Abstract
Background
Antiretroviral therapy (ART) use in pregnancy presents unquestionable benefits in preventing mother-to-child transmission (MTCT) of HIV although it is associated with maternal adverse effects. The aim of this study was to evaluate the adverse effects of antiretroviral therapy in pregnant women infected with HIV.
Methods
Cohort study of pregnant women infected with HIV followed at the CAISM/UNICAMP Obstetric Clinic from 2000 to 2015. The following maternal adverse effects were observed: anemia, thrombocytopenia, allergy, liver function test abnormalities, dyslipidemia and diabetes. Data collected from patients’ files was added to a specific database. Descriptive analysis was shown in terms of absolute (n) and relative (%) frequencies and mean, median and standard deviation calculations. Chi-square or Fisher exact test (n < 5) and relative risk (RR) with its respective p values were used for categorical variables and Student t-test (parametric data) or Mann-Whitney (non-parametric data) for the quantitative ones. A 95% confidence interval (CI) and a significant level of 0.05 were used. A multivariate Cox Logistic Regression was also done. Data analysis was conducted using SAS version 9.4.
Results
Data from 793 pregnancies were included. MTCT rate was 2.3%, with 0.8% in the last 5 years. Maternal adverse effects were: dyslipidemia (82%), anemia (56%), liver function test abnormalities (54.5%), including hyperbilirubinemia (11.6%), fasting glycemia alteration (19.2%), thrombocytopenia (14.1%), and allergic reaction (2.7%). The majority of adverse effects deemed related to ART in this study were mild according to DAIDS scale. In the multivariate analysis, co-infections and starting ART during pregnancy were risk factors for maternal anemia, while CD4 count higher than 200 cells/mm3 was protective. Nevirapine, nelfinavir and atazanavir regimens increased the risk for liver function tests abnormalities. Lopinavir use during pregnancy increased the risk for fasting glycemia alteration.
Conclusion
The evolution of the national guidelines of antiretroviral therapy for pregnant women improved adherence to the treatment and resulted in a significant reduction of MTCT. Despite the high frequency of maternal adverse effects, they are mostly of low severity. Newer ART medications with improved efficacy and significantly more favorable tolerability profiles should reduce the incidence of ART-related adverse effects.
Keywords: HIV, Toxicity, Adverse effects, Antiretroviral therapy, Pregnancy
Background
The significant progress in antiretroviral treatment caused a striking decline in morbidity and mortality associated with human immunodeficiency virus (HIV) infection and a dramatic reduction in mother-to-child transmission (MTCT) [1–3]. Antiretroviral therapy for pregnant women is proven to be the most efficient intervention by reducing the rate of MTCT to lower than 2% and decreasing maternal and child mortality [4, 5].
The World Health Organization’s (WHO) Option B+ recommends the use of antiretroviral combined therapy (ART) to all pregnant and lactating women infected with HIV, independently from the CD4 count or the disease status. It also recommends maintaining its use after birth to control maternal disease and to prevent MTCT and sexual transmission of HIV [6]. A global treatment trend followed this recommendation not only in the developed countries but also in the ones with restricted economic resources. The Brazilian protocol follows the same guidelines to prevent MTCT of HIV [7].
Nevertheless, the increased use of potent and complex antiretroviral regimens during pregnancy can cause adverse effects in pregnant women and their newborns [8, 9]. The most frequent maternal adverse effects are hematologic, hepatic and dermatologic alterations, metabolic disturbances, pre-eclampsia, and viral resistance [10–14].
The Women’s Hospital at University of Campinas School of Medical Sciences (CAISM/UNICAMP) has been running a program for pregnant women infected with HIV since 1988. The objective of this study was to evaluate the ART maternal adverse effects in a large cohort of pregnant women infected with HIV followed at the Obstetric Clinic in this Brazilian public university hospital between 2000 and 2015.
Subjects and methods
Observational analytic study based on the evaluation of a historic cohort in a population of pregnant women infected with HIV between 2000 and 2015 followed at the Obstetric Clinic at the University of Campinas School of Medical Sciences (CAISM/UNICAMP). CAISM is the reference hospital for high risk pregnancy in Campinas, the second biggest city in the State of São Paulo with 1.2 inhabitants and an Human Development Index (HDI) of 0.8. Mostly, CAISM serves pregnant women without health insurance and from low socioeconomic status. Follow-up of pregnant women infected with HIV at CAISM started in 1988. ART drugs started in 1994. The ART drug schemes varied in different periods: from 1994 to 1999 monotherapy with AZT; from 1999 to 2000 double AZT scheme with 3TC; from 2000 on, an association of AZT/3TC with a NNRTI (nevirapine) or a PI (nelfinavir). In 2006, nelfinavir was substituted for lopinavir/ritonavir (LPV/r). The AZT/3TC/LPV/r scheme used as a first line treatment for infected pregnant women since 2006 was substituted for TDF/3TC/EFV in 2015. By the end of 2017 efavirenz (EFV) was substituted for raltegravir (RAL) according to the new Brazilian guidelines for treatment. AZT was the drug of choice to compose the ART scheme during pregnancy until 2015 although tenofovir had been used as a substitute for AZT mainly in patients with anemia since 2010.
The women were selected from the clinical records in the medical files and from the epidemiologic Health Surveillance Agency data available in the clinic. A specific form was developed to collect all the information. The following variables were analyzed: pregnant women’s epidemiologic and clinical characteristics, antiretroviral treatment administrated, delivery characteristics, MTCT of HIV, adverse hematologic and hepatic effects, and glucose and lipids metabolic disorders. The time of pregnancy when antiretroviral treatment was introduced was described in terms of gestational weeks; women receiving ART in their last menstrual period were considered in treatment at conception. Peripartum viral load was measured at least 6 weeks prior to delivery. Substance use (cocaine/crack), alcohol use or smoking practice were considered previous or current in pregnancy. For the global analysis, the combination of antiretroviral drugs was classified as: monotherapy with nucleoside reverse transcriptase inhibitor (NRTI) zidovudine (AZT); double therapy with NRTI (AZT and lamivudine-3TC) or combined therapy (ART) defined as a combination of at least three drugs with no less than one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitor (NNRTI). Women using the PI darunavir (DRV), lopinavir (LPV) and atazanavir (ATV) also received booster of ritonavir (r). To evaluate the adverse effects, the main assessed outcomes were: laboratory abnormalities: anemia (hemoglobin < 11 g/dl), low platelets count (platelets < 150,000/ml), liver function test abnormalities (at least one: alanine aminotransferase-ALT > 34 U/L, aspartate aminotransferase-AST > 45 U/l, alkaline phosphatase-ALCPH > 104 U/l, gamma-glutamyl transferase-GGT > 42 U/l, bilirubin > 1,0 mg/dl); diabetes (glycemic curve with at least one abnormal value: fasting ≥92 mg/dl, after the first hour ≥180 mg/dl, after the second hour ≥152 mg/dl) and dyslipidemia (at least one abnormality: total cholesterol ≥200 mg/dl, triglyceride 150 ≥ mg/dl). Dyslipidemia criteria were used for adult population, not specified for pregnant women. Hemoglobin (Hb) level less than 11 g/dL was defined according to the Brazilian Health Ministry guidelines: mild (Hb 10–10,9 g/dL), moderate (Hb 8–9,9 g/dL) and severe (Hb ≤ 8 g/dL) [15].
The DAIDS Adverse Effect Scale (DAIDS AE) was used to quantify the severity of adverse effects. This grading scale provides an AE severity grading scale ranging from grades 1 to 5 with descriptions for each AE based on the following general guidelines: Grade 1 indicates a mild event, Grade 2 indicates a moderate event, Grade 3 indicates a severe event, Grade 4 indicates a potentially life-threatening event, Grade 5 indicates death [16].
All newborns exposed to HIV infection were followed by the Pediatric Immunodeficiency Service at the same University Hospital. The cases with no final HIV infection diagnosis due to loss of follow-up were contacted by phone or telegram. This study was approved by the Institution’s Ethics in Research Committee (Protocol #351/2006).
Descriptive analysis was shown in terms of absolute (n) and relative (%) frequencies, mean, median and standard deviation. Chi-square or Fisher exact test (n < 5) was used to analyze the association between the categorical variables. For the continuous variables, the Student t-test (parametric data) or Mann-Whitney (non-parametric data) were used. The specific effects of the different antiretroviral regimens were analyzed using relative risk (RR) with its respective p values. A multivariate Cox Logistic Regression was done. A 95% confidence interval (CI) and a significant level of 0.05 were used. Statistical analysis was performed using SAS version 9.4.
