Abstract
Background
Risk factors affecting early morality of patients with Escherichia coli bloodstream infection (BSI) were investigated including the host-pathogen-treatment tripartite components.
Methods
Six general hospitals in South Korea participated in this multicentre prospective observational study from May 2016 to April 2017 and a total of 1492 laboratory-confirmed E. coli BSI cases were studied. Cox regression was used to estimate risks of the primary endpoint, i.e., all-cause mortality within 30 days from the initial blood culture. Six multivariate analysis models were constructed in accordance to the clinical importance and intra- and inter-component multicollinearity.
Findings
Among the 1492 E. coli BSI cases, 9.5% (n = 141) patients expired within 30 days. Six models of multivariate analysis indicated risk factors of critical illness, primary infection of peritoneum, and chronic liver disease including cirrhosis for host variables; of phylogenetic group B2, ST131-sublineage H30Rx, multidrug resistance, group 1 CTX-M extended-spectrum beta-lactamase production, and having either of fyuA, afa, and sfa/foc virulence genes for causative E. coli pathogen variables; and of delayed definitive therapy for antimicrobial treatment variables. In addition, as a protective factor, primary urinary tract infection was identified.
Interpretation
Despite decades' effort searching for the risk factors for E. coli BSI, systemic understanding covering the entire tripartite component is still lacking. This study detailed the organic impact of host-pathogen-treatment tripartite components for early mortality in patients with E. coli BSI.
Keywords: Escherichia coli, Bloodstream infection, Early mortality, ST131, CTX-M ESBL, Delayed definitive treatment
Research in context.
Evidence before this study
E. coli bloodstream infection (BSI) an interesting research topic not only for the clinicians but also for the clinical microbiologists. Consequently, much research has been carried out in both fields and a lot information was available out of the reports. Since urinary tract is another habitual target for extra-intestinal pathogenic E. coli, the associated host factors were frequently assessed, such as infection of hospital/community origin, sex and age, as well as uropathogenic factors possessed by E. coli, and resistance to beta-lactams, fluoroquinolones and sulfonamides useful for both infections of bloodstream and urinary tract. The massive knowledge for the E. coli BSI was much enriched, and we now know that which circumstance is critical for the prognosis of the infectious disease. However, any complicated situation, for instance, increased rate of drug resistance and dissemination of a specific bacterial clones, prohibited any further prediction for the prognosis. This could be derived from the simple model estimating the outcomes of E. coli BSI using only the host factors.
Added value of this study
For the prospective observational study, we collected the entire E. coli blood isolates, together with clinical data, and antimicrobial treatment information from every E. coli BSI incidence. This study design allowed us a multidisciplinary analysis for the disease. In addition, owing to a fine layout of multivariate analysis, underestimation of any variables was reduced to minimum. Especially, the strong clonality of pathogen factors frequently eliminated the hazardous value and the antimicrobial resistance had strong correlation with treatment factors. We fractionized the pathogen-, and treatment-associated factors into six and the hazardous value of the factors were able to be ruled in. Finally, worrisome pathogen clones were identified, and antimicrobial resistance, not only for the number of resistant drugs but also for the resistance determinants, were listed as risk factors together with three virulence genes associated with siderophore, adhesion and invasion. In addition, the importance of prompt definitive therapy was highlighted.
Implications of all the available evidence
Collecting the clinical data, identification of the pathogen traits and proper application of antimicrobial treatment seems to be critical for the E. coli BSI. Fine prognostic estimation system could be schemed out for better estimating the outcomes of the prevailing infectious disease.
Alt-text: Unlabelled Box
1. Introduction
Bloodstream infection (BSI) is a widely aware burden causing morbidity and mortality in patients [1,2] and increasing medical costs [3]. To confirm the BSI [4], polymerase chain reaction (PCR) [5] and Matrix-Assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometer (MALDI-TOF/MS) [6] have been proposed for rapid diagnosis however, blood culture is still a gold standard to verify foreign micro-organic invaders in bloodstream. An antimicrobial susceptibility testing (AST) successive to the blood culture is indeed critical to decide a regimen active in vitro for definitive treatment.
Escherichia coli is among human intestinal microflora which is usually harmless and even has a mutual benefit through its gut habitat. However, certain serotype causes intestinal infections and extra-intestinal pathogenic E. coli (ExPEC) leads opportunistic infections including urinary tract infection (UTI) and BSI [7]. E. coli is a leading BSI-causative pathogen [8,9], and beta-lactams, fluoroqinolones, and sulfonamides are often used to treat the infections. A predominant ExPEC clone sequence type (ST) 131 [10] is commonly resistant to multiple drugs [10]. Of particular, the fluoroquinolone-resistant sublineages H30R and H30Rx frequently harbour the gene for CTX-M-type extended-spectrum beta-lactamases (ESBLs) leading to inappropriate therapy [10].
Many efforts have been made to identify the risk factors for E. coli BSI. Male patients, infection of hospital origin (HO) [11], Charlson comorbidity index [12], and Sequential Organ Failure Assessment (SOFA) score are noted host-associated hazardous factors for early mortality. In addition, resistance to fluoroquinolones and to 3rd-generation cephalosporins [13,14], ESBL production [15], and possessing siderophore genes such as fyuA [16], and ireA [17] are the pathogen-associated risk factors. The drug resistance is a decisive treatment-associated risk factor [11]. However, puzzling out those risk factors for the organic impact of host-pathogen-treatment tripartite components to the disease prognosis is difficult. The studies are often designed lacking one of the three components and the multicollinearity between variables are simply kicked out in favor of the clinically important variables for the main analysis. Hence, a multicentre prospective observational study without the conceptual difficulties was assessed for whole-year incidence of E. coli BSI to better understand the tripartite components associated with early mortality.
