Abstract
Background
Congenital diaphragmatic hernia is an orphan disease with high neonatal mortality and significant morbidity. An important cause for this is pulmonary hypertension, for which no effective postnatal therapy is available to date. An innovative strategy aiming at treating or preventing pulmonary hypertension more effectively is urgently needed. Prenatal sildenafil administration to expectant mothers prevented fetal and neonatal vascular changes leading to pulmonary hypertension in several animal models, and is, therefore, a promising approach. Before transferring this antenatal medical approach to the clinic, more information is needed on transplacental transfer and safety of sildenafil in humans.
Methods
This is a randomized, investigator-blinded, double-armed, parallel-group, phase I/IIb study with as a primary objective to measure the in-vivo transplacental transfer of sildenafil in women in the second and early third trimester of pregnancy (sub-study 1; weeks: 20.0–32.6) and at term (sub-study 2; weeks: 36.6–40). Participants will be randomized to two different sildenafil doses: 25 or 75 mg. In sub-study 1, a single dose of the investigational product will be administered to women undergoing termination of pregnancy, and maternal and fetal blood samples will be collected for determination of sildenafil concentrations. In sub-study 2, sildenafil will be administered three times daily from 3 days before planned delivery until actual delivery, following which maternal and umbilical cord samples will be collected. Proxies of maternal and fetal tolerance as well as markers of fetal pulmonary vasodilation will also be measured.
Discussion
This is the first study evaluating in-vivo transplacental passage of sildenafil in humans.
Trial registration
EU Clinical Trials Register 2016–002619-17, validated on 12 August 2016.
Trial sponsor: UZ Leuven, Herestraat 49, 3000 Leuven.
Electronic supplementary material
The online version of this article (10.1186/s13063-018-2897-8) contains supplementary material, which is available to authorized users.
Keywords: Sildenafil citrate, Congenital diaphragmatic hernia, Transplacental therapy, Placenta transfer study
Background
Congenital diaphragmatic hernia (CDH) is a birth defect occurring in 1/2500 newborns and is characterized by a failure of the diaphragm to fully form in early pregnancy. This causes herniation of the abdominal organs into the thorax, which compromises lung development, eventually leading to pulmonary hypoplasia [1]. At birth, the clinical consequence is respiratory insufficiency and persistent pulmonary hypertension (PHT) which cannot simply be solved by surgically repairing the diaphragm. Thanks to advances in postnatal management, survival of neonates with CDH has increased to 70% [2–4]. Despite that, PHT remains a major determinant of mortality and morbidity, with only expert opinions to guide treatment [5, 6].
Today, more than 70% of CDH cases are diagnosed prenatally, following which additional testing is done to estimate the degree of fetal pulmonary hypoplasia and to provide a personalized prognosis [7–11]. The ability to identify a future non-survivor during pregnancy sparks the question for a prenatal intervention to reverse the natural course of the condition. Prenatal lung growth can be stimulated by minimally invasive tracheal occlusion (TO) [12]. The efficacy of this procedure currently undergoes evaluation in a global, open-label randomized clinical trial (https://totaltrial.eu/) [13]. Although TO improves lung size and may thus increase postnatal survival, it only partially improves PHT. Furthermore, TO remains a surgical intervention, with its inherent side effects and complications, such as prematurity, partially offsetting the effect of fetal therapy [14, 15].
As an alternative or complementary treatment to TO, we will investigate the use of transplacentally administered sildenafil. Sildenafil is a selective inhibitor of phosphodiesterase-type-5. It is primarily marketed for the treatment of erectile dysfunction, but also for treating PHT in adults [16]. It enhances nitric oxide (NO)-mediated vasodilation [17], promotes pulmonary angiogenesis and decreases pulmonary arterial remodeling [18, 19]. The drug is effective in children above 1 year of age who have PHT [20]. There is a growing interest in the use of sildenafil for the treatment of PHT of various etiologies in the newborn, including in CDH [21], particularly when other therapies, such as inhaled NO, fail. Such step-up strategy has been recently incorporated in the updated European “Euro-CDH Consortium” consensus neonatal management protocol for CDH infants [22].
Sildenafil has also already been used in pregnant women for maternal PHT or for pregnancy complications such as pre-eclampsia and intrauterine growth restriction [23, 24]. A systematic review summarizing clinical studies on sildenafil use in pregnancy has shown that maternal tolerance and perinatal outcomes are comparable with controls [25]. All this suggests a safe use of sildenafil from mid-gestation onwards.
Given the maternal and fetal safety profile of sildenafil, and given its established use in the postnatal treatment of PHT, the logical next step is to consider sildenafil use prenatally. Indeed, this is when the morphologic basis for PHT is being laid down, and, as such, prenatal treatment could potentially prevent PHT.