Results
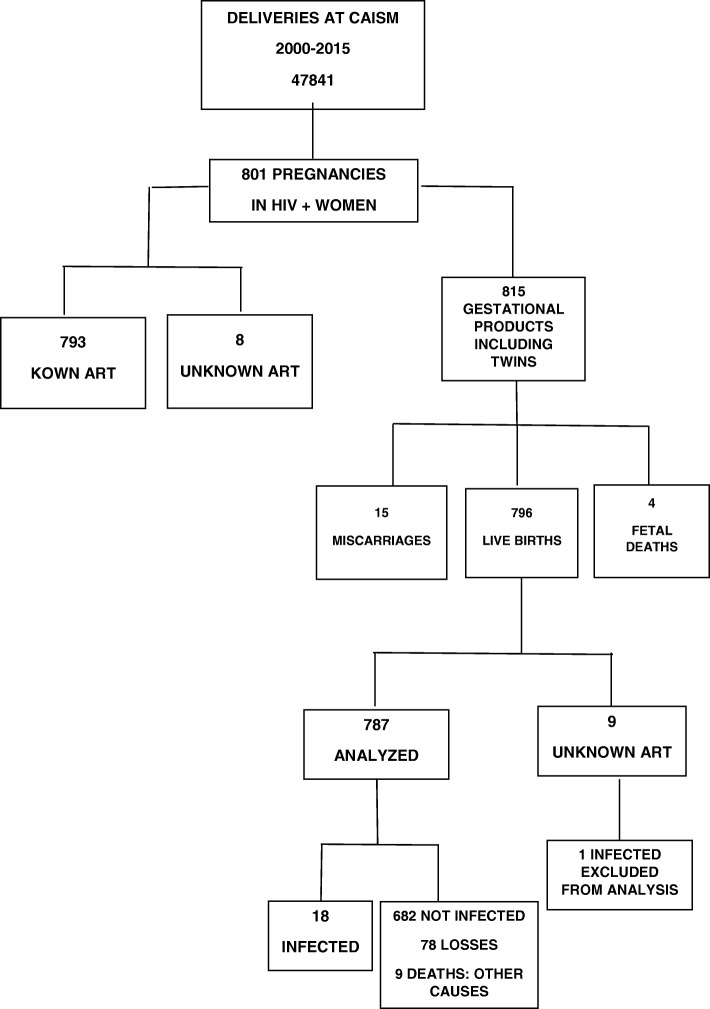
Between 2000 and 2015, there were 47,841 births at the university hospital. From these, 801 were pregnant women infected with HIV, with a 1.67% prevalence rate. Data referring to 14 multiple births were not replicated for the maternal analysis. Figure 1 shows all the eligible cases, the follow-up losses and the final number of 793 pregnancies and 787 children. The MTCT rate in this cohort was 2.3%. In the last 5 years of this study, of the 350 infected pregnant women, there were only three cases of HIV transmission, corresponding to a MTCT rate of 0.8%. For the few women who did deliver HIV positive infants three were in use of monotherapy with AZT, one was not taking any ART, one was in use of an unknown ART. The other ones were receiving three-drugs regimens.
Fig. 1.
Flowchart of the study including pregnant women and newborns between 2000 and 2015. ART antiretroviral therapy
Maternal adverse effects were: dyslipidemia (82%), anemia (56%), liver function test abnormalities (54.5%), including hyperbilirubinemia (11.6%), fasting glycemia alteration (19.2%), thrombocytopenia (14.1%), and allergic skin reaction (2.7%).
Pregnant women, prenatal care and delivery characteristics
The mean age of the pregnant women was 28 years; 61.2% were white, and 60.9% completed elementary school. Pregnancy was planned in 25.7% of the cases, but only 27.6% were in use of antiretroviral therapy at conception. The exposure category of HIV was heterosexual in 93% of the women and 10 of them (2.5%) had acquired the infection through MTCT. Regarding the HIV diagnosis, 62% were aware of their serologic status and 40.7% had already used some antiretroviral regimen previously to this pregnancy. The most common ART regimens used during pregnancy are described in Table 1. Only 15 (1.9%) women did not use antiretroviral therapy while pregnant because the HIV diagnosis was made at admission for delivery. Eighty-one women (10.2%) got pregnant using Efavirenz (Table 1). The 81 patients in use of efavirenz in the first trimester had the drug changed to PI during prenatal care, except for one which kept it through the gestational period. Four pregnant women were using EFV at the end of the gestation, including three which started EFV in the second trimester. Five women used a combination of two NRTI with NVP and PI simultaneously (3 with LPV/r, 1 with ATV/r and 1 with NFV, included in their respective groups). AZT was changed to tenofovir (TDF) in 41 cases, to stavudine (d4T) in seven cases and to abacavir (ABC) in one case. The AZT combination with TDF cases (17 patients) were excluded from the specific analysis. The integrase strand inhibitor raltegravir (RAL) was added to the ART regimen in seven cases (four cases associated with LPV/r and three cases with DRV/r), mostly in the late gestational weeks with the goal of reaching undetectable viral load at delivery (Table 1).
Table 1.
Baseline characteristics of the study population at CAISM/UNICAMP from 2000 to 2015
| Characteristics | Median (%) |
|---|---|
| Age (years) | 28 (13–46) |
| Parity | 1 (0–9) |
| Schooling years (n = 769) | |
| < 8 | 60.9 |
| 8–11 yrs. | 32.2 |
| > 12 | 5.1 |
| No schooling | 1.8 |
| Ethnicity: White | 61.2 |
| HIV diagnosis (n = 789) | |
| Prior to pregnancy | 62.5 |
| During pregnancy | 37.5 |
| In use of ART at conception (n = 787) | 27.6 |
| In use of Efavirenz at conception (n = 793) | 10.2 |
| Planned pregnancy (n = 382) | 25.7 |
| Heterosexual transmission (n = 408) | 93.1 |
| Baseline CD4 median (cells/mm3) | 444 (3–1915) |
| Peripartum CD4 median (cells/mm3) | 552 (5–2164) |
| Median time of ART use (days) | 152.5 (4–292) |
| Median of detectable peripartum viral load (copies/ml) | 815 (43–500.000) |
| Peripartum Viral Load < 50 (copies/ml) (n = 732) | 58.9 |
| CDC classification (n = 758) | |
| 1 | 29.9 |
| 2 | 51.6 |
| 3 | 18.5 |
| Substance abuse (n = 568) | 14.3 |
| Alcoholism (n = 528) | 5.7 |
| Smoking (n = 538) | 14.1 |
| ART adherence during prenatal care | 84.3 |
| Changed ART (n = 776) | 19.2 |
| Intravenous AZT (n = 775) | 94.8 |
| Antiretroviral regimens during pregnancy (n = 793) | |
| None | 1.9 |
| AZT monotherapy | 2.9 |
| Dual therapy | 1.4 |
| 2 NRTI + 1 NNRTI | 17.9 |
| AZT + 3TC + NVP | 16.8 |
| TDF + 3TC + NVP | 0.1 |
| d4T + 3TC + NVP | 0.5 |
| AZT + 3TC + EFV | 0.3 |
| TDF + 3TC + EFV | 0.3 |
| 2 NRTI + 1 PI | 74.9 |
| AZT + 3TC + LPV/r | 48.5 |
| TDF + 3TC + LPV/r | 3.4 |
| d4T + 3TC + LPV/r | 0.1 |
| TDF + AZT + 3TC + LPV/r | 1.5 |
| AZT + 3TC + ATV/r | 1.8 |
| TDF + 3TC + ATV/r | 1.0 |
| ABC + 3TC + ATV/r | 0.1 |
| TDF + AZT + 3TC + ATV/r | 0.3 |
| AZT + 3TC + NFV | 16.8 |
| d4T + 3TC + NFV | 0.1 |
| 2 NRTI + Other PI* | 1.3 |
| 2 NRTI + PI** + RAL | 0.9 |
| 2 NRTI + NNRTI + PI | 0.6 |
| Total | 100 |
ART antiretroviral therapy, NRTI nucleos(t)ide reverse transcriptase inhibitors, AZT zidovudine, 3TC lamivudine, TDF tenofovir, d4T stavudine, ABC abacavir, NNRTI non-nucleoside reverse transcriptase inhibitor NVP nevirapine, EFV efavirenz, PI protease inhibitor, NFV nelfinavir, LPV lopinavir, r ritonavir, ATV atazanavir, IDV indinavir, FPV fosamprenavir, SQV saquinavir, RAL raltegravir, CDC Centers for Disease Control and Prevention
Other PI* IDV/r (6), FPV/r (2), SQV (2)
PI** DRV/r (3), LPV/r (4) included in LPV/r group
The median duration of antiretroviral therapy during pregnancy was 152.5 days (134.5 days for monotherapy with AZT, 231 days for NNRTI and 151 days for PI). The median baseline CD4 count was 444 cells/mm3 (varying from 3 to 1915) and the median perinatal CD4 count was 552 cells/ mm3. The median baseline viral load count was 1371 copies/ml. After ART 432 (58.9%) women reached undetectable viral load in the peripartum period (50.6% in the 2000–2009 period and 68.3% in the last 5 years of the study). Women with detectable peripartum viral load presented median of 815 copies/ml (Table 1).
