and 10 years respectively and for the HPV-positive patients (n = 10) OS was 80% both after 3 and 5 years.
For Kaplan–Meier analysis SPLI expression of tumors with negative and weak expression (n = 56) and for tumors with moderate and strong expression (n = 43) was pooled. Patients with negative/weak expression showed 78.0% PSF after 3 and 5 years and of 68.9% after 10 years, while patients with moderate/strong SLPI tumor expression showed 69.0% after 3 years, 66.5% after 5 years and 52.5% PFS after 10 years (P = .044). OS of patients with negative/ weak and moderate/strong SLPI expression was not significantly different (P = .502), with OS in patients with negative/ weak SLPI expression being 78.6% after 3 years, 71.2% after 5 years and 52.0% after 10 years. OS of the patients with moderate/strong expression was 78.7%, 73.7% and 60.2% after 3, 5, and 10 years respectively.
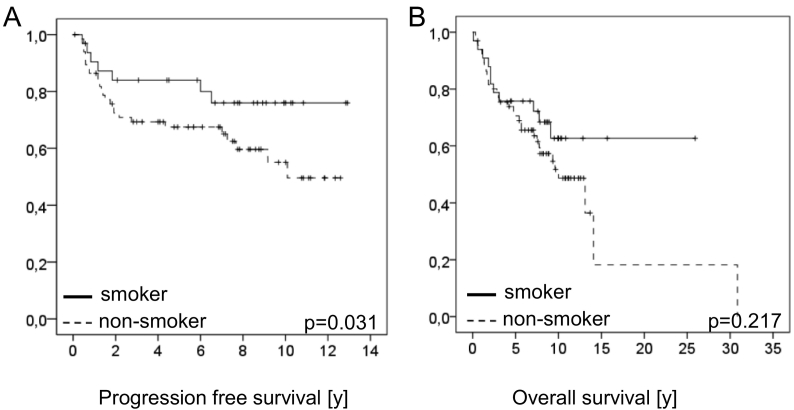
Strikingly patients reporting a smoking habit show significantly better PFS than non-smoking patients (P = .031; Figure 5A), similarly, however not significant, was the OS observed in patients with a smoking habit better than the OS of non-smoking patients (Figure 5B).
Figure 5.
Overall and progression free survival of patients with VSCC in relation to smoking habit of the patients.
In Figure 5A PFS and in Figures 5B OS is shown. PFS of the patients reporting to be non-smokers (n = 66) was 67.7%, 65.9% and 53.28% after 3, 5, and 10 years respectively, while PFS of patients reporting a smoking habit was 87.1% after 3 and 5 years and 79.2% 10 years (P = .031). OS in non-smoking patients was 78.5%, 70.5% and 51.7% after 3, 5, and 10 years respectively, while in smoking patients 3, 5 and 10 year OS survival rates were 78.8%, 75.(% and 62.7% respectively (P = 0.217).
Discussion
The results described above regarding the correlation of the SLPI and AnxA2 expression, smoking habit, and the HPV-status in 99 patients with VSCC support the hypothesis that smoking induced elevated SLPI expression hinders HPV-infections possibly by blocking AnxA2 binding sites as first suggested for HNSCCs. Even though only 10% of the here studied VSCC are attributable to HPV-infection, the correlation of either HPV-status or smoking habit to SLPI expression were highly significant. Therefore and different to previous assumptions [10], [11], [12], [13], the effect of smoking on the SLPI expression rather seems to be a systemic than a local effect.
In more detail, Table 2 shows that out of 33 smokers among this study population, 18 and 6 patients (72%) have strong or at least moderate SLPI expression, respectively, whereas with four and five cases no or only weak SLPI expression, respectively, this is a rare event among smokers. Vice versa, among non-smokers (n = 66) 47 cases (71%) are negative or show weak SLPI expression and 19 show moderate to strong SLPI expression. This correlation is highly significant with P < .0001. Additionally and as presented in Table 2, among HPV-positive cases (n = 10) seven are SLPI-negative and the other three cases show weak SLPI expression, whereas out of 89 HPV-negative cases the distribution of SLPI expression is rather even (P = .006) similar as previously observed in HNSCCs.
These results detected on protein level are corroborated by analysis of SLPI and AnxA2 gene expression, as illustrated in Figures 1 and 2. Specifically the significant lower fold change expression of SLPI and the significant higher fold change expression of AnxA2, both seen in HPV-positive cases (Figure 1), are in line with the previously postulated hypothesis. Both, data on gene and protein expression, match observations made and firstly described for HNSCCs [10], [11], [12], [13]. The results formerly obtained from HNSCC prompted us to conclude that possibly the direct contact of the ingredients of tobacco smoke to the mucosa of the upper aerodigestive tract induces elevated SLPI expression in terms of a local effect. Intriguingly, the data describe here for VSCC imply that the ingredients of tobacco smoke most likely have a systemic effect on SLPI expression, perhaps additionally to a local effect. While a correlation of decreased SLPI expression and HPV-infections in anal cancers of HIV-positive men has been shown by Nicol and co-workers [15] there is no data available concerning smoking and SLPI expression except for head and neck sites and now VSCC. The data presented here should encourage analysis of the association of smoking habit, SLPI- and AnxA2-expression, and presence of HPV-infections in all HPV-associated human cancers. Based on our ex vivo study showing that the incubation of tissue specimens derived from the lower turbinates with nicotine induces SLPI expression, we suggested that nicotine is the ingredient of interest [11]. However, other ingredients of tobacco smoke besides nicotine and, moreover, alcohol might be tested in future investigations.