In two animal models of CDH, sildenafil has been shown to rescue the pulmonary vascular bed [26, 27]. Luong et al. were the first to demonstrate that maternal sildenafil administration reverses the morphologic pulmonary vascular changes associated to CDH in the nitrofen rat model [26]. Given our focus on making fetal interventions for CDH more effective and less invasive, we felt the need to move the concept of sildenafil further along the translational research track. We demonstrated the safety and efficacy of sildenafil in the rabbit [27]. Placebo-exposed CDH fetuses had an increased wall thickness in peripheral pulmonary vessels and significantly less fifth-generation vessels compared to controls. CDH fetuses treated with sildenafil had a medial thickness in peripheral pulmonary vessels in the normal range and a normal percentage of vessels of the fifth generation or higher. Sildenafil also reversed the mean terminal bronchiolar density in CDH fetuses to normal values and improved lung mechanics. Functionally, we could demonstrate reduced in-utero pulmonary vascular resistance by micro-ultrasound Doppler examination. No maternal or fetal adverse effects were observed. In summary, both in the rodent and the rabbit model, antenatal sildenafil administration rescues vascular branching and architecture and improves airway morphometry. These preclinical findings, together with the demonstration of the safety of sildenafil during pregnancy, pave the way for clinical translation.
One last critical step prior to clinical translation is that we need a better knowledge of sildenafil pharmacokinetics and transplacental transfer in vivo. This information is currently not available in the academic literature. This knowledge gap is explained by the fact that the product is currently used for maternal indications or to improve the flow in the uterus and placenta [24, 28, 29]. In these conditions fetal effects are less relevant, hence they were less studied and fetal drug levels were not measured. We have recently performed ex-vivo placental transfer studies [30]. We demonstrated that sildenafil crosses the placenta ex vivo at a rate that is independent from the initial maternal concentration and that there is sufficient placental transfer to reach therapeutic fetal drug levels at non-toxic maternal concentrations. These results need now to be validated in vivo.
Methods and trial design
Objectives
The main objective of the study is to evaluate the transfer of sildenafil through the second and third trimester human placenta in vivo.
Secondary objectives are:
To evaluate maternal, fetal and neonatal tolerance
To evaluate the effect of sildenafil on the fetal pulmonary circulation
Trial design
This is a randomized, investigator-blinded, double-armed, parallel-group, phase I/IIb study consisting of two different sub-studies recruiting simultaneously:
-
Second trimester placental transfer study (sub-study 1)
The primary outcome of this sub-study is the fetal plasma sildenafil level after oral administration maternally. Fetal plasma will be obtained by fetal cardiocentesis or cordocentesis, required for administration of the drugs used for feticide at the time of pregnancy termination [31, 32]. Maternal blood sampling will be performed simultaneously via peripheral venous puncture.
Secondary endpoints are:- Doppler ultrasound measures of pulmonary vasodilatation: change in the acceleration time/ejection time (AT/ET) ratio of the main fetal pulmonary artery and of the pulmonary artery branch closest to the ultrasound probe between baseline and the time of fetal blood sampling. Additional measures of blood flow resistance in the fetal main pulmonary artery and in the right and left pulmonary artery branch (Pulsatility Index, Resistance Index, peak systolic velocity, peak early diastolic reversed flow) will also be collected
- Effect of sildenafil on the fetal systemic circulation: change in the Doppler waveform of the umbilical artery, between baseline and blood sampling
- Simulated maternal and fetal pharmacokinetic profiles
- Maternal tolerance, measured as change in blood pressure, heart rate and oxygen saturation compared to baseline, and incidence of side effects, including any kind of visual disturbance
-
Third trimester placental transfer and safety study (sub-study 2)
The primary endpoint is sildenafil transfer at term, expressed as a ratio of the neonatal over the maternal sildenafil plasma concentration. Neonatal venous blood samples will be collected from the umbilical cord at delivery. Maternal blood sampling will be performed via peripheral venous puncture.
Secondary endpoints are:- Doppler ultrasound measures of pulmonary vasodilatation as described above
- Effect of sildenafil on the fetal systemic circulation: change from baseline in the Doppler waveform of the umbilical artery, mean cerebral artery, ductus venosus, change in fetal heart rate monitoring pattern
- Effect of sildenafil on the maternal placental circulation: change from baseline in the Doppler waveform of the uterine arteries
- Simulated maternal and fetal pharmacokinetic profiles
- Maternal tolerance measured as change in blood pressure, heart rate and oxygen saturation compared to baseline, and incidence of side effects, including any kind of visual disturbance
- Neonatal tolerance, measured as trend in mean arterial blood pressure during the first 24 h of life, vasoactive inotropic score [33] at 24 h of life and early (< 24 h) lung function, expressed as Oxygenation Index and alveolar-arterial oxygen gradient
For both sub-studies, participants will be randomized to one of two parallel groups receiving different doses of sildenafil (sildenafil 25 or 75 mg). No placebo control is foreseen. The protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) recommendations for interventional trials (Additional file 1).