The median number of prenatal appointments was 8 and the median gestational age at the beginning of prenatal care was 16 weeks. Two hundred and thirty pregnant women presented at least one obstetrical complication, mostly preterm labor, hypertension and intrauterine grow restriction. At least 667 women presented at least one infection during pregnancy: urinary infection, bacterial vaginosis, Streptococcus B colonization, hepatitis C, latent tuberculosis and syphilis (Table 2). Only four patients presented multiresistant virus in genotyping test (3.3%).
Table 2.
Pregnancy outcomes at CAISM/UNICAMP from 2000 to 2015
| Characteristics | Median (%) |
|---|---|
| Prenatal appointments | 8 (0–18) |
| Gestational age at first appointment | 16 (3–38) |
| Gestational age at birth | 38 (22–42) |
| Obstetrical complications (n = 793) | 29 |
| Preterm labor | 14.1 |
| Hypertension | 6.1 |
| Intrauterine grow restriction | 4.4 |
| Pre-eclampsia | 1.3 |
| Co-infections during pregnancy (n = 787) | 84.8 |
| Urinary infection | 34.7 |
| Bacterial vaginosis | 33.7 |
| Streptococcus B colonization | 33.4 |
| Hepatitis C | 7.7 |
| Latent tuberculosis | 5.5 |
| Syphilis | 5.2 |
| Toxoplasmosis | 2.3 |
| Genital herpes simplex | 2.1 |
| Opportunistic disease during pregnancy (n = 785) | 6.1 |
| Active tuberculosis | 1.7 |
| Oral/esophageal candidiasis | 1.7 |
| Cerebral toxoplasmosis | 0.9 |
| Cytomegalovirosis | 0.6 |
| Pneumocystis jirovecii | 0.4 |
| Labor (n = 447) | 42.1 |
| Premature rupture of membranes (n = 773) | 15.7 |
| Preterm birth | 21.7 |
| Low birth weight | 22.5 |
| Total | 100 |
Regarding to HIV infection during pregnancy, 51.3% of pregnant women were classified into the CDC stage 2 and 18.5% were classified as having Acquired Immunodeficiency Syndrome - AIDS (stage 3). Only 48 women (6.1%) presented opportunistic infections during pregnancy, mainly active tuberculosis and oral/esophageal candidiasis (Table 2).
Intravenous AZT was used in 94.8% of the cases. Regarding mode of delivery, in 92.8% of cases it was by cesarean, mostly due to the HIV infection, according to the hospital guidelines between 2005 to 2015. There were only four episiotomies in the vaginal births. Labor occurred in 42.1% of women, and 15.7% presented premature rupture of the membrane. The mean gestational age at birth was 38 weeks. Preterm birth occurred in 21.7% and 22.5% of the newborns presented low birth weight (Table 2).
All newborns received oral AZT. Twenty-four (3.1%) children received nevirapine associated with AZT in the first week of life to reinforce the neonatal prophylaxis. All women received cabergoline for lactation inhibition, but two children were breastfed due to maternal wish.
Association between ART regimens and obstetrical characteristics
Women exposed to NVP presented lower infectious (p = 0.010, RR 0.60 CI 0.44–0.81) and obstetrical complications (p = 0.001, RR 0.6 CI 0.44–0.81), but higher risk of detectable viral load peripartum (p = 0.001, RR 1.70 CI 1.23–2.35), when compared to the ones using PI. In the same way, women exposed to NVP presented higher MTCT rate (RR 2.53 CI 1.37–4.65) compared to the ones exposed to PI. Regarding to protease inhibitors, women exposed to NFV presented lower risk for obstetrical complications (p < 0.0001, RR 0.42 CI 0.30–0.57), but higher peripartum detectable viral load (p = 0.014, RR 1.43 CI 1.06–1.95) compared to the ones using LPV/r. When evaluating the nucleosides (AZT) and nucleotides (TDF) analogues, women exposed to TDF presented increased risk for obstetrical complications (p = 0.033, RR 1.98 CI 1.04–3.77), when compared to the women exposed to AZT (data not shown).
Maternal adverse effects
During pregnancy, 56% of the women presented anemia (27.5% in the first trimester and 40.8% in the third one), 54.5% showed increased hepatic enzymes, 14.1% had low platelet count (9.5% and 6.3% in the first and third trimester, respectively), 2.7% had allergic reaction, and 11.6% presented increased bilirubin in the third trimester. Dyslipidemia was observed in 82% of cases; of those, 67.9% had increased total cholesterol and 84.4% had elevated triglycerides. Increased fasting glycemia occurred in 19.2% of the women.
The majority of pregnant women developing an adverse effect deemed related to ART in this study had mild anemia (as classified by the DAIDS toxicity and Brazilian Ministry of Health guidelines [15, 16]), with most of cases of grade 2 anemia occurring in the 3rd trimester. Most of pregnant women presented mild hepatic alteration in both first and third trimesters. On the other hand, grades 2 and 3 dyslipidemia occurred more frequently in the third trimester. There was no difference in occurrence of adverse effects between two periods of study (2000–2009 and 2010–2015) (Table 3).
Table 3.
Maternal ART-related adverse effects categorized using the DAIDS Toxicity Grading Scale according to periods of delivery
| 2000–2009 (n = 452) | 2010–2015 (n = 347) | p* | |
|---|---|---|---|
| % | % | ||
| Hemoglobin First Trimester | |||
| Grade 1 | 92.5 | 94.6 | 0.516 |
| Grade 2 | 6.4 | 4.8 | |
| Grade 3 | 1.1 | 0.6 | |
| Hemoglobin Third Trimester | |||
| Grade 1 | 83.6 | 84.9 | 0.916 |
| Grade 2 | 15.5 | 14.6 | |
| Grade 3 | 0.9 | 0.5 | |
| AST First Trimester | |||
| Grade 1 | 98.4 | 98.9 | |
| Grade 2 | 0.7 | 0.7 | 1.000 |
| Grade 3 | 0.7 | 0.4 | |
| Grade 4 | 0.3 | 0.0 | |
| AST Third Trimester | |||
| Grade 1 | 99.4 | 100.0 | |
| Grade 2 | 0.6 | 0.0 | 1.000 |
| Grade 3 | 0.0 | 0.0 | |
| Grade 4 | 0.0 | 0.0 | |
| ALT First Trimester | |||
| Grade 1 | 98.7 | 98.9 | |
| Grade 2 | 0.7 | 0.7 | 1.000 |
| Grade 3 | 0.3 | 0.4 | |
| Grade 4 | 0.3 | 0.0 | |
| ALT Third Trimester | |||
| Grade 1 | 99.4 | 99.4 | |
| Grade 2 | 0.6 | 0.6 | 1.000 |
| Grade 3 | 0.0 | 0.0 | |
| Grade 4 | 0.0 | 0.0 | |
| Cholesterol First Trimester | |||
| Grade 1 | 91.1 | 90.6 | 0.631 |
| Grade 2 | 6.9 | 8.4 | |
| Grade 3 | 2.0 | 1.0 | |
| Cholesterol Third Trimester | |||
| Grade 1 | 69.7 | 64.9 | 0.792 |
| Grade 2 | 21.2 | 24.7 | |
| Grade 3 | 9.1 | 10.3 | |
| Triglyceride First Trimester | |||
| Grade 1 | 95.5 | 94.2 | 0.820 |
| Grade 2 | 4.5 | 5.3 | |
| Grade 3 | 0.0 | 0.5 | |
| Grade 4 | 0.0 | 0.0 | |
| Triglyceride Third Trimester | |||
| Grade 1 | 83.3 | 77.1 | 0.514 |
| Grade 2 | 15.6 | 20.8 | |
| Grade 3 | 1.0 | 2.1 | |
| Grade 4 | 0.0 | 0.0 | |
| Platelets First Trimester | |||
| Grade 1 | 98.4 | 97.2 | 0.496 |
| Grade 2 | 1.1 | 2.2 | |
| Grade 3 | 0.0 | 0.3 | |
| Grade 4 | 0.5 | 0.3 | |
| Platelets Third Trimester | |||
| Grade 1 | 99.5 | 98.8 | 0.480 |
| Grade 2 | 0.5 | 0.5 | |
| Grade 3 | 0.0 | 1.0 | |
| Grade 4 | 0.0 | 0.0 | |
| Bilirrubin First Trimester | |||
| Grade 1 | 98.6 | 95.2 | 0.264 |
| Grade 2 | 1.4 | 3.0 | |
| Grade 3 | 0.0 | 1.8 | |
| Grade 4 | 0.0 | 0.0 | |
| Bilirrubin Third Trimester | |||
| Grade 1 | 97.6 | 93.0 | 0.200 |
| Grade 2 | 1.2 | 6.1 | |
| Grade 3 | 1.2 | 0.9 | |
| Grade 4 | 0.0 | 0.0 | |
| Total | 100 | 100 | |
ALT alanine aminotransferase, AST aspartate aminotransferase
During pregnancy, 19.2% of ART were changed as shown on Table 1. AZT was changed to tenofovir (TDF) in 41 cases, to stavudine in seven cases and to abacavir in one case, because of anemia. NVP was changed in 11 cases (9 for ART medication-related drug hypersensitivity/allergic reaction, with three of them Stevens-Johnsons Syndrome). The adverse effects were mild or moderate in most of cases. None was associated with maternal mortality during pregnancy or after birth and subsided after change. EFV was changed in 80 cases when the risk for fetal malformation associated with it was considered high. Later, it was proved safe. All cases of stavudine and/or didanosine use were substituted preventively, due to their high risk of severe adverse effects during pregnancy. After delivery, all women were referred to the infectious disease clinic for follow-up. Allergic reaction was associated with ART in 22 cases, 18 of which associated with nevirapine use. Most of the allergy cases were mild or moderate (grades 1 or 2) skin/allergic hypersensitivity reactions. In 9 cases, NVP was substituted for protease inhibitor (including three cases of grade 4 Stevens-Johnsons Syndrome according to DAIDS Scale) and the symptoms subsided.
