Abstract
Background
We explored the relation between blood concentrations of monocyte/lymphocyte subsets and carotid artery plaque macrophage content, measured by positron emission tomography (PET) with 11C-PK11195.
Methods and results
In 9 patients with carotid plaques we performed 11C-PK11195-PET/computed tomography angiography imaging and measurement of absolute concentrations and frequencies of circulating monocytes and T-cell subsets. Plaque standardized uptake value (SUV) for 11C-PK11195 was negatively correlated with concentrations of total monocytes (r = −0.58, p = 0.05) and CD14++CD16−HLA-DR+ classical subset (r = −0.82, p = 0.005). These correlations hold true also in relation to plaque target to background ratio. No correlation was observed between plaque SUV and CD3+T lymphocytes, CD4+T lymphocytes nor with activated CD3+CD4+T cells expressing HLA-DR.
Conclusions
We first demonstrated a reduction in the absolute concentration of monocytes and particularly in classical monocytes expressing HLA-DR in the presence of an increased uptake of 11C-PK11195 in carotid plaques. The present work, despite being a pilot study comprising only a small number of subjects provides new insights in the search for specific cellular biomarkers with potential diagnostic and prognostic value in patients with a known carotid plaque.
Keywords: Carotid plaque, 11C-PK11195 uptake, Classical monocytes, Positron emission tomography, Atherosclerosis
1. Introduction
Monocytes are among the first cells to be recruited into dysfunctional arterial walls, and are involved in atherosclerotic plaque formation and development [1]. Their contribution spans from secretion of inflammatory mediators to local differentiation into lesional macrophages [2], that make up a large proportion of atherosclerotic plaque cell constituents [3]. Activated macrophages have been demonstrated in vulnerable carotid plaques [4,5]. 11C-PK11195 is a selective ligand for the translocator protein (18 kDa) (TSPO), which is highly expressed in activated cells of the mononuclear phagocyte lineage [6]. Hybrid imaging with positron emission tomography and computed tomography angiography (PET/CTA) using 11C-PK11195 detects and quantifies vascular inflammation [7,8], and discriminates between vulnerable and non-vulnerable atherosclerotic lesions within the carotid arteries [9], We recently demonstrated an inverse correlation between plaque neovascularization, an index of plaque vulnerability, and blood concentrations of both pro-inflammatory CD14++CD16− classical monocytes and activated CD4+HLA-DR+T cells in asymptomatic patients with moderate carotid plaques [10,11]. Whether the reduction of circulating classical monocytes is due to a potential redistribution of these cell types into active lesions remains to be explored. In this proof of principle study, we sought to explore the relation between peripheral blood concentrations of classical monocyte subsets and carotid artery plaque macrophage content, measured by PET/CTA with 11C-PK11195.
2. Methods
2.1. Study design and population
The population of the study consisted of 9 patients with carotid plaques referred for routine carotid ultrasound evaluation (8 asymptomatic subjects, 1 with a previous history of ischemic stroke). The study was approved by the Ethics Committee of the San Raffaele Institute and all patients signed a written consent. This substudy is part of the IMaging Della PLAcca Carotidea (IMPLAC) study, ClinicalTrials.gov registration number was NCT03333330 and EudraCT number was 2012-000648-83.