2. Methods
2.1. Study design
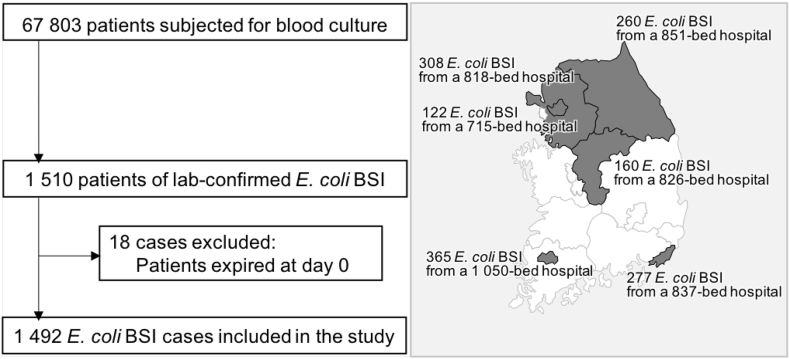
The study was conducted as a prospective observational study for entire episodes of E. coli BSI occurred between May 2016 and April 2017 in six general hospitals of 715 to 1050 beds (mean, 849.5 beds) participating in a national surveillance system in South Korea compatible to the Global Antimicrobial Surveillance System (GLASS), namely Kor-GLASS (Fig. 1) [18]. All the local ethical committee approval at each participating hospital was expedited or waived owing to the purely observational nature of the study. Cases were detected through the daily review of blood culture results. Among a total of 67,803 patients subjected for blood culture, E. coli isolates were recovered from 1510 patients (Fig. 1) and, following the elimination of 18 episodes of expirations at day 0, 1492 cases were included in the analysis. Of note, 19 follow-up-loss cases either by transfer (n = 12) or by discharge (n = 7) within 2 days of initial blood culture were excluded for the analysis of antimicrobial treatment. For the sequential isolation of duplicate E. coli from one patient, first isolate was taken for analysis and the followings were discarded. One investigator of each hospital reviewed the patients' medical records for demographic characteristics, infection-related clinical data, morbidities and underlying illness. Charlson comorbidity index [19], and SOFA [20] score were calculated at the analyzing centre for the day of initial blood culture. Prognoses were followed-up until hospital discharge or at least for 30 days. All data were recorded on a pre-formatted spreadsheet and submitted via an email to the analyzing centre without monitoring. All blood isolates were transferred to the analyzing centre and the microbiological assessment was carried out in the laboratory. Performing the microbiological assessment was blinded to patient information.
Fig. 1.
Flowchart for the case selection of E. coli bloodstream infection for analysis. Number of E. coli blood isolates and the bed size are indicated by hospital with a line-association of the district served by either in gray or in black. BSI, bloodstream infection.
2.2. Definitions
A laboratory-confirmed E. coli BSI was defined as positive E. coli culture identified from one or more blood specimens [4]. HO infection was specified as the case that a patient admitted for ≥2 calendar days at hospitals including the previous health care facility before transfer at the day of initial blood culture. Early mortality was defined as all-cause mortality within 30 days from the initial blood culture. Critically ill patients were defined as those with SOFA score at ≥9. Non-susceptibility (NS) to a drug class was defined as NS in vitro to at least one drug in a class and the resistance phenotypes were classified as drug susceptible (DS, susceptible to all drugs tested), drug resistant (DR, NS to one or two drug classes) and multidrug resistance (MDR, NS to more than three drug classes but two) following Magiorakos et al. [21] with few modifications. Empirical antimicrobial therapy was defined as an initial therapy without any AST results of the causative pathogen, and definitive antimicrobial therapy as administration of revised antimicrobial regimen based on the AST results. Delayed definitive therapy was if revised antimicrobial regimen was administered after 72 h. Adequate antimicrobial therapy was if the treatment regimen included at least one antimicrobial drug active in vitro and both the dosage and route of administration were in conformity with current medical standards.
2.3. Microbiological assessment
Bacterial species of the collected isolates were identified by MALDI-TOF/MS using Bruker Biotyper™ system (Billerica, MA, USA). Strain typing was conducted by multilocus sequence typing (MLST) [22], and by phylogenetic typing [23] for every E. coli blood isolate and, for ST131 E. coli isolates, sublineage was assessed [24]. Antimicrobial susceptibility was tested by a disk diffusion method [25] for 18 drugs belonging to 12 drug classes, and the beta-lactamase genes were identified by PCR and direct sequencing using gene-specific primers (Appendix Table S1) [26]. Twenty-three virulence-associated genes associated with 12 virulence characteristics were searched for by PCR. Details of the microbiological assessment is available in the Appendix.
2.4. Statistical analysis
All statistical analyses were performed in SPSS statistics (version 23, IBM Corp., Armonk, NY, USA). Data were expressed as mean ± SD or number (%) as appropriate. Categorical variables were compared by Pearson's chi-square test or Fisher's exact test and continuous variables were compared by independent two sample t-test. Associations between early mortality and risk factors were evaluated by using univariate and multivariate Cox proportional hazard regression model. For multivariate analysis, variables having P value <0·1 on the univariate analysis were considered. To avoid multicollinearity, six multivariate Cox models were constructed. All tests of significance were two-tailed; P values <0·05 were considered to be significant. Proportional-hazards assumption was evaluated by including an interaction term of covariate*log-transformed time and by log-minus-log survival plots (Appendix Fig. S1). Six models for Cox proportional multivariate analysis were constructed considering both clinical importance and multicollinearity between variables evaluated by chi-square test and t-test (Appendix Fig. S2): model 1 including variables selected by stepwise selection method; model 2 to model 5 including fixed host variables and adaptable pathogen variables mandatorily one strain type and one other genotype devoid of multicollinearity; and model 6 including fixed host variables and adaptable pathogen variables and mandatorily a treatment variable devoid of multicollinearity (Appendix Figure).