Study population
Second trimester placental transfer study (sub-study 1)
Inclusion criteria
Volunteer with singleton pregnancy undergoing second trimester or early third trimester termination of pregnancy for fetal anomalies not interfering with normal lung development
Maternal age > 18 and < 45 years (to allow consent and avoid age-related cardiac risks, respectively)
Exclusion criteria
Multiple pregnancy
Maternal diseases (such as hypertension, diabetes mellitus, infections), pregnancy complications (such as pre-eclampsia or growth restriction) or use of medication that could interfere with placental transfer of the study drug
Contraindication to sildenafil therapy: renal or hepatic failure, maternal cardiac disease, hypotension (blood pressure < 90/60 mmHg), stroke, retinitis pigmentosa, sickle cell disease, bleeding disorders, active peptic ulcer, concomitant treatment with nitrates, previous serious adverse reaction or allergy to sildenafil, use of other vasodilator therapy, alpha-blockers, protease inhibitors, erythromycin or cimetidine
History of psychiatric or psychological disease or concerns about psychological eligibility
Previous loss of vision because of suspected non-arteritic, anterior, ischemic optic neuropathy (NAION)
Third trimester placenta transfer and safety study
Inclusion criteria
Singleton pregnancy with isolated fetal CDH, left- or right-sided, with any degree of lung hypoplasia
Gestational age ≥ 36 + 6 and ≤ 40 weeks
Planned delivery at least 3 days after study initiation
Maternal age ≥ 18 and ≤ 45 years (to allow consent and avoid age-related cardiac risks respectively)
Exclusion criteria
Multiple pregnancy, chromosomal or major structural fetal abnormality
Maternal conditions (such as hypertension, diabetes mellitus, infections), pregnancy complications (such as pre-eclampsia or growth restriction) or use of medication that could interfere with placental transfer of the study drug
Any contraindication to sildenafil therapy as in sub-study 1
Preterm premature rupture of membranes (pPROM)
Interventions
In both sub-studies, sildenafil tablets will be administered orally to the mother as this administration route is the most practical and clinically most acceptable for sustained administration, especially in pregnant women. The half-life of sildenafil after oral administration is about 4 h [34]. Therefore, the most commonly used administration schemes for chronical conditions even in pregnancy consist in three administrations daily [20, 35–37].
Two sildenafil doses have been selected according to previously published literature and to our ex-vivo data on placental transfer [30], i.e., 25 and 75 mg. A dose of 25 mg three times daily has been proven safe in the treatment of pregnancies complicated by intrauterine growth retardation (IUGR) [24]. A dose of 75 mg three times daily is the maximum dose used in a trial on pregnant women affected by pre-eclampsia, without any fetal or maternal side effects being reported under that dose [23].
Second trimester placental transfer study (sub-study 1)
A single administration of sildenafil is planned before maternal and fetal blood sampling. Participants will be randomized to receive either a 25-mg or a 75-mg dose. For each dose, participating women will additionally be randomized to receive sildenafil either 2 h or 8 h before the termination. These time points have been selected because they represent the peak time and the trough time, respectively [34].
Third trimester placental transfer and safety study (sub-study 2)
Participants will be allocated to receive sildenafil tablets at a dose of 25 mg or 75 mg three times daily from 3 days prior to planned delivery until delivery.
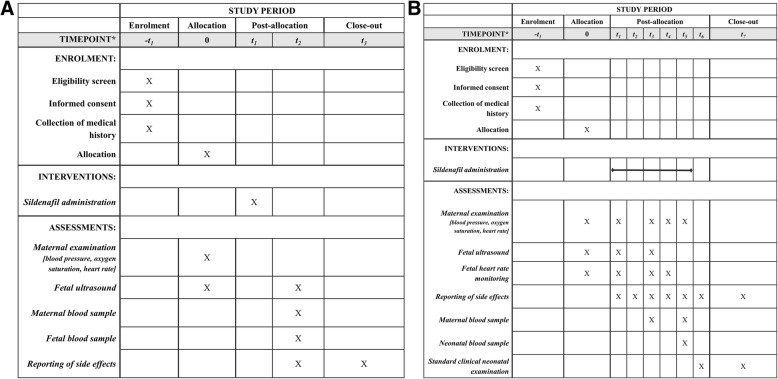
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Diagram in Fig. 1 summarizes trial procedures for sub-studies 1 (Fig. 1A) and 2 (Fig. 1B) [38]. For both sub-studies, collection of routine clinical information from participants’ medical records to determine eligibility, informed consent and patient randomization will take place during the screening visit. A web-based randomization tool will be used in order to assign each patient to a study arm.