Kaplan–Meier survival analysis of the here described 99 VSCC demonstrates an involvement of HPV-status and SLPI expression. PFS stratified for SLPI expression and HPV-status show a significant survival benefit for HPV-positives (P = .023) and for cases with negative or weak SLPI expression (P = .044), respectively (Figure 4), with similar results obtained in HNSCC patients. This significant survival benefit as well as all other parameters with significant impact on PFS was lost, when calculating OS. The latter is even true for survival analysis stratifying for the lymph nodal status of the patients (c or pN0 vs. pN1 vs. pN1/N3) showing significance for PFS but not for OS (Table 3). Possibly, the size of the study population is the reason for these unclear results. The only exception with significant differences in OS but not in PFS is seen when stratifying for the T-category of the tumors (Table 3). Significance for both, OS and PFS, however, can exclusively be described when the HPV-status is correlated to the N-category of patients with a clear survival advantage of HPV-positives among the different N-category groups (Table 3), with again similar results shown for HNSCCs [20]. Whether or not the results for VSCC indicate that HPV-positive cases respond better to treatment as repeatedly suggested for HNSCC patients will have to be clarified in future.
VSCC show striking congruities with specifically OSCC in terms of a divergence in HPV prevalence, the role of p16INK4A as surrogate marker including the issue of mismatches (HPV-DNA/RNA-positive, yet p16-negative cases and vice versa), and in terms of the impact of an HPV-infection on survival. While a PubMed search for the terms “OSCC and HPV” shows 320 publications, only 42 publications match the criteria “VSCC and HPV” reflecting the sparseness of data for the latter. In fact, to the best of our knowledge, there is only one publication [21] on HPV in VSCC considering, among other parameters, smoking habit of the patients. With regard to the HPV-part, the design of the study of Wakeham and co-workers [21] investigating 62 VSCC from Scotland is comparable to the study presented here. Some results of the two studies show similarities whereas other results, however, are conflicting. In the present study, investigating 99 VSCC the HPV-prevalence rate is with 10% rather low; in fact the lowest in comparison to other studies describing prevalence rates from 15 to 100% (summarized in [6]). It is, however, established that with age the HPV prevalence rate in VSCC is decreasing [7]. Wakeham and co-workers [21] report a high risk HPV-detection rate of 52%, yet not specifying the patients´ age in the context of HPV prevalence in VSCC. The median age of the here described population is 60.2 and, thus, age might contribute to the low HPV detection rate in the present study. For HNSCC and also discussed for VSCC [6], divergence of HPV-prevalence rates is well established and is most likely depending on the geographical region the investigated patients live in; the reason for these geographical differences, however, is still a matter of debate.
Another matching result is the survival of HPV-positive cases with significant benefit in OS and PFS in the Scottish study [21]. In the German study population there is only significance in OS survival which is due to the fact that only 2/10 HPV-positive patients die in the course of disease. The latter is reason for the positive effect of HPV on survival data although only 10/99 cases show HPV-infection. Surprisingly, in the present study OS and PFS analysis revealed that smokers survive better than non-smokers, with PFS data being significant.
In conclusion, the here described data on VSCC underline the assumed involvement of SLPI and AnxA2 in the mode of HPV-infections and, furthermore, add data to the positive correlation of SLPI-expression and smoking habit of patients. Both should encourage intensive scientific effort investigating all HPV-associated cancers for these parameters shedding further light onto the field of HPV perception.
Acknowledgement
The authors thank Gudrun Scherer (Dept. of ORL) and Monika Kunz (Institute of Immunology) for skillful technical assistance with immunohistochemistry and RNA-isolation, cDNA synthesis and qPCR, respectively.