2.5. Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Characteristics of enrolled patients
From six general hospitals, 1492 laboratory-confirmed E. coli BSI cases were enrolled: mean 248·7 ± 91·5 cases/hospital, ranged from 122 to 365 by hospital (Fig. 1). Among the patients with E. coli BSI, 39·7% (n = 592) were male and their age was mean 69·0 ± 15·6 years resulting in 69·7% (n = 1040) being ≥65 years old (Table 1). Of total, 19·4% (290/1492) cases were HO infection, 58·4% (n = 871) were inpatients and 18·4% of those (161/871) stayed in intensive care units (ICUs). SOFA scoring had a mean value of 3·6 ± 3·0 and 7·6% (n = 114) patients were critically ill (SOFA [9]. Charlson comorbidity index was mean 3·3 ± 1·8. Diabetes mellitus was the most observed underlying disease (15·4%, 231/1492), of which 15 with complications, and solid tumor (12·5%, n = 187) was followed. Around half (49.5%, n = 738) of the BSI cases were secondary infection confirmed by culture and the most identified primary site of infection was urinary tract (90.9%, 671/738).
Table 1.
Patient-associated factors related to early mortality in patients with E. coli BSI.
| Characteristics | Totala |
Survival |
Early deathb |
Pc |
|---|---|---|---|---|
| (n = 1492) | 1351 (90·5%) | 141 (9·5%) | ||
| Demographic information | ||||
| Sex (male) | 592 (39.7%) | 520 (38.5%) | 72 (51.1%) | 0.005 |
| Age (in years, mean ± SD) | 69·1 ± 15·7 | 69·0 ± 15·6 | 70·4 ± 15·8 | 0·287 |
| Hospital origin | 293 (19.6%) | 241 (17.8%) | 52 (36.9%) | <0.001* |
| Comorbid condition | ||||
| Inpatients | 871 (58.4%) | 773 (57.2%) | 98 (69.5%) | 0.005 |
| ICU inpatients | 161 (10.8%) | 116 (8.6%) | 45 (31.9%) | <0.001* |
| Polymicrobial infection | 86 (5.8%) | 73 (5.4%) | 13 (9.2%) | 0.084 |
| SOFA score (mean ± SD) | 3·6 ± 3·0 | 3·2 ± 2·8 | 7·1 ± 3·2 | <0·001* |
| Underlying diseased | ||||
| Charlson comorbidity index (mean ± SD) | 3·3 ± 1·8 | 3·3 ± 1·8 | 3·6 ± 1·8 | 0·095 |
| Cerebrovascular diseases | 36 (2.4%) | 34 (2.5%) | 2 (1.4%) | 0.571 |
| Peripheral vascular diseases | 29 (1.9%) | 29 (2.1%) | 0 (0%) | 0.104 |
| Myocardial infarction | 39 (2.6%) | 37 (2.7%) | 2 (1.4%) | 0.576 |
| Congestive cardiac insufficiency | 6 (0.4%) | 6 (0.4%) | 0 (0%) | >0.999 |
| Hemiplegia | 2 (0.1%) | 2 (0.1%) | 0 (0%) | >0.999 |
| Solid tumor | 187 (12.5%) | 164 (12.1%) | 23 (16.3%) | 0.180 |
| Lymphoma | 5 (0.3%) | 4 (0.3%) | 1 (0.7%) | 0.392 |
| Leukemia | 11 (0.7%) | 9 (0.7%) | 2 (1.4%) | 0.279 |
| Diabetes mellitus | 231 (14·6%) | 210 (15.5%) | 21 (14.9%) | 0·900 |
| Moderate or severe kidney diseases | 88 (5.9%) | 74 (5.5%) | 14 (9.9%) | 0.039 |
| Chronic liver diseases including cirrhosis | 49 (3.3%) | 40 (3%) | 9 (6.4%) | 0.043 |
| Chronic pulmonary diseases | 17 (1.1%) | 16 (1.2%) | 1 (0.7%) | >0.999 |
| Dementia | 22 (1.5%) | 20 (1.5%) | 2 (1.4%) | >0.999 |
| Ulcer | 11 (0.7%) | 10 (0.7%) | 1 (0.7%) | >0.999 |
| Primary site of infectionc | ||||
| Blood | 33 (2.2%) | 32 (2.4%) | 1 (0.7%) | 0.360 |
| Urinary tract | 671 (45.0%) | 627 (46.4%) | 44 (31.2%) | 0.001 |
| Biliary tract | 26 (1.7%) | 25 (1.9%) | 1 (0.7%) | 0.504 |
| Respiratory tract | 15 (1.0%) | 9 (0.7%) | 6 (4.3%) | 0.002 |
| Peritoneum | 12 (0.8%) | 7 (0.5%) | 5 (3.5%) | 0.003 |
| Wound | 11 (0.7%) | 9 (0.7%) | 2 (1.4%) | 0.279 |
| Otherse | 3 (0.2%) | 3 (0.2%) | 0 (0%) | >0.999 |
| Unknown | 744 (49.9%) | 659 (48.8%) | 85 (60.3%) | 0.010 |
| Laboratory data (mean ± SD)f | ||||
| WBC (103/μL) | 12·5 ± 10·9 | 12·3 ± 8·9 | 14·2 ± 22 | 0·038 |
| Hemoglobin (g/dL) | 11·4 ± 4·9 | 11·5 ± 5·1 | 10·1 ± 2·3 | 0·001 |
| Platelet (109/L) | 172·2 ± 94·2 | 177·2 ± 93·2 | 126·0 ± 91·8 | 0·001 |
| Total bilirubin (mg/dL) | 2·0 ± 3·6 | 1·8 ± 3·4 | 3·2 ± 5·0 | <0·001* |
| Creatinine clearance (mg/dL) | 1·5 ± 3·1 | 1·5 ± 3·2 | 1·9 ± 2·1 | 0·164 |
| Prothrombin time (seconds) | 14·5 ± 5·9 | 14·2 ± 5·6 | 18·6 ± 7·1 | 0·225 |
SD, standard deviation; WBC, white blood cells.
The percentage was calculated out of total number of E. coli BSI cases.
All-cause mortality within 30 days from the initial blood culture.