Fig. 1.
A; * − t1: screen visit; t0: allocation/baseline visit; t1: 2 or 8 h before termination of pregnancy; t2: at the time of feticide; t3: at patient’s discharge. B: * − t1: screen visit; t0: allocation/baseline visit; t1: 3 days before induction; t2: day 1 after first sildenafil administration; t3: 50 h after first sildenafil administration; t4: at beginning of labor; t5: at delivery; t6: day 1 after delivery; t7: at maternal and neonatal discharge
Second trimester placental transfer study (sub-study 1)
Eligible patients will be randomly assigned to one of four different study groups: dose 1 (25 mg) with sampling at 2 h, dose 2 (75 mg) with sampling at 2 h, dose 1 with sampling at 8 h or dose 2 with sampling at 8 h
Baseline visit: maternal blood pressure, heart rate and oxygen saturation will be measured and Doppler examination of the fetal pulmonary circulation and umbilical artery will be performed
Day 0: at the time of pregnancy termination, the same procedures as in the baseline visit will be performed. At this time point, one maternal and one fetal blood sample will be collected for analysis of sildenafil concentration
Third trimester placental transfer and safety study (sub-study 2)
Participants will be randomly assigned to one of two study groups: dose 1 (25 mg three times daily) and dose 2 (75 mg three times daily)
Baseline visit: the study procedures will involve measurements of maternal blood pressure, heart rate and oxygen saturation; fetal ultrasound for assessment of fetal wellbeing and Doppler examination of the fetal main pulmonary artery and contralateral pulmonary artery branch, mean cerebral artery, umbilical artery, ductus venosus; Doppler examination of the uterine arteries; fetal heart rate monitoring (cardiotocography)
During sildenafil administration (from day 0 until occurrence of labor, specifically at 2 and 50 h after the first administration) the study procedures will be the same as in the baseline visit. Treatment compliance and any side effect will also be reported. One maternal blood sample will be collected on day 2 (50 h after the first administration) for determination of maternal plasma sildenafil concentration
During labor and at delivery fetal heart rate, maternal blood pressure, heart rate and oxygen saturation, and eventual side effects will be recorded as part of the clinical protocol. At the moment of delivery, one maternal and one umbilical cord blood sample will be collected for determination of sildenafil concentration
At the neonatal follow-up visit (day 1 after birth), mean arterial blood pressure, vasoactive inotropic score [33] and early (< 24 h) lung function, expressed as Oxygenation Index and alveolar-arterial oxygen gradient will be recorded. Adverse effects, if any, will be recorded
Sample size calculation
This is a phase II, dose-finding study and there is no information in the literature about transplacental sildenafil transfer in vivo. A formal power calculation has, therefore, not been performed [39, 40]. The number of cases is driven by the practices commonly used to document pharmacokinetics in a rather homogeneous population with simultaneous collection of maternal and fetal effects.
Second trimester placental transfer study (sub-study 1)
We assume that a sample size of six participants per dose for each time point (total = 24 women) is an ethical balance between the research question and the exposure of healthy volunteers to the drug. In addition we will perform an interim analysis after having obtained all data in 16 women (four per group) to potentially conclude the study prematurely in case of a low variability (< 20% variability in fetal sildenafil concentrations) within the groups. To ensure a balance in sample size across groups over time, a block size randomization of 8 with equal numbers for each group is chosen [41, 42].
We expect a 2% incidence of maternal conditions representing an exclusion criteria for the study. Furthermore, since this is a study on pregnant women without medical conditions, we expect a high rejection rate (~ 50%). Since this is a short-term study, we do not expect any drop-out after allocation. Therefore, the plan is to assess a total of 49 women for eligibility for recruitment of up to 24 women.
Third trimester placental transfer and safety study (sub-study 2)
To obtain reliable pharmacokinetic data, we plan to include six patients for each tested dose (total = 12 patients). For the reasons given above in sub-study 1, block randomization (block size = 4) will be used.
We expect a 50% incidence of maternal or fetal (i.e., not isolated cases, fetuses having had fetal therapy) conditions representing an exclusion criterion for the study and a 50% rejection rate. Speculating that the drop-out rate after inclusion will be less than 10%, we will need to assess for eligibility a total of 52 women with ongoing pregnancies and CDH at term.