2.2. Flow cytometry
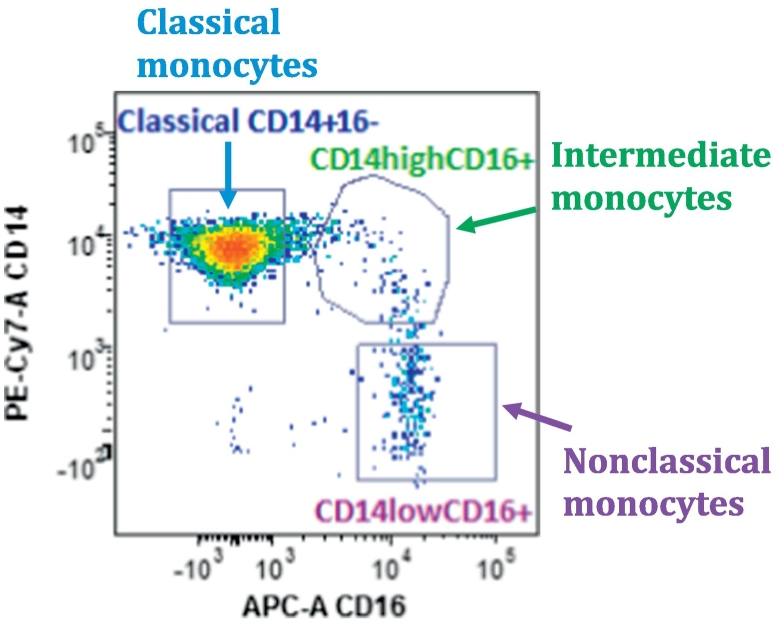
Absolute concentrations of circulating monocytes and T-cell subsets were assessed by flow cytometry on a LSRFortessa Flow Cytometer (BD Biosciences) equipped with 5 lasers and standard optics. Whole blood was collected on the same day of the ultrasonographic evaluation using EDTA-anticoagulated vacutainer tubes. The samples were stained and analyzed within 24 h of collection. CD3 and CD4 were used to identify CD4+T cells. CD14 and CD16 were used to identify classical, intermediate and non-classical monocyte subsets as previous described following the classification of the International Union of Immunologic Societies [4,12]: classical CD14++CD16− monocytes; intermediate CD14+CD16+; non-classical CD14+CD16+ (representative case in Fig. 1). HLD-DR was used as a marker of cellular activation. Absolute monocytes/T-cells concentration was obtained with the aid of Flow-Count beads (Beckman Coulter) as previously described [10]. Frequencies of cellular subsets were also reported as percentage of the total number of monocytes for monocyte subsets. Similarly, frequencies were calculated for lymphocytes subsets.
Fig. 1.
Monocyte subsets identified by flow cytometry in a representative patient. Gating strategy used to identify monocytes based on forward and orthogonal scatter; then differential identification of monocytes subsets based on surface markers CD14 (antibody: PE-Cy7, M5E2, BD-Pharmingen), and CD16 (antibody: APC, B73.1, BD Pharmingen). Classical monocytes are CD14++CD16−; intermediate monocytes are CD14++CD16+; and non-classical monocytes are CD14+CD16+.
2.3. 11C-PK11195-PET/CTA imaging
11C-PK11195-PET/CTA imaging of the carotid arteries was performed according to the protocol previously described by our group using a 64-slice CT scanner (VCT Lightspeed, GE Healthcare, USA) administering non-ionic, iso-osmolar contrast Iodixanol (Visipaque 320; GE) [9].
2.4. Brain Magnetic resonance imaging
Asymptomatic patients (n = 8) underwent a brain MRI using a 1.5 Tesla scanner (ACHIEVA Philips Medical Systems) to assess the presence of silent white matter hyperintensities (WMHs). The protocol that was used has been previously described [13].
2.5. Statistical analysis
One-way Spearman's test was used for correlations. Correlations with p < 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, USA).
3. Results
3.1. Patients' characteristics
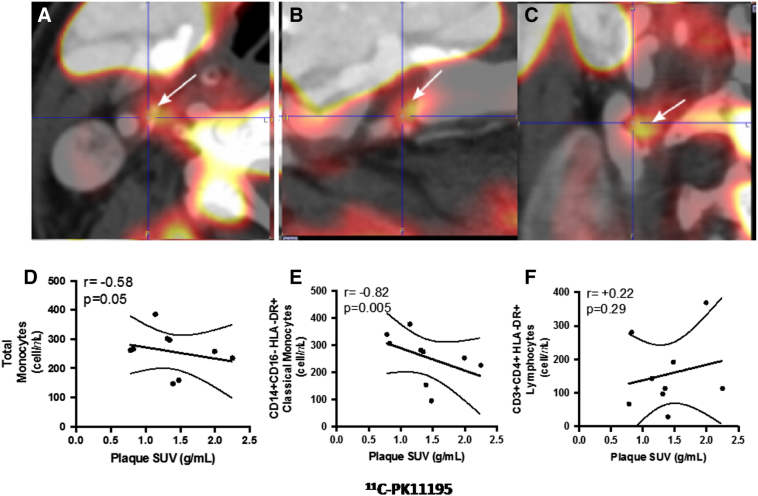
Patients' clinical characteristics (5 men, median age 71 years, interquartile [IQR] 65–76 years) are summarized in Table 1. Patients had a median carotid stenosis of 60% (IQR 53%–70%) using European Carotid Surgery Trial (ECST) method on CTA images and a median main carotid plaque standardized uptake value (SUV) of 1.35 g/mL (IQR 0.98–1.74 g/mL) and target-to-background ratio (TBR) of 0.94 (IQR 0.87–1.01) for 11C-PK11195 (representative case is reported in Fig. 2A–C). Only 1 patient was symptomatic for ischemic stroke, whereas the other 8 subjects were asymptomatic. Nevertheless, 7 out of 8 (87.5%) asymptomatic patients presented brain WMHs, with a median number of WMH of 23 (interquartile range [Q1–Q3]: 7–56).