P value is either from Pearson's chi-square test for categorical data or from t-test for continuous data. Bonferroni corrected significance level was considered as P < 0.001 and the significant P values are indicated with asterisks.
Underlying disease and primary site of infection could be multiple by patient.
Cervix (n = 2) and eye (n = 1) were included.
Five of total bilirubin data, two of creatinine clearance data, and ten of prothrombin time data were absent.
3.2. Characteristics of E. coli blood isolates
3.2.1. Strain type
E. coli blood isolates were characterized as described in the Appendix. More than half of the isolates belonged to the phylogenetic group B2 (55·8%, 833/1492) and the remaining was composed with either the group D (24·7%, n = 368) or A (19·4%, n = 289) (Table 2) with exceptional two isolates (0·1%) of the group B1. Among the identified 338 STs including 212 known-STs and 126 not-yet-numbered STs, ST131 was prevalent (24·1%, 359/1492) followed by ST95 (9·6%, n = 143), ST69 (8·6%, n = 128), ST1193 (6·4%, n = 96), ST73 (4·6%, n = 69), and ST38 (3·8%, n = 56). The ST131-sublineages were comprised with non-H30 (5·3%, 19/359), H30 (11·1%, 40/359), H30R (33·7%, 121/359), and H30Rx (49·9%, 179/359).
Table 2.
Pathogen-associated factors related to early mortality in patients with E. col BSI.
| Characteristics | Totala |
Survival |
Early deathb |
Pc |
|---|---|---|---|---|
| (n = 1492) | 1351 (90·5%) | 141 (9·5%) | ||
| Strain type | ||||
| Phylogenetic group A | 289 (19·4%) | 275 (20·4%) | 14 (9·9%) | 0·002 |
| B2 | 833 (55·8%) | 741 (54·8%) | 92 (65·2%) | 0·020 |
| D | 368 (24·7%) | 333 (24·6%) | 35 (24·8%) | 0·999 |
| ST131 | 359 (24·1%) | 310 (22·9%) | 49 (34·8%) | 0·003 |
| Non-H30 | 19 (1·3%) | 17 (1·3%) | 2 (1·4%) | 0·699 |
| H30 | 40 (2·7%) | 34 (2·5%) | 6 (4·3%) | 0·264 |
| H30R | 121 (8·1%) | 108 (8·0%) | 13 (9·2%) | 0·626 |
| H30Rx | 179 (12·0%) | 151 (11·2%) | 28 (19·9%) | 0·004 |
| ST95 | 143 (9·6%) | 135 (10%) | 8 (5·7%) | 0·131 |
| ST69 | 128 (8·6%) | 117 (8·7%) | 11 (7·8%) | 0·874 |
| ST1193 | 96 (6·4%) | 83 (6·1%) | 13 (9·2%) | 0·151 |
| ST73 | 69 (4·6%) | 63 (4·7%) | 6 (4·3%) | >0·999 |
| ST38 | 56 (3·8%) | 53 (3·9%) | 3 (2·1%) | 0·359 |
| Antimicrobial resistance categoryd | ||||
| DS | 330 (22·1%) | 306 (22·6%) | 24 (17·0%) | 0·136 |
| DR | 297 (19·9%) | 277 (20·5%) | 20 (14·2%) | 0·077 |
| MDR | 865 (58·0%) | 768 (56·8%) | 97 (68·8%) | 0·007 |
| Non-susceptible toe | ||||
| Penicillins | 1024 (68·6%) | 918 (67·9%) | 106 (75·2%) | 0·086 |
| Penicillins/beta-lactamase inhibitors | 546 (36·6%) | 476 (35·2%) | 70 (49·6%) | 0·001 |
| 1st-generation cephalosporins | 987 (66·2%) | 885 (65·5%) | 102 (72·3%) | 0·112 |
| 3rd/4th-generation cephalosporins | 524 (35·1%) | 457 (33·8%) | 67 (47·5%) | 0·002 |
| Monobactams | 402 (26·9%) | 349 (25·8%) | 53 (37·6%) | 0·004 |
| Cephamycins | 77 (5·2%) | 69 (5·1%) | 8 (5·7%) | 0·692 |
| Carbapenems | 3 (0·2%) | 3 (0·2%) | 0 (0%) | 0·999 |
| Aminoglycosides | 463 (31·0%) | 409 (30·3%) | 54 (38·3%) | 0·056 |
| Fluoroquinolones | 590 (39·5%) | 521 (38·6%) | 69 (48·9%) | 0·019 |
| Sulfonamides | 494 (33·1%) | 445 (32·9%) | 49 (34·8%) | 0·707 |
| Glycylcyclines | 3 (0·2%) | 3 (0·2%) | 0 (0%) | >0·999 |
| Polymyxins | 3 (0·2%) | 3 (0·2%) | 0 (0%) | >0·999 |
| Resistance determinant | ||||
| CTX-M ESBL | 463 (31·0%) | 403 (29·8%) | 60 (42·6%) | 0·003 |
| Group 1 | 254 (17·0%) | 216 (16·0%) | 38 (27·0%) | 0·002 |
| Group 9 | 225 (15·1%) | 202 (15·0%) | 23 (16·3%) | 0·710 |
| Plasmid-mediated AmpC | 37 (2·5%) | 32 (2·4%) | 5 (3·5%) | 0·388 |
| Carbapenemase | 1 (0·1%) | 0 (0%) | 1 (0·7%) | 0·095 |
| Virulence determinants | ||||
| No· of virulence determinantsf (mean ± SD) | 3·3 ± 2·4 | 3·3 ± 2·4 | 3·9 ± 2·5 | 0·004 |
| Haemolysis | ||||
| hlyA, haemolysin A | 107 (7·2%) | 92 (6·8%) | 15 (1·1%) | 0·120 |
| hlyE, avian E. coli haemolysin | 52 (3·5%) | 45 (3·3%) | 7 (5·0%) | 0·329 |
| Toxins | ||||
| sat, serine protease autotransporter | 332 (22·3%) | 293 (21·7%) | 39 (27·7%) | 0·111 |
| vat, vacuolating autotransporter | 298 (20·0%) | 261 (19·3%) | 37 (26·2%) | 0·059 |
| astA, heat-stable enterotoxin | 34 (2·3%) | 29 (2·1%) | 5 (3·5%) | 0·245 |