The Data Safety and Monitoring Committee will evaluate drop-out rate ad interim, and is authorized to adjust sample size, if appropriate.
Statistical analysis plan
For all pharmacokinetic, demographic, safety and efficacy outputs, data will be summarized by sub-study and dose group. For continuous variables, summary statistics will include the number of observations, the arithmetic mean, median, arithmetic standard deviation, minimum and maximum. For categorical variables, frequency counts and percentages will be used.
The measured sildenafil concentrations will be used to develop a physiologically based pharmacokinetic (PBPK) model allowing prediction of the maternal dose that will result in therapeutic concentrations in the fetus [43]. The effect of covariates (gestational age, maternal height and weight) will also be assessed.
For efficacy and tolerability endpoints, baseline is defined as the last value for each assessment prior to the subject’s first dose. Each individual change from baseline will be calculated by subtracting the individual subject’s baseline value from the value at the time point. The individual subject’s change from baseline values will be used to calculate the mean change from baseline. Missing values will not be imputed.
Adverse events will be summarized for each of the sub-studies and of the dose groups. Summaries will include the number of patients who have at least one occurrence of the adverse event. In addition, the total number of adverse events with a first reporting of the event while on treatment will be displayed. The analysis will be for descriptive purpose only and no inferential testing was performed.
Discussion
The mortality from CDH-related PHT unresponsive to medical therapy remains around 60% [44], and infants with PHT require prolonged mechanical ventilation, need extracorporeal life support, or progress into right-sided heart failure [44, 45]. PHT also adversely affects the quality of life in CDH survivors [46]. CDH, certainly when complicated by PHT, also is a serious socio-economic burden. The postnatal management costs of a CDH patient is estimated to exceed US$200,000 [47], making it the costliest non-cardiac birth defect [48]. When the baby also has PHT, costs are significantly higher. For instance, when extra-corporeal membrane oxygenation is used (which is more likely in cases of PHT), in-hospital costs rise fourfold. To this, the burden of chronic PHT must be added [49]. Chronic PHT is associated with an increased risk of death, cardiac and pulmonary complications and re-hospitalization. It requires chronic medical treatment, an intense clinical follow-up often with additional testing and investigations.
Therefore, effective treatment or prevention of PHT is urgently needed. SToP-PH introduces the concept of a medical approach that acts already during fetal life hence preventing the ill-development of pulmonary vasculature. The herein described phase I studies are required to properly design a randomized controlled trial on maternal sildenafil administration for the prevention of PHT in CDH. If proven effective, oral administration of a safe drug will also allow for a wider implementation than fetal surgery, including in less privileged countries. Furthermore, sildenafil is generically available. Therefore, this would be the first affordable therapy for a fetal surgical condition ever.
The European Commission has supported the establishment of virtual thematic networks across Europe (European Reference Networks), aimed at sharing knowledge, exchanging information and facilitating collaborations between specialized centers. “ERNICA” is the European reference network on rare diseases of the foregut grouping skills from 20 centers in 11 countries. It covers malformations of the digestive system, the diaphragm (including CDH) and the abdominal wall. Such network will make a randomized clinical trial on CDH fetuses possible, despite the rarity of the condition. We also have already obtained Orphan Designation for this indication by the European Medical Agency (http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2017/07/WC500231579.pdf). The present phase I/IIb trial should allow to determine the effective maternal dosage to be used in the subsequent phase III efficacy study. Moreover, it will investigate and provide first data on maternal and fetal tolerance and on the effect of the drug on the fetal pulmonary circulation. This knowledge will provide safety information also for other maternal and placental diseases, for which sildenafil is currently being explored.
Trial status
Recruiting
Approved by the Belgian National Authorities: 20 July 2017.
Date of local Ethics Committee approval (Leuven): 22 September 2017 (protocol version 5, dated 28 July 2017).
Recruitment starting date: 1 February 2018.
Additional file
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Checklist: recommended items to address in a clinical trial protocol and related documents. (DOC 124 kb)
Acknowledgments
Funding
This clinical trial is funded by the Katholieke Universiteit of Leuven, C3 Internal Funding grant number C32/17/054, and by the “klinische onderzoeks-en opleidingsraad” (KOOR) funding of the University Hospitals of Leuven.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AT/ET
Acceleration time/ejection time ratio
- CDH
Congenital diaphragmatic hernia
- NO
Nitric oxide
- PHT
Pulmonary hypertension
- TO
Tracheal occlusion
Authors’ contributions
FMR participated in the design and coordination of the study and drafted the manuscript. AB, JDH, KVC, PA, KA, TVM and JMT contributed to the development of the study and reviewed the manuscript. JD conceived the study idea, participated in the design and coordination of the study and provided final review of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The protocol and investigational product information have been reviewed and approved by the Belgian Federal Agency for Medicines and Health products and by the Ethics Committee of the University Hospitals of Leuven (internal number S59204). The Ethics Committee has also approved patient information, consent form and other accompanying material that will be provided to participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francesca Maria Russo, Email: francescamaria.russo@kuleuven.be.