References
- 1.Faraji F, Zaidi M, Fakhry C, Gaykalova DA. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 2017;19:464–475. doi: 10.1016/j.micinf.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quabius ES, Haag J, Kuhnel A, Henry H, Hoffmann AS, Gorogh T, Hedderich J, Evert M, Beule AG, Maune S. Geographical and anatomical influences on human papillomavirus prevalence diversity in head and neck squamous cell carcinoma in Germany. Int J Oncol. 2015;46:414–422. doi: 10.3892/ijo.2014.2697. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann M, Ihloff AS, Gorogh T, Weise JB, Fazel A, Krams M, Rittgen W, Schwarz E, Kahn T. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595–1602. doi: 10.1002/ijc.25174. [DOI] [PubMed] [Google Scholar]
- 4.Klussmann JP, Weissenborn SJ, Wieland U, Dries V, Eckel HE, Pfister HJ, Fuchs PG. Human papillomavirus-positive tonsillar carcinomas: a different tumor entity? Med Microbiol Immunol. 2003;192:129–132. doi: 10.1007/s00430-002-0126-1. [DOI] [PubMed] [Google Scholar]
- 5.Prigge ES, von Knebel DM, Reuschenbach M. Clinical relevance and implications of HPV-induced neoplasia in different anatomical locations. Mutat Res Rev Mutat Res. 2017;772:51–66. doi: 10.1016/j.mrrev.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Rakislova N, Saco A, Sierra A, Del PM, Ordi J. Role of human papillomavirus in vulvar cancer. Adv Anat Pathol. 2017;24:201–214. doi: 10.1097/PAP.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 7.Alkatout I, Schubert M, Garbrecht N, Weigel MT, Jonat W, Mundhenke C, Gunther V. Vulvar cancer: epidemiology, clinical presentation, and management options. Int J Womens Health. 2015;7:305–313. doi: 10.2147/IJWH.S68979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farsi NJ, Rousseau MC, Schlecht N, Castonguay G, Allison P, Nguyen-Tan PF, Soulieres D, Coutlee F, Hier M, Madathil S. Aetiological heterogeneity of head and neck squamous cell carcinomas: the role of human papillomavirus infections, smoking and alcohol. Carcinogenesis. 2017;38:1188–1195. doi: 10.1093/carcin/bgx106. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M, Quabius ES, Tribius S, Gebhardt S, Gorogh T, Hedderich J, Huber K, Dunst J, Ambrosch P. Influence of HPV-status on survival of patients with tonsillar carcinomas (TSCC) treated by CO2-laser surgery plus risk adapted therapy - A 10 year retrospective single centre study. Cancer Lett. 2018;413:59–68. doi: 10.1016/j.canlet.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 10.Quabius ES, Gorogh T, Fischer GS, Hoffmann AS, Gebhard M, Evert M, Beule A, Maune S, Knecht R, Ovari A. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett. 2015;357:339–345. doi: 10.1016/j.canlet.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Quabius ES, Moller P, Haag J, Pfannenschmidt S, Hedderich J, Gorogh T, Rocken C, Hoffmann M. The role of the antileukoprotease SLPI in smoking-induced human papillomavirus-independent head and neck squamous cell carcinomas. Int J Cancer. 2014;134:1323–1334. doi: 10.1002/ijc.28462. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann M, Quabius ES, Tribius S, Hebebrand L, Gorogh T, Halec G, Kahn T, Hedderich J, Rocken C, Haag J. Human papillomavirus infection in head and neck cancer: the role of the secretory leukocyte protease inhibitor. Oncol Rep. 2013;29:1962–1968. doi: 10.3892/or.2013.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quabius ES, Merz I, Gorogh T, Hedderich J, Haag J, Rocken C, Ambrosch P, Hoffmann M. miRNA-expression in tonsillar squamous cell carcinomas in relation to HPV infection and expression of the antileukoproteinase SLPI. Papillomavirus Res. 2017;4:26–34. doi: 10.1016/j.pvr.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodham AW, DA Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand HE, Isas JM, Langen R, Kast WM. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicol AF, Brunette LL, Nuovo GJ, Grinsztejn B, Friedman RK, Veloso VG, Cunha CB, Coutinho JR, Vianna-Andrade C, Oliveira NS. Secretory Leukocyte Protease Inhibitor Expression and High-Risk HPV Infection in Anal Lesions of HIV-Positive Patients. J Acquir Immune Defic Syndr. 2016;73:27–33. doi: 10.1097/QAI.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remmerbach TW, Brinckmann UG, Hemprich A, Chekol M, Kuhndel K, Liebert UG. PCR detection of human papillomavirus of the mucosa: comparison between MY09/11 and GP5+/6+ primer sets. J Clin Virol. 2004;30:302–308. doi: 10.1016/j.jcv.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Quabius ES, Ossenkop L, Harder S, Kern M. Dental implants stimulate expression of Interleukin-8 and its receptor in human blood--an in vitro approach. J Biomed Mater Res B Appl Biomater. 2012;100:1283–1288. doi: 10.1002/jbm.b.32693. [DOI] [PubMed] [Google Scholar]
- 18.Cordes C, Hasler R, Werner C, Gorogh T, Rocken C, Hebebrand L, Kast WM, Hoffmann M, Schreiber S, Ambrosch P. The level of secretory leukocyte protease inhibitor is decreased in metastatic head and neck squamous cell carcinoma. Int J Oncol. 2011;39:185–191. doi: 10.3892/ijo.2011.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M, Gorogh T, Gottschlich S, Lohrey C, Rittgen W, Ambrosch P, Schwarz E, Kahn T. Human papillomaviruses in head and neck cancer: 8 year-survival-analysis of 73 patients. Cancer Lett. 2005;218:199–206. doi: 10.1016/j.canlet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Wakeham K, Kavanagh K, Cuschieri K, Millan D, Pollock KG, Bell S, Burton K, Reed NS, Graham SV. HPV status and favourable outcome in vulvar squamous cancer. Int J Cancer. 2017;140:1134–1146. doi: 10.1002/ijc.30523. [DOI] [PubMed] [Google Scholar]