| Siderophore | ||||
| ireA, siderophore receptor | 193 (12·9%) | 176 (13·0%) | 17 (12·1%) | 0·895 |
| iro, siderophore esterase | 184 (12·3%) | 165 (12·2%) | 19 (13·5%) | 0·686 |
| fyuA, yersiniabactin | 421 (28·2%) | 366 (27·1%) | 55 (39·0%) | 0·004 |
| iutA, ion uptake system | 120 (8·0%) | 105 (7·8%) | 15 (10·6%) | 0·253 |
| Cytotoxicity | ||||
| ETTT, type III secretion system | 295 (19·8%) | 268 (19·8%) | 27 (19·1%) | 0·912 |
| Colibactin, genotoxic metabolite | 240 (16·1%) | 215 (15·9%) | 25 (17·7%) | 0·549 |
| cnf1, cytotoxic necrotizing factor | 173 (11·6%) | 151 (11·2%) | 22 (15·6%) | 0·128 |
| Bacteriocin | ||||
| cvaC, colicin V | 82 (5·5%) | 78 (5·8%) | 4 (2·8%) | 0·175 |
| Serum resistance | ||||
| traT, outer membrane protein | 870 (58·3%) | 790 (58·5%) | 80 (56·7%) | 0·720 |
| Invasion | ||||
| afa, invasion protein | 80 (5·4%) | 66 (4·9%) | 14 (9·9%) | 0·017 |
| ibeA, brain microvascular endothelial cell invasion | 68 (4·6%) | 61 (4·5%) | 7 (5·0%) | 0·831 |
| ompT, outermembrane protease T | 263 (17·6%) | 238 (17·6%) | 25 (17·7%) | >0·999 |
| Encapsulation | ||||
| kpsMII, capsule synthesis | 142 (9·5%) | 123 (9·1%) | 19 (13·5%) | 0·097 |
| Adhesion | ||||
| papA, P fimbriae | 28 (1·9%) | 25 (1·9%) | 3 (2·1%) | 0·743 |
| sfa/foc, S fimbriae minor subunit | 108 (7·2%) | 92 (6·8%) | 16 (11·3%) | 0·059 |
| Others | ||||
| usp, uropathogenic protein | 681 (45·6%) | 601 (44·5%) | 80 (56·7%) | 0·006 |
| PAI, pathogenic island marker | 83 (5·6%) | 74 (5·5%) | 9 (6·4%) | 0·698 |
| pic, protease involved in intestinal colonization | 103 (6·9%) | 96 (7·1%) | 7 (5·0%) | 0·483 |
SD, standard deviation.
The percentage was calculated out of total number of E. coli blood isolates.
All-cause mortality within 30 days from the initial blood culture.
P value is either from Pearson's chi-square test for categorical data or from t-test for continuous data. Bonferroni corrected significance level was considered as P < 0.0009 and no variable was statistically significant.
Isolates categorized as XDR and PDR were not identified. DS, drug susceptible; DR, drug resistant; MDR, multi-drug resistant; XDR, extensively drug resistant; PDR, pan-drug resistant [21].
Ampicillin and piperacillin for penicillins; amoxicillin-sulbactam for penicillins/beta-lactamase inhibitors; cephazolin for 1st-generation cephalosporins; cefotaxime, ceftazidime, and cefepime for 3rd/4th-generation cephalosporins; cefoxitin for cephamycins; aztreonam for monobactam; imipenem, meropenem, and ertapenem for carbapenems; gentamicin and amikacin for aminoglycosides; ciprofloxacin for fluoroquinolone; trimethoprim-sulfamethoxazole for sulfonamides; tigecycline for glycylcyclines; colistin for polymyxins.
Number of identified virulence determinants among the 23 genes tested.
3.2.2. Antimicrobial resistance
More than half of the E. coli blood isolates were MDR (58·0%, 865/1492) and the rest was either DR (19·9%, n = 297) or DS (22·1%, n = 330) (Table 2, Appendix). Of total, 31·0% (463/1492) E. coli blood isolates harboured the blaCTX-M gene: 54·9% (254/463) were group 1 blaCTX-M gene and 48·9% (225/460) were group 9 blaCTX-M. Sixteen isolates including 13 ST131-sublineage H30Rx, one ST38, one ST196, and one ST1456 co-carried both genes for groups 1 and 9. Less frequently, 2·5% (34/1492) isolates harboured genes for plasmid-mediated AmpC, and three isolates overexpressed the chromosomal ampC gene. One isolate carried the blaKPC-2 gene.
3.2.3. Virulence determinants
Among the 23 virulence genes tested, mean 3·3 ± 2·4 were identified per isolate (Table 2). The most identified gene was traT (58·3%, n = 870), and usp (45·6%, n = 681), followed by fyuA (28·2%, n = 421), sat (22·3%, n = 332), vat (20·0%, n = 298), ETTT (19·8%, n = 295), ompT (17·6%, n = 263) and colibactin (16·1%, n = 240).
3.2.4. Clonal traits
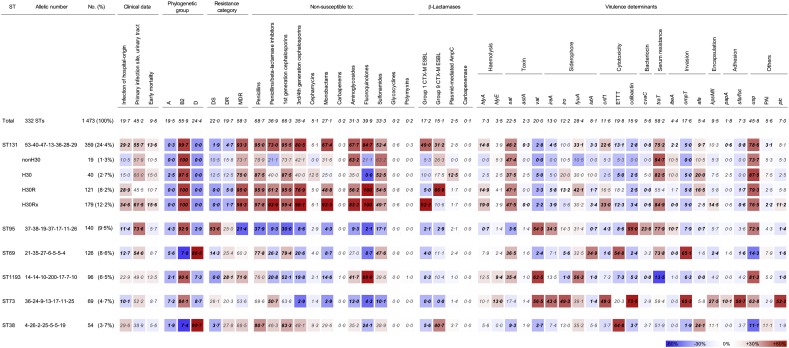
Clonal traits of top six predominant STs, ST131, ST95, ST69, ST1193, ST73, and ST38, covering 57·3% (n = 844) isolates were presented in Fig. 2. The six clones largely belonged either to the phylogenetic group B2 (ST131, ST95, ST1193, and ST73) or D (ST69, and ST38).