Alexandra Benachi, Email: alexandra.benachi@aphp.fr.
Tim Van Mieghem, Email: Tim.VanMieghem@sinaihealthsystem.ca.
Jan De Hoon, Email: jan.dehoon@uzleuven.be.
Kristel Van Calsteren, Email: kristel.vancalsteren@uzleuven.be.
Pieter Annaert, Email: pieter.annaert@kuleuven.be.
Jean-Marc Tréluyer, Email: jean-marc.treluyer@aphp.fr.
Karel Allegaert, Email: karel.allegaert@uzleuven.be.
Jan Deprest, Phone: +32 16 34 42 15, Email: jan.deprest@uzleuven.be.
References
- 1.Kinsella JP, Ivy DD, Abman SH. Pulmonary vasodilator therapy in congenital diaphragmatic hernia: acute, late, and chronic pulmonary hypertension. Semin Perinatol. 2005;29:123–128. doi: 10.1053/j.semperi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Grushka JR, Laberge JM, Puligandla P, Skarsgard ED, Canadian Pediatric Surgery N. Effect of hospital case volume on outcome in congenital diaphragmatic hernia: the experience of the Canadian Pediatric Surgery Network. J Pediatr Surg. 2009;44:873–876. doi: 10.1016/j.jpedsurg.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 3.van den Hout L, Schaible T, Cohen-Overbeek TE, Hop W, Siemer J, van de Ven K, Wessel L, Tibboel D, Reiss I. Actual outcome in infants with congenital diaphragmatic hernia: the role of a standardized postnatal treatment protocol. Fetal Diagn Ther. 2011;29:55–63. doi: 10.1159/000322694. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa M, Ito M, Hattori T, Kanamori Y, Okuyama H, Inamura N, Takahashi S, Nagata K, Taguchi T, Usui N, Japanese Congenital Diaphragmatic Hernia Study Group effect of hospital volume on the mortality of congenital diaphragmatic hernia in Japan. Pediatr Int. 2013;55:190–196. doi: 10.1111/ped.12059. [DOI] [PubMed] [Google Scholar]
- 5.Pierro M, Thebaud B. Understanding and treating pulmonary hypertension in congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19:357–363. doi: 10.1016/j.siny.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, Gorett Silva M, Greenough A, Tibboel D, Consortium CE. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010;98:354–364. doi: 10.1159/000320622. [DOI] [PubMed] [Google Scholar]
- 7.Claus F, Sandaite I, DeKoninck P, Moreno O, Cruz Martinez R, Van Mieghem T, Gucciardo L, Richter J, Michielsen K, Decraene J, et al. Prenatal anatomical imaging in fetuses with congenital diaphragmatic hernia. Fetal Diagn Ther. 2011;29:88–100. doi: 10.1159/000320605. [DOI] [PubMed] [Google Scholar]
- 8.Mayer S, Klaritsch P, Petersen S, Done E, Sandaite I, Till H, Claus F, Deprest JA. The correlation between lung volume and liver herniation measurements by fetal MRI in isolated congenital diaphragmatic hernia: a systematic review and meta-analysis of observational studies. Prenat Diagn. 2011;31:1086–1096. doi: 10.1002/pd.2839. [DOI] [PubMed] [Google Scholar]
- 9.Cannie M, Jani J, Chaffiotte C, Vaast P, Deruelle P, Houfflin-Debarge V, Dymarkowski S, Deprest J. Quantification of intrathoracic liver herniation by magnetic resonance imaging and prediction of postnatal survival in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2008;32:627–632. doi: 10.1002/uog.6146. [DOI] [PubMed] [Google Scholar]
- 10.Jani J, Cannie M, Sonigo P, Robert Y, Moreno O, Benachi A, Vaast P, Gratacos E, Nicolaides KH, Deprest J. Value of prenatal magnetic resonance imaging in the prediction of postnatal outcome in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol. 2008;32:793–799. doi: 10.1002/uog.6234. [DOI] [PubMed] [Google Scholar]
- 11.Jani J, Nicolaides KH, Keller RL, Benachi A, Peralta CF, Favre R, Moreno O, Tibboel D, Lipitz S, Eggink A, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol. 2007;30:67–71. doi: 10.1002/uog.4052. [DOI] [PubMed] [Google Scholar]
- 12.DiFiore JW, Fauza DO, Slavin R, Peters CA, Fackler JC, Wilson JM. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:248–256. doi: 10.1016/0022-3468(94)90328-X. [DOI] [PubMed] [Google Scholar]