Co-infection with hepatitis C virus (61 women) did not show a significant association with hepatic alteration (p = 0.063) or ART-related allergic reaction (p = 0.065). Gestational CD4 values higher than 250 cells/mm3 in the baseline (RR 0.91 CI 0.71–1.16) and in the last count (RR 0.94 CI 0.71–1.24) were not associated with a higher risk for hepatic changes. The pregnant women exposed to nevirapine with CD4 > = 250 cells/mm3 did not present a higher occurrence of hepatic alteration (p = 1.00) or ART medication-related allergic reaction (p = 1.00) (data not shown).
In the multivariate analysis, the occurrence of co-infections during gestation increased the risk of developing maternal anemia (RR 1.52 CI 1.04–2.21). Having a peripartum CD4 higher or equal to 200 cells/mm3 was associated with having a lower occurrence of ART-related anemia (RR 0.67 CI 0.51–0.90) (Table 5).
Table 5.
Multivariate analysis of risk factors associated with maternal adverse effects
| Risk Factor | Anemia | Relative Risk | Logistic Regression* | Hepatic alterations | Relative Risk | Logistic Regression* | Dyslipidemia | Relative risk | Fasting glycemia alterations | Relative risk | Logistic Regression* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | ||||||||||||||||
| n | % | n | % | RR CI 95% | RR CI 95% | n | % | n | % | RR CI 95% | RR CI 95% | n | % | n | % | RR CI 95% | n | % | n | % | RR CI 95% | RR CI 95% | |
| Co-infection during pregnancy | |||||||||||||||||||||||
| No | 35 | 9.2 | 63 | 23.8 | 1 | 1 | |||||||||||||||||
| Yes | 378 | 99.7 | 263 | 99.2 | 1.65(1.17–2.33) | 1.52(1.04–2.21) | |||||||||||||||||
| No information | 1 | 2 | |||||||||||||||||||||
| Baseline CD4 (cells/mm3) | |||||||||||||||||||||||
| < 200 | 66 | 19.0 | 19 | 6.1 | 1 | 52 | 14.2 | 32 | 10.4 | 1 | 82 | 17.7 | 21 | 21.4 | 1 | 15 | 12.2 | 59 | 11.5 | 1 | |||
| > = 200 | 330 | 94.8 | 286 | 92.6 | 0.69(0.53–0.90) | 315 | 85.8 | 276 | 89.6 | 0.86(0.64–1.16) | 381 | 82.3 | 77 | 78.6 | 1.01(0.75–1.35) | 108 | 87.8 | 453 | 88.5 | 0.95(0.55–1.63) | |||
| No information | 18 | 23 | 15 | 9 | 7 | 5 | 4 | 24 | |||||||||||||||
| Peripartum CD4 (cells/mm3) | |||||||||||||||||||||||
| < 200 | 59 | 16.6 | 13 | 4.1 | 1 | 1 | 43 | 11.7 | 29 | 9.4 | 1 | 65 | 14.0 | 13 | 13.1 | 1 | 12 | 9.8 | 48 | 9.4 | 1 | ||
| > = 200 | 337 | 94.9 | 291 | 92.4 | 0.66(0.50–0.86) | 0.67(0.51–0.90) | 323 | 88.3 | 281 | 90.6 | 0.9(0.65–1.23) | 399 | 86.0 | 86 | 86.9 | 0.98(0.71–1.34) | 111 | 90.2 | 464 | 90.6 | 0.97(0.53–1.75) | ||
| No information | 18 | 24 | 16 | 7 | 6 | 4 | 4 | 24 | |||||||||||||||
| CDC classification | |||||||||||||||||||||||
| 1 | 97 | 30.6 | 117 | 55.5 | 1 | 107 | 28.8 | 96 | 30.5 | 1 | 141 | 30.2 | 28 | 27.5 | 1 | 39 | 31.0 | 154 | 29.4 | 1 | |||
| 2 | 211 | 66.6 | 158 | 74.9 | 1.26(0.99–1.60) | 191 | 51.3 | 160 | 50.8 | 1.03(0.82–1.31) | 242 | 51.8 | 58 | 56.9 | 0.97(0.78–1.19) | 63 | 50.0 | 274 | 52.3 | 0.93(0.62–1.38) | |||
| 3 | 95 | 30.0 | 41 | 19.4 | 1.54(1.16–2.05) | 74 | 19.9 | 59 | 18.7 | 1.06(0.79–1.42) | 84 | 18.0 | 16 | 15.7 | 1.01(0.77–1.32) | 24 | 19.0 | 96 | 18.3 | 0.99(0.60–1.65) | |||
| No information | 11 | 12 | 10 | 2 | 3 | 1 | 1 | 12 | |||||||||||||||
| Peripartum viral load | |||||||||||||||||||||||
| Undetectable (< 50 copies/ml) | 215 | 108.0 | 205 | 166.7 | 1 | 309 | 66.3 | 54 | 56.3 | 1 | 81 | 66.4 | 314 | 61.4 | 1 | ||||||||
| Detectable (> = 50 copies/ml) | 178 | 89.4 | 102 | 82.9 | 1.24(1.02–1.52) | 157 | 33.7 | 42 | 43.8 | 0.93(0.77–1.12) | 41 | 33.6 | 197 | 38.6 | 0.84(0.58–1.22) | ||||||||
| No information | 21 | 21 | 4 | 7 | 5 | 25 | |||||||||||||||||
| Start of ART use | |||||||||||||||||||||||
| Before pregnancy | 58 | 16.3 | 80 | 32.3 | 1 | 1 | 58 | 15.4 | 77 | 24.3 | 1 | 97 | 20.7 | 21 | 20.4 | 1 | 37 | 29.6 | 95 | 18.0 | 1 | 1 | |
| During pregnancy | 350 | 98.3 | 240 | 96.8 | 1.41(1.07–1.86) | 1.35(1.02–1.80) | 319 | 84.6 | 240 | 75.7 | 1.33(1–1.76) | 371 | 79.3 | 82 | 79.6 | 1(0.80–1.25) | 88 | 70.4 | 434 | 82.0 | 0.6(0.41–0.88) | 0.67 (0.44–1.01) | |
| No information | 6 | 8 | 5 | 2 | 0 | 2 | 7 | ||||||||||||||||
| NRTI | |||||||||||||||||||||||
| AZT | 373 | 909.8 | 291 | 786.5 | 1 | ||||||||||||||||||
| TDF | 21 | 51.2 | 20 | 54.1 | 0.91(0.59–1.42) | ||||||||||||||||||
| No information | 20 | 17 | |||||||||||||||||||||
| ART during pregnancy | |||||||||||||||||||||||
| NRTI + NRTI + NVP | 72 | 21.1 | 49 | 17.6 | 1 | 92 | 26.0 | 17 | 5.6 | 2.04(1.58–2.63) | 2.04(1.58–2.63) | 52 | 11.5 | 19 | 19.8 | 1 | 10 | 8.3 | 87 | 17.5 | 1 | 1 | |
| NRTI + NRTI + NFV | 77 | 22.5 | 50 | 17.9 | 1.02(0.74–1.41) | 77 | 21.8 | 47 | 15.5 | 1.5(1.15–1.97) | 1.5(1.15–1.97) | 86 | 19.0 | 18 | 18.8 | 1.13(0.80–1.59) | 15 | 12.4 | 99 | 19.9 | 1.28(0.57–2.84) | 1.31(0.59–2.92) | |
| NRTI + NRTI + LPV/r | 228 | 66.7 | 183 | 65.6 | 0.93(0.72–1.22) | 165 | 46.6 | 234 | 77.2 | 1 | 1 | 295 | 65.3 | 57 | 59.4 | 1.14(0.85–1.54) | 91 | 75.2 | 292 | 58.8 | 2.31(1.20–4.43) | 2.08(1.07–4.04) | |
| NRTI + NRTI + ATV/r | 11 | 3.2 | 14 | 5.0 | 0.74(0.39–1.40) | 20 | 5.6 | 5 | 1.7 | 1.94(1.22–3.08) | 1.94(1.22–3.08) | 19 | 4.2 | 2 | 2.1 | 1.24(0.73–2.09) | 5 | 4.1 | 19 | 3.8 | 2.02(0.69–5.91) | 1.58(0.52–4.76) | |
| No information | 26 | 32 | 28 | 14 | 18 | 7 | 6 | 39 | |||||||||||||||
| Hepatitis C | |||||||||||||||||||||||
| No | 345 | 90.6 | 299 | 94.3 | 1 | ||||||||||||||||||
| Yes | 36 | 9.4 | 18 | 5.7 | 1.25(0.88–1.75) | ||||||||||||||||||
| No information | 1 | 0 | |||||||||||||||||||||
* Cox Logistic Regression
ART antiretroviral therapy, NRTI nucleos(t)ide reverse transcriptase inhibitors, AZT zidovudine, TDF tenofovir, NVP nevirapine, NFV nelfinavir, LPV lopinavir, ATV atazanavir, r ritonavir, CDC Centers for Disease Control and Prevention
Association between ART regimens and maternal adverse effects
Pregnant women exposed to NVP presented higher risk for elevated hepatic enzymes (p < 0.0001, RR 4.26 CI 2.67–6.81) and for allergic reaction (p = 0.002, RR 2.71 CI 1.69–4.37), but lower risk for dyslipidemia (p = 0.015, RR 0.56 CI 0.35–0.89) and for alteration in fasting glycemia (p = 0.032, RR 0.55 CI 0.31–0.97), when compared with the ones using PI (Table 4).