Fig. 2.
Genotypic and phenotypic diversity of E. coli blood isolates by six most strain types. The proportion of isolates having the geno/phenotype is indicated and statistical significance (P < 0.05) determined by Pearson's chi-square test is in bold. A smaller proportion compared to that of total is indicated in blue-gradients and a greater proportion than that of total is indicated in red-gradients. ST, sequence type; DS, drug-susceptible; DR, drug-resistant; MDR, multidrug resistance; ESBL, extended-spectrum beta-lactamase.
The ST131 was frequently associated with HO (29·2%, 205/359), especially the sublineages H30R (28·9%, 35/121), and H30Rx (34·6%, 62/179) were. Proportion of MDR showed gradual increase by sublineages as 73·7% (14/19) non—H30, 75·0% (30/40) H30, 95·0% (115/121) H30R, and 98·3% (176/179) H30Rx. Most of the ST131 carried the group 1 (49·0%, 179/359) and/or the group 9 blaCTX-M genes (31·2%, 112/359): 66·9% (82/121) H30R with the group 9, and 92·2% (165/179) H30Rx with the group 1 blaCTX-M genes. Besides, relatively a greater proportion of H30 (12·5%, 5/40) harboured the blaAmpC genes. The ST131 carried the hlyA (14·8%, 53/359), sat (46·2%, n = 166), fyuA (33·1%, n = 119), cnf1 (22·6%, n = 81), traT (75·2%, n = 270), afa (9·7%, n = 35), and usp (78·6%, n = 282) virulence genes more frequently than other STs.
The second most ST95 was associated exceedingly with primary UTI (73·6%, 103/140) and majority of the clone was DS (53·6%, n = 75). For the third most ST69, a half of the isolates were linked with primary UTI (54·0%, 68/126). Two thirds of the ST1193 were MDR (71·9%, 69/96) and the ST73 possessed much more genes for virulence (mean, 7·1 ± 3·7) compared to the other STs. Finally, ST38 frequently carried the group 9 blaCTX-M gene (40·7%, 22/54).
3.3. Characteristics of antimicrobial regimen
3.3.1. Antimicrobial regimen for empirical therapy
Among the 1473 antimicrobial treatment follow-up cases, 1443 patients (98·0%) received empirical antimicrobial therapy for Gram-negative bacteria and 1142 of those were adequate (Table 3). The other 30 patients took either not-for-Gram-negative antimicrobial therapies (n = 29) or none by refusal (n = 1). More than half of the patients were treated with 3rd/4th-generation cephalosporins (55·6%, n = 819), and 20·0% (n = 295) with fluoroquinolones, followed by carbapenems (14·0%, n = 206), penicillins/beta-lactamase inhibitors (12·9%, n = 190), penicillins (2·4%, n = 35) and 1st-generation cephalosporins (1·0%, n = 14). The rate of adequacy for antimicrobial treatment was greater in combination therapy (90·2%, 138/153) than in monotherapy (81·2%, 1042/1284). Exceptionally, choice of carbapenems, which all but three E. coli isolates were susceptible to, was always adequate for treatment whether they used alone or in combination.
Table 3.
Empirical antimicrobial therapy for the characteristic patients with E. coli BSIa.
| Antimicrobial choice for empirical therapy | Total |
Early mortality |
Adequate treatment |
Critically illb |
ICU inpatients |
HO infection |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 1473 | n = 141 | Pc | n = 1142 | Pc | n = 111 | Pc | n = 161 | Pc | n = 290 | Pc | ||
| Penicillins | Monotherapy | 31 (2·1%) | 4 (2.8%) | 0·531 | 8 (0.7%) | <0·001* | 5 (4.5%) | 0·078 | 14 (8.7%) | <0·001* | 14 (4.8%) | <0·001* |
| Combination therapy | 4 (0·3%) | 1 (0.7%) | 0·332 | 3 (0.3%) | 1·000 | 0 (0%) | 1·000 | 4 (2.5%) | <0·001* | 1 (0.3%) | 0·584 | |
| Penicillins/beta-lactamase inhibitors | Monotherapy | 161 (10·9%) | 22 (15.6%) | 0·066 | 147 (12.9%) | <0·001* | 19 (17.1%) | 0·039 | 25 (15.5%) | 0·060 | 5 (1.7%) | 0·528 |
| Combination therapy | 29 (2·0%) | 7 (5·0%) | 0·016 | 27 (2.4%) | 0·043 | 6 (5.4%) | 0·018 | 4 (2.5%) | 0·549 | 9 (3.1%) | 0·153 | |
| 1st-generation cephalosporins | Monotherapy | 7 (0·5%) | 0 (0%) | 1·000 | 6 (0.5%) | 1·000 | 0 (0%) | 1·000 | 1 (0.6%) | 0·556 | 4 (1.4%) | 0·031 |
| Combination therapy | 7 (0·5%) | 1 (0.7%) | 0·506 | 7 (0.6%) | 0·360 | 1 (0.9%) | 0·423 | 0 (0%) | 1·000 | 2 (0.7%) | 0·630 | |
| 3rd/4th-generation cephalosporins | Monotherapy | 747 (50·7%) | 49 (34.8%) | <0·001* | 572 (50.1%) | 0·383 | 38 (34.2%) | <0·001* | 68 (42.2%) | 0·024 | 116 (40·0%) | <0·001* |
| Combination therapy | 72 (4·9%) | 9 (6.4%) | 0·408 | 60 (5.3%) | 0·250 | 11 (9.9%) | 0·019 | 6 (3.7%) | 0·565 | 20 (6.9%) | 0·093 | |
| Carbapenems | Monotherapy | 165 (11·2%) | 25 (17.7%) | 0·016 | 165 (14.4%) | <0·001* | 25 (22.5%) | <0·001* | 22 (13.7%) | 0·290 | 39 (13.4%) | 0·178 |