- 13.Dekoninck P, Gratacos E, Van Mieghem T, Richter J, Lewi P, Ancel AM, Allegaert K, Nicolaides K, Deprest J. Results of fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia and the set up of the randomized controlled TOTAL trial. Early Hum Dev. 2011;87:619–624. doi: 10.1016/j.earlhumdev.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Doné E., Gratacos E., Nicolaides K. H., Allegaert K., Valencia C., Castañon M., Martinez J.-M., Jani J., Van Mieghem T., Greenough A., Gomez O., Lewi P., Deprest J. Predictors of neonatal morbidity in fetuses with severe isolated congenital diaphragmatic hernia undergoing fetoscopic tracheal occlusion. Ultrasound in Obstetrics & Gynecology. 2013;42(1):77–83. doi: 10.1002/uog.12445. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez JA, Eixarch E, DeKoninck P, Bennini JR, Devlieger R, Peralta CF, Gratacos E, Deprest J. Balloon removal after fetoscopic endoluminal tracheal occlusion for congenital diaphragmatic hernia. Am J Obstet Gynecol. 2017;217:78.e1–78.e11. doi: 10.1016/j.ajog.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 16.Wharton J, Strange JW, Moller GM, Growcott EJ, Ren X, Franklyn AP, Phillips SC, Wilkins MR. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005;172:105–113. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- 17.Perez Krystle M., Laughon Matthew. Sildenafil in Term and Premature Infants: A Systematic Review. Clinical Therapeutics. 2015;37(11):2598-2607.e1. doi: 10.1016/j.clinthera.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 19.Enomoto M, Jain A, Pan J, Shifrin Y, Van Vliet T, McNamara PJ, Jankov RP, Belik J. Newborn rat response to single vs. combined cGMP-dependent pulmonary vasodilators. Am J Physiol Lung Cell Mol Physiol. 2014;306:L207–L215. doi: 10.1152/ajplung.00164.2013. [DOI] [PubMed] [Google Scholar]
- 20.Barst RJ, Ivy DD, Gaitan G, Szatmari A, Rudzinski A, Garcia AE, Sastry BK, Pulido T, Layton GR, Serdarevic-Pehar M, Wessel DL. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez Luna M, Franco ML, Bernardo B. Therapeutic strategies in pulmonary hypertension of the newborn: where are we now? Curr Med Chem. 2012;19:4640–4653. doi: 10.2174/092986712803306475. [DOI] [PubMed] [Google Scholar]
- 22.Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, Storme L, Deprest J, Schaible T, van Heijst A, Tibboel D. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus—2015 update. Neonatology. 2016;110:66–74. doi: 10.1159/000444210. [DOI] [PubMed] [Google Scholar]
- 23.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, Baker PN. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28:369–382. doi: 10.3109/10641950802601278. [DOI] [PubMed] [Google Scholar]
- 24.von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, Lim KI, Liston RM, Miller SP, Rurak D, et al. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG. 2011;118:624–628. doi: 10.1111/j.1471-0528.2010.02879.x. [DOI] [PubMed] [Google Scholar]
- 25.Dunn Liam, Greer Ristan, Flenady Vicki, Kumar Sailesh. Sildenafil in Pregnancy: A Systematic Review of Maternal Tolerance and Obstetric and Perinatal Outcomes. Fetal Diagnosis and Therapy. 2016;41(2):81–88. doi: 10.1159/000453062. [DOI] [PubMed] [Google Scholar]
- 26.Luong C, Rey-Perra J, Vadivel A, Gilmour G, Sauve Y, Koonen D, Walker D, Todd KG, Gressens P, Kassiri Z, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123:2120–2131. doi: 10.1161/CIRCULATIONAHA.108.845909. [DOI] [PubMed] [Google Scholar]
- 27.Russo Francesca M, Toelen Jaan, Eastwood M Patrice, Jimenez Julio, Miyague Andre Hadyme, Vande Velde Greetje, DeKoninck Philip, Himmelreich Uwe, Vergani Patrizia, Allegaert Karel, Deprest Jan. Transplacental sildenafil rescues lung abnormalities in the rabbit model of diaphragmatic hernia. Thorax. 2016;71(6):517–525. doi: 10.1136/thoraxjnl-2015-207949. [DOI] [PubMed] [Google Scholar]
- 28.Molelekwa V, Akhter P, McKenna P, Bowen M, Walsh K. Eisenmenger’s syndrome in a 27 week pregnancy—management with bosentan and sildenafil. Ir Med J. 2005;98:87–88. [PubMed] [Google Scholar]
- 29.Lacassie HJ, Germain AM, Valdes G, Fernandez MS, Allamand F, Lopez H. Management of Eisenmenger syndrome in pregnancy with sildenafil and L-arginine. Obstet Gynecol. 2004;103:1118–1120. doi: 10.1097/01.AOG.0000125148.82698.65. [DOI] [PubMed] [Google Scholar]