Table 4.
Comparative analysis between pregnant women in use of NNRTI and PI
| NNRTI | PI | p* | RR | CI 95% | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Co-infections during pregnacy | 0.010 | ||||||
| Yes | 111 | 78.2 | 515 | 86.7 | 0.60 | 0.44–0.81 | |
| No | 31 | 21.8 | 79 | 13.3 | 1.00 | ||
| Obstetrical complications | 0.001 | ||||||
| Yes | 57 | 40.1 | 334 | 56.0 | 0.60 | 0.44–0.81 | |
| No | 85 | 59.9 | 262 | 44.0 | 1.00 | ||
| ART adherence during pregnancy | 0.387 | ||||||
| Yes | 121 | 86.4 | 494 | 83.4 | 1.21 | 0.78–1.88 | |
| No | 19 | 13.6 | 98 | 16.6 | 1.00 | ||
| ART changes during pregnancy | < 0.0001 | ||||||
| Yes | 10 | 7.1 | 134 | 22.5 | 0.31 | 0.17–0.58 | |
| No | 131 | 92.9 | 462 | 77.5 | 1 | ||
| Viral load after ART | 0.001 | ||||||
| Undetectable | 57 | 47.5 | 365 | 63.4 | 1 | ||
| Detectable | 63 | 52.5 | 211 | 36.6 | 1.70 | 1.23–2.35 | |
| Anemia | 0.664 | ||||||
| Yes | 73 | 58.4 | 323 | 56.3 | 1.07 | 0.78–1.48 | |
| No | 52 | 41.6 | 251 | 43.7 | 1 | ||
| Thrombocytopenia | 0.479 | ||||||
| Yes | 15 | 12.1 | 83 | 14.5 | 0.08 | 0.51–1.38 | |
| No | 109 | 87.9 | 488 | 85.5 | 1 | ||
| Hepatic alteration | < 0.0001 | ||||||
| Yes | 94 | 83.2 | 267 | 47.8 | 4.26 | 2.67–6.81 | |
| No | 19 | 16.8 | 292 | 52.2 | 1 | ||
| Dyslipidemia | 0.015 | ||||||
| Yes | 52 | 72.2 | 406 | 83.9 | 0.56 | 0.35–0.89 | |
| No | 20 | 27.8 | 78 | 16.1 | 1 | ||
| Allergic reaction | 0.002 | ||||||
| Yes | 10 | 7.5 | 11 | 1.9 | 2.71 | 1.69–4.37 | |
| No | 123 | 92.5 | 578 | 98.1 | 1 | ||
| Fasting glycemia alteration | 0.032 | ||||||
| Yes | 12 | 11.9 | 112 | 21.1 | 0.55 | 0.31–0.97 | |
| No | 89 | 88.1 | 418 | 78.9 | 1 | ||
| Total | 142 | 100 | 597 | 100 | |||
(#) Numbers are different due to the lack of information on some patients
* Chi-square
ART antiretroviral therapy, NNRTI non-nucleoside reverse transcriptase inhibitor, PI protease inhibitor
In the protease inhibitors evaluation, women exposed to NFV presented increased risk for hepatic enzymes elevation (p < 0.0001, RR 1.90 CI 1.38–2.62) and for allergic reaction (p < 0.0001, RR 4.07 CI 3.19–5.20), but a lower risk for fasting glycemia alteration (p = 0.015, RR 0.56 CI 0.34–0.92), when compared with the ones exposed to LPV/r. Pregnant women exposed to ATV/r presented higher risk for elevated bilirubin (p = 0.0002, RR 5.17 CI 1.98–13.51) when compared to the ones exposed to LPV/r (data not shown).
In the nucleosides (AZT) and nucleotides (TDF) analogues comparison, there was no difference in adverse effects occurrence between AZT and TDF regimens (data not shown).
In the evaluation of the adverse effects associated with the timing of ART introduction, the women who initiated treatment during pregnancy presented increased risk for anemia (p = 0.0002), for hepatic changes (p = 0.003) and for allergic reaction (p = 0.025) than the ones who were already in use at conception (data not shown).
In the multivariate analysis, starting ART during pregnancy (RR 1.35 CI 1.02–1.80) increased the risk for developing maternal anemia. The use of NVP (RR 2.04 CI 1.58–2.63), NFV (RR 1.50 CI 1.15–1.97) and ATV (RR 1.94, CI 1.22–3.08) increased the risk for hepatic alterations in pregnancy. The use of LPV (RR 2.08 CI 1.07–4.04) increased the risk for alteration in fasting glycemia during pregnancy (Table 5).
Discussion
In our study pregnant women infected with HIV presented significant rates of adverse effects, most of low severity. Anemia and hepatic alteration occurred in more than half of the cases and lower platelets count in almost 15%. Less than 3% presented ART-related allergic reaction.
The Obstetric Clinic in CAISM/UNICAMP is a reference service for HIV infected women in the Campinas region and serves a low-income population with less schooling. The great majority of the cases are highly complex including users of psychoactive substances (mainly crack). In this updated cohort of 15 years of medical care, the great majority of women were exposed to ART during pregnancy, delivered by C-section, received intravenous zidovudine during labor, did not breastfeed and their newborns received oral AZT for 6 weeks.
The antiretroviral drugs used in pregnant women during the study years varied according to the Brazilian Ministry of Health’s recommendations. Since 2000, an ART regimen with three combined drugs has been in use [7].
One unquestionable benefit of using ART during pregnancy is the viral load control which directly impacted MTCT. In our study, analyzing 793 pregnancies of women infected with HIV, the MTCT rate was 2.3%, close to the World Health Organization (WHO) Global Plan’s goal [17, 18]. Nevertheless, it is important to stress that among the 350 infected pregnant women in the cohort’s last 5 years, there were only three cases of MTCT, all from women with lower adherence to treatment and in abuse of substances. Previous studies from the same clinic showed MTCT rates of 2.9% in 2000 and 3.7% in 2009, the last one strongly related to no adherence to antiretroviral treatment during pregnancy [19, 20].
It is known that using complex and potent antiretroviral regimens during pregnancy can cause adverse effects for the mother and the newborn, including the organogenesis period [8, 9]. The physiologic changes in pregnancy affect the kinetics of absorption, distribution, biotransformation and elimination of medication, potentially altering pregnant woman’s susceptibility to the toxicity of different drugs [7]. These changes cause pharmacokinetic alterations with consequent reduction of exposure to most of the drugs during the gestational period. However, it is not always necessary to adjust their dosages until the end of pregnancy [21].