Table 1.
Clinical characteristics of the study population. Symptoms refer to previous history of cerebrovascular accidents. BMI = body mass index; SUV = standardized uptake value; LDL = low density lipoprotein; HDL = high density lipoprotein; Hs-CRP = high sensitivity C-reactive protein.
| N | 9 |
|---|---|
| Sex (M/F) | 5/4 |
| Age (years) | 71 (65–76) |
| Asymptomatic/symptomatic | 8/1 |
| BMI (Kg/m2) | 25 (20–28) |
| Plaque SUV (g/mL) | 1.35 (0.98–1.74) |
| Total cholesterol (mg/dL) | 172 (156–182) |
| LDL cholesterol (mg/dL) | 102 (91–112) |
| HDL cholesterol (mg/dL) | 48 (40–58) |
| Hs-CRP (mg/dL) | 1.56 (1.18–7.45) |
Fig. 2.
11C-PK11195 carotid plaque uptake and graphs summarizing the main findings of the study. Panels A–C display PET/CT hybrid imaging of a plaque located at the right carotid bifurcation in a representative patient. The plaque shows intense 11C-PK11195 uptake (white arrows). Panel D shows the significant negative correlation between plaque SUV for 11C-PK11195 and circulating total monocytes, Panel E the correlation between SUV and CD14++CD16−HLA-DR+ activated classical monocytes and Panel F the absence of correlation between plaque SUV and activated CD3+CD4+T cells expressing HLA-DR.
3.2. Correlations between 11C-PK11195 plaque uptake and circulating inflammatory cells
Plaque SUV for 11C-PK11195 was negatively correlated with circulating concentrations of total monocytes (r = −0.58, p = 0.05), and in particular with the CD14++CD16−HLA-DR+ classical monocytes (r = −0.82, p = 0.005, Table 2 and Fig. 2D–E). A correlation was also observed between the TBR in the main carotid artery plaques with total monocytes (r = −0.58, p = 0.05) and CD14+CD16−HLA-DR+ classical monocytes (r = −0.58, p = 0.05). No significant changes in the frequencies of total monocytes in relation to the whole blood count or of CD14+CD16−HLA-DR+ classical monocytes in relation to the total monocytes were observed (Table 2).
Table 2.
Correlations between monocyte subpopulation and carotid plaque standardized uptake value (SUV) and target-to-background ratio (TBR). * indicates significant result. WBC indicates whole blood count.