- 30.Russo FM, Conings S, Allegaert K, van Mieghem T, Toelen J, van Calsteren K, Annaert P, Deprest J. Sildenafil crosses the placenta at therapeutic levels in a dually perfused human cotyledon model. Am J Obstet Gynecol; 2018. [Epub ahead of print] [DOI] [PubMed]
- 31.Lopez-Cepero R, Lynch L, de la Vega A. Effectiveness and safety of lidocaine in the induction of fetal cardiac asystole for second trimester pregnancy termination. Bol Asoc Med P R. 2013;105:14–17. [PubMed] [Google Scholar]
- 32.Pasquini L, Pontello V, Kumar S. Intracardiac injection of potassium chloride as method for feticide: experience from a single UK tertiary centre. Bjog. 2008;115:528–531. doi: 10.1111/j.1471-0528.2007.01639.x. [DOI] [PubMed] [Google Scholar]
- 33.Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, Gall C, Rice TB, Thiagarajan RR. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med. 2014;15:529–537. doi: 10.1097/PCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker DK, Ackland MJ, James GC, Muirhead GJ, Rance DJ, Wastall P, Wright PA. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 35.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 36.Ganzevoort W, Alfirevic Z, von Dadelszen P, Kenny L, Papageorghiou A, van Wassenaer-Leemhuis A, Gluud C, Mol BW, Baker PN. STRIDER: sildenafil therapy in dismal prognosis early-onset intrauterine growth restriction—a protocol for a systematic review with individual participant data and aggregate data meta-analysis and trial sequential analysis. Syst Rev. 2014;3:23. doi: 10.1186/2046-4053-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldenberg MM. Safety and efficacy of sildenafil citrate in the treatment of male erectile dysfunction. Clin Ther. 1998;20:1033–1048. doi: 10.1016/S0149-2918(98)80103-3. [DOI] [PubMed] [Google Scholar]
- 38.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, Hrobjartsson A, Mann H, Dickersin K, Berlin JA, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shannon C, Jauniaux E, Gulbis B, Thiry P, Sitham M, Bromley L. Placental transfer of fentanyl in early human pregnancy. Hum Reprod. 1998;13:2317–2320. doi: 10.1093/humrep/13.8.2317. [DOI] [PubMed] [Google Scholar]
- 40.Jauniaux E, Jurkovic D, Lees C, Campbell S, Gulbis B. In-vivo study of diazepam transfer across the first trimester human placenta. Hum Reprod. 1996;11:889–892. doi: 10.1093/oxfordjournals.humrep.a019272. [DOI] [PubMed] [Google Scholar]
- 41.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43:215–221. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4:8–11. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Dallmann A, Pfister M, van den Anker J, Eissing T. Physiologically based pharmacokinetic modeling in pregnancy: a systematic review of published models. Clin Pharmacol Ther. 2018. [Epub ahead of print] [DOI] [PubMed]
- 44.Lusk LA, Wai KC, Moon-Grady AJ, Steurer MA, Keller RL. Persistence of pulmonary hypertension by echocardiography predicts short-term outcomes in congenital diaphragmatic hernia. J Pediatr. 2015;166:251–256. doi: 10.1016/j.jpeds.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:307–312. doi: 10.1016/j.jpedsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Behrsin J, Cheung M, Patel N. Sildenafil weaning after discharge in infants with congenital diaphragmatic hernia. Pediatr Cardiol. 2013;34:1844–1847. doi: 10.1007/s00246-013-0725-1. [DOI] [PubMed] [Google Scholar]
- 47.Raval MV, Wang X, Reynolds M, Fischer AC. Costs of congenital diaphragmatic hernia repair in the United States-extracorporeal membrane oxygenation foots the bill. J Pediatr Surg. 2011;46:617–624. doi: 10.1016/j.jpedsurg.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 48.Metkus AP, Esserman L, Sola A, Harrison MR, Adzick NS. Cost per anomaly: what does a diaphragmatic hernia cost? J Pediatr Surg. 1995;30:226–230. doi: 10.1016/0022-3468(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 49.Sikirica M, Iorga SR, Bancroft T, Potash J. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res. 2014;14:676. doi: 10.1186/s12913-014-0676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Checklist: recommended items to address in a clinical trial protocol and related documents. (DOC 124 kb)
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.