The most frequent and significant maternal adverse effects studied are hematologic, hepatic and dermatologic alterations, metabolic disturbances like diabetes mellitus and dyslipidemia, pre-eclampsia and viral resistance [8, 22].
Our study demonstrated a global anemia rate of more than 50% in pregnant women exposed to ART, which is in accordance with the international and national literature. Recent study showed high anemia occurrence in Brazilian pregnant women [23]. This adverse effect was not associated with any specific antiretroviral drug in our multivariate data analysis but was associated with the starting of ART during pregnancy, with the occurrence of infectious complications and with peripartum CD4 count lower than 200 cells/mm3.
It is known that anemia in pregnant women infected with HIV can be associated with other causes besides ART, such as advanced maternal disease, iron and folic acid deficiency, malnutrition, intestinal parasitosis, and increased intravascular volume (hemodilution); all significantly associated with increased risk for maternal mortality [24, 25].
The use of zidovudine is one of the causes of cytopenia in pregnant women infected with HIV. In general anemia occurs at the beginning of treatment, in the first 12 weeks of use, and it is frequently associated with low CD4 count and advanced disease [26–28], as demonstrated in our data.
Even when it is known that AZT is not the only cause of anemia in pregnancy, and that ART is linked to improved health, particularly in lower income countries, anemia is the most common adverse effect associated with AZT use in pregnancy, independently of the regimen [29–31]. A study with 1070 pregnant women in sub-Saharan Africa between 2005 and 2008 reported the same results [32]. Many studies have been demonstrating high rates of anemia independently from the treatment regimen, as the one in South Africa with 408 pregnant women and 64.2% rate of anemia [33]. Similarly, our multivariate analysis showed that low CD4 count is a risk factor associated with anemia.
Our data did not show a significant difference in the occurrence of anemia between pregnant women exposed to AZT or TDF regimens. We did not compare the use of monotherapy with AZT and combined ART because of the small number of cases using only AZT (23 cases). A clinical trial (PROMISE Trial) in six sub-Saharan African countries and India compared monotherapy with combined regimens (AZT/3TC/LPV/r or TDF/Emtricitabine/LPV/r) and demonstrated that maternal adverse effects were more frequent with combined regimens, although the difference was not significant [34].
A study with 117 pregnant women in 2011 demonstrated adverse hematologic and hepatic effects and gestational cholestasis particularly associated with nevirapine, zidovudine and nelfinavir use. The higher rates of discontinuation or substitution due to adverse effects or intolerance occurred with these same drugs, while only 1% of patients in use of lopinavir/ritonavir had the drugs changed [35]. Our data also evidenced an increased risk of elevated hepatic enzymes with the use of combined ART. In the multivariate analysis, the use of nevirapine was associated with increased risk for hepatic alteration during pregnancy, although NFV and ATV were also associated with these adverse effects.
In our cohort, nevirapine was the most utilized NNRTI. It was associated with increased hepatic alterations and hypersensitivity skin reactions, in agreement with recently published researches. Using NVP in pregnant women, especially with CD4 count higher or equal to 250 cells/mm3 is debatable, due to elevated risk for hepatic toxicity and skin allergic reaction already demonstrated [36, 37]. However, an elevated risk for hepatic alteration and allergic reaction was not demonstrated in pregnant women exposed to nevirapine with CD4 > = 250 cells/mm3 in our cohort. This drug is considered an alternative, seldom recommended for pregnant women in Brazil [7].
Data from a 2006 Brazilian study evaluating NVP adverse effects in 197 pregnant women reported a 5.5% toxicity rate, 4.5% of exanthema with only one case of Stevens-Johnson Syndrome, and 1.5% of hepatotoxicity including one severe case [38]. That study also described hepatitis C virus as the only risk factor for NVP toxicity, which was also demonstrated in recent studies [39, 40]. In our cohort, pregnant women co-infected with hepatitis C virus did not present higher hepatotoxicity due to ART use.
In our study, despite the low rates of maternal skin allergic reaction associated with NVP, there were three cases of Stevens-Johnson Syndrome with total recovery.
A clinical trial in Uganda compared LPV/r and efavirenz (EFV) use in 389 women infected with HIV during pregnancy and puerperium and found no difference in the adverse effects such as anemia and neutropenia related to the two drugs. On the other hand, the pregnant women exposed to EFV presented higher viral suppression at delivery compared to the ones in use of LPV/r, although this difference was extinguished one year after birth [41]. In our study only four pregnant women were using EFV by pregnancy’s conclusion and only one of them used it continuously from the beginning to the end. Although for years EFV has been largely used in adults’ antiretroviral regimens due mainly to its highly potent effects and its simpler posology, only recently and after its security was demonstrate, was this drug suggested as the preferable regimen for pregnant women [6, 42]. For this reason, in 2015 the Brazilian Health Ministry recommended EFV as the chosen drug for initial treatment [43]. Nevertheless, recent studies discussed its significant collateral effects, in special the neuropsychiatric one and the increased risk for suicide [44, 45], besides its lower potentiality when compared to the promising integrase strand inhibitors [46]. Because of this, the most recent Brazilian recommendation is to use raltegravir as first choice to complete ART regimen in pregnancy [7].
Until 2015 the main recommended drug for pregnant women in Brazil was lopinavir/ritonavir (LPV/r). Atazanavir/ritonavir (ATV/r), which was highly recommended in international guidelines, was restricted in Brazil to first line PI intolerance cases and was associated with higher risk for elevated bilirubin, without clinical implications to the newborn. Bilirrubin elevation is a known adverse effect of atazanavir during pregnancy or anytime of use. It is known that protease inhibitors can cause lipid metabolic changes, metabolic syndrome, lipodystrophy, changes in the carbohydrate metabolism and increased cardiovascular risk in every HIV infected patient [47–49]. Some studies had demonstrated that during pregnancy women in use of PI also presented higher rates of dyslipidemia, metabolic syndrome, lipodystrophy, diabetes and pre-eclampsia [50–52], as some of our data also demonstrated.
When evaluating the different ART classes, PI use was associated with elevated risk for dyslipidemia and fasting glycemia alterations when compared to NVP use. In the multivariate analysis, LPV use was associated with elevated risk for alteration in fasting glycemia, demonstrating the need for its early evaluation and diagnosis in the pregnant women exposed to PI, and the need to revise the criteria for indicating PI to women with known risk factors for diabetes, such as obesity.
On carbohydrates metabolism, a study showed that some antiretroviral drugs increased the TNFα, IL-6 e IL-1β pro-inflammatory cytokines involved in changes of adipocytes function and in the decrease of adiponectin, a positive modulator for insulin sensitivity [53]. A multi-center randomized clinical trial with 1407 women observed a general rate of gestational diabetes in 2.1%, higher with PI use (4.6%) [54].
Regarding birth outcomes, the authors used the same pregnant women’s cohort and their newborns to analyze neonatal adverse effects such as prematurity, low birth weight, small for gestational age week, neonatal anemia and hepatic alterations. The results on birth outcomes are described in another article which has just been published [55].
The high Caesarian section rate in this cohort occurred because from 2005 to 2015 most of pregnant women infected with HIV delivered by C-section following changes in the service’s protocol after the European Collaborative Group published their study [56]. On the other hand, prematurity was not considered iatrogenic and was not associated with C-section as shown in the recently published article by the same authors [55].
Our study focus was on the evaluation of adverse effects to which we produced consistent data on a clinical cohort in use of ART following the Brazilian Health Ministry’s recommendations, using guidelines very similar to the international ones. Data collection based on information registered in the women’s and children’s clinical records might have contributed to a possible register bias. Another limitation was having no HIV negative controls. The authors did not compare adverse effects in HIV negative control women because some tests to evaluate toxicity (hepatic alterations and dyslipidemia) are done only when the pregnant woman is in use of specific medication such as ART.
Our data showed more than 700 evaluations of pregnant women infected with HIV followed in a specialized clinic showing that the ART choices recommended by the Brazilian Health Ministry were adequate, leading to low gravity adverse effects and reaching excellent rates of MTCT.
Conclusions
Despite the growing evidence on the unquestionable benefits of antiretroviral treatment during pregnancy, the clinical monitoring on drug safety and efficacy is of extreme importance to optimize the treatment recommendations.
The evolution of the national guidelines of antiretroviral therapy for pregnant women improved adherence to the treatment and resulted in a significant reduction of mother-to-child transmission of HIV throughout the years. Despite the high frequency of maternal adverse effects, they are mostly of low severity. Parallelally, recommending newer ART medications with improved efficacy and significantly more favorable tolerability profiles (introduction of tenofovir/lamivudine plus raltegravir for first line) should reduce the incidence of maternal ART-related adverse effects, which will reduce maternal as well as infant morbidity and potentially mortality.