| Cellular populations | Plaque SUV |
Plaque TBR |
||
|---|---|---|---|---|
| r Spearman | p | r Spearman | p | |
| Total monocytes (count) | −0.58 | 0.05 | −0.58 | 0.05 |
| Monocytes/WBC (%) | 0.08 | 0.42 | −0.33 | 0.19 |
| CD14++CD16− classical (% of monocytes) | 0.57 | 0.06 | −0.10 | 0.40 |
| CD14++CD16− classical (count) | −0.25 | 0.26 | −0.47 | 0.11 |
| CD14++CD16+ intermediate (% of monocytes) | −0.05 | 0.46 | −0.20 | 0.30 |
| CD14++CD16+ intermediate (count) | −0.36 | 0.16 | −0.40 | 0.13 |
| CD14+CD16+ nonclassical (% of monocytes) | −0.48 | 0.10 | 0.12 | 0.39 |
| CD14+CD16+ nonclassical (count) | −0.73 | 0.01* | 0.01 | 0.50 |
| HLADR+CD14++CD16− classical (% of monocytes) | −0.50 | 0.09 | 0.18 | 0.32 |
| HLADR+CD14++CD16− classical (count) | −0.82 | 0.005* | −0.58 | 0.05 |
| HLA-DR+CD14++CD16+ intermediate (% of monocytes) | −0.53 | 0.07 | −0.27 | 0.23 |
| HLA-DR+CD14++CD16+ intermediate (count) | −0.66 | 0.03* | −0.38 | 0.15 |
| HLA-DR+CD14+CD16+ nonclassical (% of monocytes) | −0.17 | 0.34 | 0.28 | 0.23 |
| HLA-DR+CD14+CD16+ nonclassical (count) | −0.65 | 0.03* | 0.05 | 0.46 |
| Total lymphocytes (count) | −0.07 | 0.44 | 0.07 | 0.44 |
| Lymphocytes/WBC (%) | 0.22 | 0.29 | 0.30 | 0.22 |
| CD3+CD4+ T cells (% of lymphocytes) | 0.27 | 0.25 | 0.20 | 0.31 |
| CD3+CD4+ T cells (count) | 0.25 | 0.26 | 0.13 | 0.37 |
| HLA-DR+CD3+CD4+ T cells (% of lymphocytes) | 0.12 | 0.39 | 0.42 | 0.13 |
| HLA-DR+ CD3+CD4+ T cells (count) | 0.22 | 0.29 | 0.47 | 0.10 |
In bold are underlined correlations with a p-value of 0.05 or inferior.
No correlation was observed between background SUV in the internal jugular vein and total monocytes (r = +0.05, p = 0.46) and CD14++CD16−HLA-DR+ classical monocytes (r = −0.23, p = 0.28) supporting the specificity of the signal observed in the plaque. Both the count of CD14++CD16+HLA-DR+ intermediate and CD14+CD16+HLA-DR+ non-classical monocytes negatively correlated with plaque SUV (respectively r = −0.66, p = 0.03 and r = −0.65, p = 0.03), but the correlation was not confirmed in relation with the plaque TBR (Table 2). This finding was specific for monocytes; in fact, no correlation was observed between plaque SUV and CD3+T lymphocytes, CD4+T lymphocytes nor with activated CD3+CD4+T cells expressing HLA-DR (Table 2 and Fig. 2F), that were previously found to be reduced in patients with signs of neovascularization in carotid plaques [10]. Plaque SUV of 11C-PK11195 was not associated with other patients' characteristics nor with inflammatory biomarkers (high-sensitivity C-reactive protein [hs-CRP] and interleukin-6 [IL-6] levels).
4. Discussion
We described for the first time the reverse correlation between total monocytes, and in particular the CD14++CD16−HLA-DR+ classical subset with the carotid plaque uptake of 11C-PK11195. 11C-PK11195 selectively marks TPSO, that is expressed by activated monocytes/macrophages. Total monocytes and CD14++CD16−HLA-DR+ classical subset are decreased in patients with plaque activation measured using a novel and specific non-invasive diagnostic tool able to demonstrate the accumulation of activated mononuclear cells within atherosclerotic lesions [9]. The fact, that 8 out of 9 patients were symptomatic or had silent WMHs (7/8 among those patients without previous cerebrovascular events had WMHs) supports the idea that the investigated carotid plaques can be considered vulnerable, as suggested by the association between the presence of WMHs with the risk of ischemic stroke [14,15]. The observed reduction in the absolute concentration of circulating HLA-DR expressing classical monocytes, in the presence of an increased uptake of 11C-PK11195 in the plaques, supports the hypothesis that selected monocytes subsets are recruited from the circulation into more inflamed, vulnerable lesions, even if no demonstration of cell tracking has been shown in this setting. Our data show an association but are unable to implicate cell recruitment into the plaque as it is not the only mechanism to explain the decrease in monocyte counts. The phenotype of reduction of classical monocytes could result from