| Combination therapy | 41 (2·8%) | 10 (7.1%) | 0·004 | 41 (3.6%) | <0·001* | 2 (1.8%) | 0·764 | 3 (1.9%) | 0·614 | 8 (2.8%) | >0·999 | |
| Fluoroquinolones | Monotherapy | 165 (11·2%) | 4 (2.8%) | <0·001* | 98 (8.6%) | <0·001* | 2 (1.8%) | <0·001* | 7 (4.3%) | <0·001* | 26 (9.0%) | 0·212 |
| Combination therapyd | 130 (8·8%) | 25 (17.7%) | <0·001* | 122 (10.7%) | <0·001* | 15 (13.5%) | 0·081 | 11 (6.8%) | 0·461 | 30 (10.3%) | 0·300 | |
| Otherse | 8 (0·5%) | 5 (3.5%) | <0·001* | 5 (0.4%) | 0·389 | 1 (0.9%) | 0·467 | 3 (1.9%) | 0·047 | 7 (2.4%) | <0·001* | |
| Not-for-Gram-negatives | 29 (2·0%) | 2 (1.4%) | – | 0 (0%) | – | 1 (0.9%) | – | 3 (1.9%) | – | 8 (2.8%) | – | |
| Refusal of antimicrobial therapy | 1 (0·1%) | 0 (0%) | – | 0 (0%) | – | 0 (0%) | – | 1 (0.6%) | – | 0 (0%) | – | |
ICU, intensive care unit; HO, hospital-origin.
Nineteen cases of the follow-up-loss before 2nd day of the treatment were excluded from the analysis. The percentage was calculated out of total number of E. coli BSI cases.
Critically ill patients were defined as those with SOFA score at ≥9.
P value is from Pearson's chi-square test. Bonferroni corrected significance level was considered as P < 0.003 and the significant P values are indicated with asterisks.
Combination fluoroqniolone therapy was always with beta-lactams.
Glycylcyclines (n = 4), rifampicin (n = 2), cephamycins (n = 1), and sulfonamides (n = 1) were included.
Increased preference of both carbapenems monotherapy (22·5%, 25/111), and penicillins/beta-lactamase inhibitors monotherapy (17·1%, n = 19) were notable for the critically ill patients. On the contrary, fluoroquinolones monotherapy (1·8%, n = 2), and 3rd/4th-generation cephalosporines monotherapy (34·2%, n = 38) were less preferred options targeting on those patients than those for total. For ICU inpatients (n = 161), both fluoroquinolones monotherapy (4·3%, n = 7), and 3rd/4th-generation cephalosporines monotherapy (42·2%, n = 68) were also less favored. Preference of 3rd/4th-generation cephalosporines was dropped from 55.6% (819/1473) of total to 40·0% (116/290) targeting HO infection.
3.3.2. Antimicrobial regimen for definitive therapy
Among 331 patients taking inadequate empirical therapy, 56·8% (n = 188) were treated with definitive antimicrobial therapy within 72 h. Carbapenems (70·7%, monotherapy:combination therapy = 130:3) were a leading choice for the revision and the followings were 3rd/4th-generation cephalosporins (14·9%, monotherapy:combination therapy = 26:2), penicillins/beta-lactamase inhibitor (10·6%, monotherapy:combination therapy = 14:5), and fluoroquinolones (9·0%, monotherapy:combination therapy = 8:9).
3.4. Risk factors for early mortality in patients with E. coli BSI
3.4.1. Factors associated with early mortality in patients with E. coli BSI
Overall early mortality was observed in 9·5% (141/1492) cases. Comparison of the demographic data from all survival and early death patients are summarized in Table 1. Age distribution was similar between survival and early death groups, while patients with early death than those of survival were more likely to be male (51·1% vs. 38·5%, P = 0.005). A greater percentage of patients with early death were associated with HO infection (36·9% vs. 17·8%, P < 0.001), hospitalization (69·5% vs. 57·2%, P = 0.005) and ICU hospitalization (31·9% vs. 8·6%, P < 0.001). Patients with early death were more likely to have moderate or severe kidney diseases (9·9% vs. 5·5%, P = 0.039), chronic liver diseases including cirrhosis (6·4% vs. 3·0%, P = 0·043), and primary infection of peritoneum (3·5% vs. 0·5%, P = 0.003), while those were less likely than survivals to be associated with primary UTI (31·2% vs. 46·4%, P = 0.001).
Comparison of the microbiological data for causative E. coli blood isolates from patient groups of survival and early death are in Table 2. A higher percentage of patients with early death were with MDR E. coli (68·8% vs. 56·8%, P = 0.007) and CTX-M ESBL-producing E. coli (42·6%, vs. 29·8%, P = 0.003), especially group 1 CTX-M producers (27·0% vs. 16·0%, P = 0.002). A greater percentage of patients with early death were with E. coli harboring the fyuA gene for yersiniabactin (39·0% vs. 27·1%, P = 0.004), the afa gene for invasion protein (9·9% vs. 4·9%, P = 0·017), and the usp gene for uropathogenic protein (56·7% vs. 44·5%, P = 0.006). Although patients with early death were more likely to have E. coli carrying the vat gene for vacuolating autotransporter (26·2% vs. 19·3%) and the sfa/foc gene for S fimbriae (11·3% vs. 6·8%), the difference did not reach statistical significance (P = 0.059 for both).