Acknowledgments
The authors would like to thank Marina Polydoro for the data collecting, Gislaine Carvasan for the statistical analysis, and Maria Sílvia Setubal for the language editing.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3TC
Lamivudine
- ABC
Abacavir
- AIDS
Acquired immunodeficiency syndrome
- ALCPH
Alkaline phosphatase
- ALT
Alanine aminotransferase
- ART
Antiretroviral therapy
- AST
Aspartate aminotransferase
- ATV
Atazanavir
- AZT
Zidovudine
- CAISM/UNICAMP
Women’s Hospital at University of Campinas School of Medical Sciences
- CI
Confidence interval
- d4T
Stavudine
- DRV
Darunavir
- EFV
Efavirenz
- FPV
Fosamprenavir
- GGT
Gamma-glutamyl transferase
- HIV
Human immunodeficiency virus
- IDV
Indinavir
- LPV
Lopinavir
- MTCT
Mother-to-child transmission
- NFV
Nelfinavir
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- NRTI
Nucleoside reverse transcriptase inhibitors
- NVP
Nevirapine
- PI
Protease inhibitor
- r
Ritonavir
- RAL
Raltegravir
- RR
Relative risk
- SQV
Saquinavir
- TDF
Tenofovir
- WHO
World Health Organization
Authors’ contributions
AMD and HM participated in all steps of the study, including research planning, data collection, analysis and interpretation of data, writing the manuscript and revising it for intellectual content. GJL and EA participated in research planning, interpretation of data, and revising the manuscript for intelectual content. FC, IM and FL participated in data collection and interpretation of data. All authors gave suggestions, read the manuscript carefully, fully agreed on its content and approved the final version.
Ethics approval and consent to participate
This research project was approved by the research committee at the Department of Gynecology and Obstetrics CAISM/UNICAMP and by the University of Campinas School of Medical Sciences ethics review board (Project number 351/2006) and since it is a retrospective study, it was exempt from an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adriane M. Delicio, Email: adri36_unicamp@hotmail.com
Giuliane J. Lajos, Email: giuliane@unicamp.br
Eliana Amaral, Email: elianaa@unicamp.br.
Fabia Lopes, Email: lopesfabia1977@hotmail.com.
Fernanda Cavichiolli, Email: fecavichiolli@gmail.com.
Isabeli Myioshi, Email: isabelimiyoshi@gmail.com.
Helaine Milanez, Email: helaine@caism.unicamp.br.
References
- 1.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. The INSIGHT START study group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggaley R, Doherty M, Ball A, Ford N, Gottfried HG. Department of HIV/AIDS, World Health Organization, Geneva, Switzerland. The strategic use of antiretrovirals to prevent HIV infection: a converging agenda. Clin Infect Dis. 2015;60(Suppl 3):159–160. doi: 10.1093/cid/civ091. [DOI] [PubMed] [Google Scholar]
- 3.The Working Group on Mother-To-Child Transmission of HIV Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: results from 13 perinatal studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(Suppl 5):506–510. doi: 10.1097/00042560-199504120-00011. [DOI] [PubMed] [Google Scholar]
- 4.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan M, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 5.French CE, Thorne C, Byrne L, Cortina-Borja M, Tookey PA. Presentation for care and antenatal management of HIV in the UK, 2009-2014. HIV Med. 2017;18(Suppl 3):161–170. doi: 10.1111/hiv.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: WHO Guidelines Review Committee; 2013. [PubMed]
- 7.Brasil. Ministério da Saúde. Departamento de DST, Aids e Hepatites Virais. Protocolo Clínico e Diretrizes Terapêuticas para Prevenção da transmissão vertical do HIV, sífilis e hepatites virais. Brasília. 2017.
- 8.Newell ML, Bunders MJ. Safety of antiretroviral drugs in pregnancy and breastfeeding for mother and child. Curr Opin HIV AIDS. 2013;8(Suppl 5):504–510. doi: 10.1097/COH.0b013e3283632b88. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Sando MM, Spiegelman D, Hertzmark E, Liu E, Sando D, et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis. 2016;213(Suppl 7):1057–1064. doi: 10.1093/infdis/jiv389. [DOI] [PubMed] [Google Scholar]
- 10.Morén C, Noguera-Julián A, Garrabou G, Rovira N, Catalán M, Bañó M, et al. Mitochondrial disturbances in HIV pregnancies. AIDS. 2015;29(Suppl 1):5–12. doi: 10.1097/QAD.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 11.Cohan D, Mwesigwa J, Natureeba P, Aliba Luwedde F, Ades V, et al. WHO option B+: early experience of antiretroviral therapy sequencing after cessation of breastfeeding and risk of dermatologic toxicity. J Acquir Immune Defic Syndr. 2013;62(Suppl 3):101–103. doi: 10.1097/QAI.0b013e31828011ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browne JL, Schrier VJ, Grobbee DE, Peters SA, Klipstein-Grobusch K. Antiretroviral therapy, and hypertensive disorders in pregnancy: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2015;70(Suppl 1):91–98. doi: 10.1097/QAI.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann CJ, Cohn S, Mashabela F, Hoffmann JD, McIlleron H, Denti P, et al. Treatment failure, drug resistance, and CD4 T-cell count decline among postpartum women on antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2016;71(Suppl 1):31–37. doi: 10.1097/QAI.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santini-Oliveira M, Friedman RK, Veloso VG, Cunha CB, Pilotto JH, Marins LM, et al. Incidence of antiretroviral adverse drug reactions in pregnant women in two referral centers for HIV prevention of mother-to-child-transmission care and research in Rio de Janeiro, Brazil. Braz J Infect Dis. 2014;18(Suppl 4):372–378. doi: 10.1016/j.bjid.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde . Departamento de Ações Programáticas Estratégicas. Brasília: Gestação de alto risco: manual técnico; 2012. [Google Scholar]
- 16.Division of AIDS. National Institute of Allergy and Infectious Diseases. National Institutes of Health. US Department of Health and Human Services. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. Version 2.0. Bethesda: NIH; 2014.
- 17.WHO . Global Plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: UNAIDS; 2011. [Google Scholar]
- 18.WHO . Progress Report on the Global Plan towards the elimination of new HIV infections among children and keeping their mothers alive. Geneva: UNAIDS; 2015. [Google Scholar]
- 19.Amaral E, Assis-Gomes F, Milanez H, Cecatti JG, Vilela MM, Pinto e Silva JL. Timely implementation of interventions to reduce vertical HIV transmission: a successful experience in Brazil, Rev Panam Salud Publica. 2007;21(Suppl 6):357–64. [DOI] [PubMed]
- 20.Delicio AM, Milanez H, Amaral E, Morais SS, Lajos GJ, Pinto e Silva JLC, et al. Mother-to-child transmission of human immunodeficiency virus in a ten years period. Reprod Health. 2011;8:35. doi: 10.1186/1742-4755-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colbers A, Greupink R, Burger D. Pharmacological considerations on the use of antiretrovirals in pregnancy. Curr Opin Infect Dis. 2013;26(Suppl 6):575–588. doi: 10.1097/QCO.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 22.Senise JF, Castelo A, Martínez M. Current treatment strategies, complications and considerations for the use of HIV antiretroviral therapy during pregnancy. AIDS Rev. 2011;13(Suppl 4):198–213. [PubMed] [Google Scholar]
- 23.Pinho-Pompeu M, Surita FG, Pastore DA, Paulino DS, Pinto E Silva JL. Anemia in pregnant adolescents: impact of treatment on perinatal outcomes. J Matern Fetal Neonatal Med. 2016;17:1–5. doi: 10.1080/14767058.2016.1205032. [DOI] [PubMed] [Google Scholar]
- 24.WHO . Regional Office for Africa: Health Situation Analysis in the African Region. Geneva: Atlas of Health Statistics; 2011. [Google Scholar]
- 25.Saving Mothers 2008–2010. Fifth Report on Confidential Enquiries into Maternal Deaths in South Africa. Comprehensive Report. Compiled by the National Committee for Confidential Enquiry into Maternal Deaths. Department Health Republic of South Africa. South Africa. 2012. http://sanac.org.za/wp-content/uploads/2015/12/Report_on_Confidential_Enquiries_into_Maternal_Deaths_in_South_Africa.pdf.