their conversion into intermediate and eventually nonclassical subsets in the circulation as described by Patel et al. [16]. Nevertheless, the frequencies of HLA-DR expressing classical, intermediate and nonclassical monocyte subsets are not associated with plaque SUV. In addition, we describe a negative correlation between total monocytes and plaque SUV, a situation in which the reciprocal conversion between different cellular subsets cannot be held responsible. Thus, the explanation of monocyte conversion as a potential mechanism to explain CD14++CD16−HLA-DR+ classical monocyte reduction in patients with activated carotid plaque is less likely compared with a reduction of this specific cellular subset from the blood due to an accumulation in a potential target as the atherosclerotic plaque. Furthermore, this finding seems specific for activated monocyte subsets since no association was found between plaque 11C-PK11195 uptake and reduction of circulating activated CD4+HLA-DR+T cells. The present finding appears in line with our previous study that have shown a reverse association between circulating classical monocytes and presence of signs of neovascularization in patients with asymptomatic carotid plaques, demonstrated by the use of contrast enhanced ultrasound (CEUS) [10]. In fact, it has been shown that plaque vascularization measured by CEUS correlates positively with fludeoxyglucose (FDG) uptake measured by PET/CT in humans, suggesting an association between vascularization and inflammation [17]. Of note, in the present study the ligand used to measure the plaque activity was specific for the detection of activated monocytes [9]. Thus, it is not surprising that we did not observe a reverse correlation also with CD4+HLA-DR+T cells. On the contrary in a previous study, we have shown a decrease of circulating CD4+HLA-DR+T cells in patients with carotid plaques with signs of plaque vascularization measured by CEUS [11]. Finally, no association was found between plaque SUV and hs-CRP or IL-6 further supporting the concept that these markers are not sufficient to identify patients with activated plaques [18], whereas specific cellular biomarkers appear more promising.
In conclusion, positive studies investigating the relation between signs of plaque activation by means of molecular marker of inflammation (i.e. TPSO) and specific monocytic subsets are limited [18]. Thus, the present work, despite being a pilot study comprising only a small number of subjects provides new insights in the search for specific cellular biomarkers with potential diagnostic and prognostic value in patients with known carotid plaque.
Disclosures and conflict of interests
Dr. Ammirati received financial support from the “Giovane Ricercatore 2009 Grant” from Italian Health Ministry (GR-2009-1608780). Dr. Camici has served as a consultant for Laboratoires Servier. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
Enrico Ammirati, Email: ammirati.enrico@hsr.it.
Paolo G. Camici, Email: camici.paolo@hsr.it.
References
- 1.Swirski F.K., Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. doi: 10.1126/science.1230719. (Epub 2013/01/12, PubMed PMID: 23307733; PubMed Central PMCID: PMCPMC3891792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz C., Massberg S. Atherosclerosis—multiple pathways to lesional macrophages. Sci. Transl. Med. 2014;6(239):239ps2. doi: 10.1126/scitranslmed.3008922. (Epub 2014/06/06, PubMed PMID: 24898743) [DOI] [PubMed] [Google Scholar]
- 3.Virmani R., Burke A.P., Farb A., Kolodgie F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006;47(Suppl. 8) doi: 10.1016/j.jacc.2005.10.065. (PubMed PMID: 16631505) [DOI] [PubMed] [Google Scholar]
- 4.Weber C., Shantsila E., Hristov M., Caligiuri G., Guzik T., Heine G.H. Role and analysis of monocyte subsets in cardiovascular disease. Joint consensus document of the European Society of Cardiology (ESC) Working Groups “Atherosclerosis & Vascular Biology” and “Thrombosis”. Thromb. Haemost. 2016;116(4):626–637. doi: 10.1160/TH16-02-0091. (Epub 2016/07/15, PubMed PMID: 27412877) [DOI] [PubMed] [Google Scholar]