- 26.Volberding PA, Lagakos SW, Grimes JM, et al. The duration of zidovudine benefit in persons with asymptomatic HIV infection. Prolonged evaluation of protocol 019 of the AIDS Clinical Trials Group. JAMA. 1994;272:437–442. doi: 10.1001/jama.1994.03520060037029. [DOI] [PubMed] [Google Scholar]
- 27.Mildvan D, Creagh T, Leitz G, Group APS Prevalence of anemia and correlation with biomarkers and specific antiretroviralregimens in 9690 human-immunodeficiency-virus-infectedpatients: findings of the Anemia prevalence study. Curr Med Res Opin. 2007;23:343–355. doi: 10.1185/030079906X162683. [DOI] [PubMed] [Google Scholar]
- 28.Iuliano AD, Weidle PJ, Brooks JT, Masaba R, Girde S, Ndivo R, et al. Neutropenia in HIV-infected Kenyan women receiving triple antiretroviral prophylaxis to prevent mother-to-child HIV transmission is not associated with serious clinical sequelae. J Int Assoc Provid AIDS Care. 2015;14(Suppl 3):261–268. doi: 10.1177/2325957413502543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzi P, Spicher VM, Laubereau B, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. AIDS. 1998;12:241–247. doi: 10.1097/00002030-199818000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Tuomala RE, Watts DH, Li D, Vajaranant M, Pitt J, Hammill H, Landesman S, Zorrilla C, Thompson B. Women and Infants Transmission Study. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antirretroviral therapy during pregnancy. J Acquir Immune Defic Syndr. 2005;38:449–473. doi: 10.1097/01.qai.0000139398.38236.4d. [DOI] [PubMed] [Google Scholar]
- 31.Odhiambo C, Zeh C, Angira F, Opollo V, Akinyi B, Masaba R, et al. Anaemia in HIV-infected pregnant women receiving triple antiretroviral combination therapy for prevention of mother-to-child transmission: a secondary analysis of the Kisumu breastfeeding study (KiBS) Tropical Med Int Health. 2016;21(Suppl 3):373–384. doi: 10.1111/tmi.12662. [DOI] [PubMed] [Google Scholar]
- 32.Benn KD, Chersich MF, Mary M, Nicolas M, Marleen T, et al. Maternal anaemia and duration of zidovudine in antiretroviral regimens for preventing mother-to-child transmission: a randomized trial in three African countries. BMC Infect Dis. 2013;13:522. doi: 10.1186/1471-2334-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandlal V, Moodley D, Grobler A, Bagratee J, Maharaj NR, Richardson P. Anaemia in pregnancy is associated with advanced HIV disease. PLoS One. 2014;9(Suppl 9):e106103. doi: 10.1371/journal.pone.0106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. IMPAACT 1077BF/1077FF PROMISE Study Team. N Engl J Med. 2016;375(Suppl 18):1726–1737. doi: 10.1056/NEJMoa1511691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg A, Forster-Harwood J, Davies J, McFarland EJ, Pappas J, Kinzie K, et al. Safety and tolerability of antiretrovirals during pregnancy. Infect Dis Obstet Gynecol. 2011;2011:867674. doi: 10.1155/2011/867674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coster LO, Kumar PN. Contemporary role of Nevirapine in HIV treatment. AIDS Rev. 2012;14(Suppl 2):132–144. [PubMed] [Google Scholar]
- 37.Cohan D, Mwesigwa J, Natureeba P, Aliba Luwedde F, Ades V, et al. WHO option B+: early experience of antiretroviral therapy sequencing after cessation of breastfeeding and risk of dermatologic toxicity. J Acquir Immune Defic Syndr. 2013;62(Suppl 3):e101–e103. doi: 10.1097/QAI.0b013e31828011ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.João ES, Calvet GA, Menezes JA, D’ippolito MM, Cruz MLS, Salgado LAT, et al. Nevirapine toxicity in a cohort of HIV-1–infected pregnant women. Am J Obstet Gynecol. 2006;194:199–202. doi: 10.1016/j.ajog.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Snijdewind IJ, Smit C, Godfried MH, Nellen JF, de Wolf F, Boer K, et al. HCV coinfection, an important risk factor for hepatotoxicity in pregnant women starting antiretroviral therapy. J Inf Secur. 2012;64(Suppl 4):409–416. doi: 10.1016/j.jinf.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Andreotti M, Pirillo MF, Liotta G, Jere H, Maulidi M, Sagno JB, et al. The impact of HBV or HCV infection in a cohort of HIV-infected pregnant women receiving a nevirapine-based antiretroviral regimen in Malawi. BMC Infect Dis. 2014;14:180. doi: 10.1186/1471-2334-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohan D, Natureeba P, Koss CA, Plenty A, Luwedde F, Mwesigwa J, et al. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS. 2015;29(Suppl 2):183–191. doi: 10.1097/QAD.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford Nathan, Mofenson Lynne, Shubber Zara, Calmy Alexandra, Andrieux-Meyer Isabelle, Vitoria Marco, Shaffer Nathan, Renaud Françoise. Safety of efavirenz in the first trimester of pregnancy. AIDS. 2014;28:S123–S131. doi: 10.1097/QAD.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 43.Brasil. Ministério da Saúde. Departamento de DST, Aids e Hepatites Virais. Protocolo Clínico e Diretrizes Terapêuticas para Prevenção da transmissão vertical do HIV, sífilis e hepatites virais. Brasília. 2015.
- 44.Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav. 2011;15:1803–1818. doi: 10.1007/s10461-011-9939-5. [DOI] [PubMed] [Google Scholar]
- 45.Smith C, Ryom L, Monforte AD, Reiss P, Mocroft A, El-Sadr W, et al. Lack of association between use of efavirenz and death from suicide: evidence from the D:A:D study. J Int AIDS Soc. 2014;17(4 Suppl 3):19512. doi: 10.7448/IAS.17.4.19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rockstroh JK, DeJesus E, Lennox JL, Yazdanpanah Y, Saag MS, Wan H, Rodgers AJ, Walker ML, Miller M, DiNubile MJ, Nguyen BY, Teppler H, Leavitt R, Sklar P, STARTMRK Investigators Durable efficacy and safety of raltegravir versus efavirenz when combined with Tenofovir/Emtricitabine in treatment-naive HIV-1–infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63(1):77–85. doi: 10.1097/QAI.0b013e31828ace69. [DOI] [PubMed] [Google Scholar]
- 47.Rakotoambinina B, Médioni J, Rabian C, Jubault V, Jais JP, Viard JP. Lipodystrophic syndromes and hyperlipidemia in a cohort of HIV-1-infected patients receiving triple combination antiretroviral therapy with a protease inhibitor. J Acquir Immune Defic Syndr. 2001;27(Suppl 5):443–449. doi: 10.1097/00126334-200108150-00004. [DOI] [PubMed] [Google Scholar]
- 48.Justman JE, Benning L, Danoff A, Minkoff H, Levine A, Greenblatt RM, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. J Acquir Immune Defic Syndr. 2003;32(Suppl 3):298–302. doi: 10.1097/00126334-200303010-00009. [DOI] [PubMed] [Google Scholar]
- 49.Guira O, Tiéno H, Diendéré AE, Sagna Y, Diallo I, Yaméogo B, et al. Features of metabolic syndrome and its associated factors during highly active antiretroviral therapy in Ouagadougou (Burkina Faso) J Int Assoc Provid AIDS Care. 2016;15(Suppl 2):159–163. doi: 10.1177/2325957415601503. [DOI] [PubMed] [Google Scholar]
- 50.Andany N, Loutfy MR. HIV protease inhibitors in pregnancy: pharmacology and clinical use. Drugs. 2013;73(Suppl 3):229–247. doi: 10.1007/s40265-013-0017-3. [DOI] [PubMed] [Google Scholar]
- 51.Chougrani I, Luton D, Matheron S, Mandelbrot L, Azria E. Safety of protease inhibitors in HIV-infected pregnant women. HIV/ AIDS Res Palliative Care. 2013;5:253–262. doi: 10.2147/HIV.S33058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guaraldi G, Stentarelli C, Da Silva AD, Luzi K, Neri I, Cellini M, et al. Metabolic alterations in HIV-infected pregnant women: moving to metabolic tailoring of antiretroviral drugs. AIDS Rev. 2014;16(Suppl 1):14–22. [PubMed] [Google Scholar]
- 53.Lagathu C, Kim M, Maachi M, Vigouroux C, Cervera P, Capeau J, et al. HIV antiretroviral treatment alters adipokine expression and insulin sensitivity of adipose tissue in vitro and in vivo. Biochimie. 2005;87(Suppl 1):65–71. doi: 10.1016/j.biochi.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Watts H, Balasubramanian R, Maupin RT, Jr, Delke I, Dorenbaum A, Fiore S, et al. PACTG 316 Study Team. Maternal toxicity and pregnancy complications in human immunodeficiency viruseinfected women receiving antiretroviral therapy: PACTG 316. Am J Obstet Gynecol. 2004;190(Suppl 2):506–516. doi: 10.1016/j.ajog.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Delicio AM, Lajos GJ, Amaral E, Cavichiolli F, Polydoro M, Milanez H. Adverse effects in children exposed to maternal HIV and antiretroviral therapy during pregnancy in Brazil: a cohort study. Reprod Health. 2018;15(1):76. doi: 10.1186/s12978-018-0513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The European Collaborative Study Mother to child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–465. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.