- 5.Magnoni M., Ammirati E., Camici P.G. Non-invasive molecular imaging of vulnerable atherosclerotic plaques. J. Cardiol. 2015;65(4):261–269. doi: 10.1016/j.jjcc.2015.01.004. (PubMed PMID: 25702846) [DOI] [PubMed] [Google Scholar]
- 6.Camici P.G., Rimoldi O.E., Gaemperli O., Libby P. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur. Heart J. 2012;33(11):1309–1317. doi: 10.1093/eurheartj/ehs067. (PubMed PMID: 22507974) [DOI] [PubMed] [Google Scholar]
- 7.Pugliese F., Gaemperli O., Kinderlerer A.R., Lamare F., Shalhoub J., Davies A.H. Imaging of vascular inflammation with [(11)C]-PK11195 and positron emission tomography/computed tomography angiography. J. Am. Coll. Cardiol. 2010;56(8):653–661. doi: 10.1016/j.jacc.2010.02.063. (PubMed PMID: 20705222) [DOI] [PubMed] [Google Scholar]
- 8.Ammirati E., Moroni F., Pedrotti P., Scotti I., Magnoni M., Bozzolo E.P. Non-invasive imaging of vascular inflammation. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00399. (PubMed PMID: 25183963; PubMed Central PMCID: PMC4135304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaemperli O., Shalhoub J., Owen D.R., Lamare F., Johansson S., Fouladi N. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur. Heart J. 2012;33:1902–1910. doi: 10.1093/eurheartj/ehr367. (PubMed PMID: 21933781) [DOI] [PubMed] [Google Scholar]
- 10.Ammirati E., Moroni F., Magnoni M., Di Terlizzi S., Villa C., Sizzano F. Circulating CD14+ and CD14highCD16-classical monocytes are reduced in patients with signs of plaque neovascularization in the carotid artery. Atherosclerosis. 2016;255:171–178. doi: 10.1016/j.atherosclerosis.2016.10.004. (PubMed PMID: 27751505) [DOI] [PubMed] [Google Scholar]
- 11.Ammirati E., Magnoni M., Moroni F., Di Terlizzi S., Scotti I., Villa C. Reduction of circulating HLA-DR T cell levels correlates with increased carotid intraplaque neovascularization and atherosclerotic burden. JACC Cardiovasc. Imaging. 2016 doi: 10.1016/j.jcmg.2015.10.010. (PubMed PMID: 26777226) [DOI] [PubMed] [Google Scholar]
- 12.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. doi: 10.1182/blood-2010-02-258558. (Epub 2010/07/16. PubMed PMID: 20628149) [DOI] [PubMed] [Google Scholar]
- 13.Ammirati E., Moroni F., Magnoni M., Rocca M.A., Messina R., Anzalone N. Relation between characteristics of carotid atherosclerotic plaques and brain white matter hyperintensities in asymptomatic patients. Sci. Rep. 2017;7(1):10559. doi: 10.1038/s41598-017-11216-x. (Epub 2017/09/07, PubMed PMID: 28874779; PubMed Central PMCID: PMCPMC5585357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moroni F., Ammirati E., Magnoni M., D'Ascenzo F., Anselmino M., Anzalone N. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: a systematic review and meta-analysis. Int. J. Cardiol. 2016;223:681–687. doi: 10.1016/j.ijcard.2016.08.234. (PubMed PMID: 27568989) [DOI] [PubMed] [Google Scholar]
- 15.Moroni F., Ammirati E., Rocca M.A., Filippi M., Magnoni M., Camici P.G. Cardiovascular disease and brain health: focus on white matter hyperintensities. Int. J. Cardiol. Heart Vasc. 2018;19:63–69. doi: 10.1016/j.ijcha.2018.04.006. (Epub 2018/06/28. PubMed PMID: 29946567; PubMed Central PMCID: PMCPMC6016077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel A.A., Zhang Y., Fullerton J.N., Boelen L., Rongvaux A., Maini A.A. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017;214(7):1913–1923. doi: 10.1084/jem.20170355. (Epub 2017/06/14. PubMed PMID: 28606987; PubMed Central PMCID: PMCPMC5502436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjelmgren O., Johansson L., Prahl U., Schmidt C., Freden-Lindqvist J., Bergstrom G.M. A study of plaque vascularization and inflammation using quantitative contrast-enhanced US and PET/CT. Eur. J. Radiol. 2014;83(7):1184–1189. doi: 10.1016/j.ejrad.2014.03.021. (PubMed PMID: 24767629) [DOI] [PubMed] [Google Scholar]
- 18.Ammirati E., Moroni F., Norata G.D., Magnoni M., Camici P.G. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/718329. (PubMed PMID: 25960621; PubMed Central PMCID: PMC4415469) [DOI] [PMC free article] [PubMed] [Google Scholar]