Adequacy of empirical antimicrobial treatment had no critical impact on early mortality (Table 3): 9·5% (108/1142) patients treated with adequate empirical therapy and 10·0% (33/331) patients treated with inadequate antimicrobials expired within 30 days. Definitive therapy within 72 h was rather critical to early mortality: 9·2% (13/188) patients taking punctual definitive therapy and 14·2% (20/143) patients treated by delayed definitive therapy expired within 30 days. Even so, adequacy of empirical antimicrobial treatment and punctuality of definitive treatment were neither the risk nor the protective factors for early mortality through the analysis using Cox regression model and only the delayed definitive treatment had the risk tendency of 1·54 [1·96–2·48] with P = 0·073 (Appendix Fig. S2, Table 4).
Table 4.
Uni- and multivariate analysis by Cox-regression model for early mortality in patients with E. col BSIa.
| Variables for | Univariate analysis |
Multivariate model 1 |
Multivariate model 2 |
Multivariate model 3 |
Multivariate model 4 |
Multivariate model 5 |
Multivariate model 6 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | P | HR [95% CI] | P | VIF | HR [95% CI] | P | VIF | HR [95% CI] | P | VIF | HR [95% CI] | P | VIF | HR [95% CI] | P | VIF | HR [95% CI] | P | VIF | |
| Host | ||||||||||||||||||||
| Critically ill | 6·78 [4·75–9·69] | <0·001 | 5·85 [4·06–8·42] | <0·001* | 1·01 | 5·76 [3·98–8·35] | <0·001* | 1·02 | 5·67 [3·92–8·21] | <0·001* | 1·02 | 5·55 [3·82–8·05] | <0·001* | 1·02 | 5·72 [3·94–8·29] | <0·001* | 1·02 | 5·99 [4·13–8·68] | <0·001* | 1·02 |
| Polymicrobial infection | 1·72 [0·97–3·05] | 0·061 | 1·63 [0·91–2·89] | 0·098 | 1·01 | 1·57 [0·87–2·84] | 0·137 | 1·01 | 1·58 [0·87–2·85] | 0·130 | 1·01 | 1·49 [0·83–2·68] | 0·185 | 1·01 | 1·53 [0·84–2·77] | 0·164 | 1·01 | 1·53 [0·85–2·76] | 0·159 | 1·01 |
| Primary infection of peritoneum | 4·82 [1·98–11·78] | <0·001 | 2·80 [1·10–7·15] | 0·031 | 1·01 | 2·85 [1·13–7·18] | 0·026 | 1·01 | 3·04 [1·21–7·69] | 0·018 | 1·01 | 2·88 [1·12–7·42] | 0·028 | 1·01 | 3·21 [1·27–8·11] | 0·013 | 1·01 | 3·02 [1·20–7·63] | 0·019 | 1·01 |
| Primary infection of urinary tract | 0·54 [0·38–0·77] | <0·001 | 0·56 [0·39–0·81] | <0·001* | 1·03 | 0·55 [0·38–0·79] | 0·001* | 1·07 | 0·55 [0·38–0·79] | <0·001* | 1·04 | 0·59 [0·41–0·85] | 0·005* | 1·03 | 0·56 [0·39–0·81] | 0·002* | 1·03 | 0·61 [0·42–0·87] | 0·007* | 1·03 |
| Moderate to severe kidney disease | 1·81 [1·04–3·14] | 0·037 | 1·24 [0·69–2·22] | 0·465 | 1·02 | 1·18 [0·66–2·12] | 0·582 | 1·03 | 1·19 [0·66–2·15] | 0·553 | 1·02 | 1·10 [0·61–1·98] | 0·746 | 1·03 | 1·08 [0·59–1·96] | 0·802 | 1·03 | |||
| Chronic liver disease incl. Cirrhosis | 1·80 [1·04–3·13] | 0·027 | 2·09 [1·06–4·12] | 0·033 | 1·00 | 2·11 [1·06–4·20] | 0·033 | 1·02 | 1·86 [0·93–3·71] | 0·080 | 1·02 | 1·97 [0·99–3·94] | 0·055 | 1·02 | 1·97 [0·99–3·94] | 0·053 | 1·02 | 1·84 [0·91–3·73] | 0·089 | 1·02 |
| White blood cells | 1·01 [1·00–1·02] | 0·020 | 1·01 [1.00–1·02] | 0·148 | 1·01 | 1·01 [1·00–1·01] | 0·167 | 1·02 | 1·01 [1·00–1·02] | 0·072 | 1·01 | 1·00 [1·00–1·01] | 0·328 | 1·02 | 1·01 [1·00–1·01] | 0·157 | 1·02 | |||
| Pathogen | ||||||||||||||||||||
| Phylogenetic group B2 | 1·50 [1·06–2·12] | 0·022 | 1·69 [1·18–2·42] | 0·004* | 1·06 | |||||||||||||||
| ST131-sublineage H30Rx | 1·89 [1·25–2·86] | 0·003 | 2·09 [1·37–3·20] | <0·001* | 1·02 | |||||||||||||||
| MDR | 1·62 [1·13–2·31] | 0·008 | 1·59 [1·10–2·29] | 0·014 | 1·02 | |||||||||||||||
| Group 1 CTX-M ESBL | 1·87 [1·29–2·71] | <0·001 | 2·05 [1·40–3·01] | <0·001* | 1·02 | 1·94 [1·33–2·84] | <0·001* | 1·02 | ||||||||||||
| fyuA for siderophore | 1·65 [1·18–2·32] | 0·004 | 1·53 [1·08–2·16] | 0·020 | 1·02 | 1·61 [1·14–2·27] | 0·007* | 1·01 | 1·60 [1·14–2·26] | 0·007* | 1·01 | 1·59 [1·13–2·25] | 0·008* | 1·01 | ||||||
| afa for invasion | 1·99 [1·15–3·45] | 0·015 | 2·21 [1·27–3·86] | 0·005* | 1·01 | 2·11 [1·19–3·73] | 0·010 | 1·01 | 2·08 [1·17–3·67] | 0·012 | 1·01 | 2·03 [1·14–3·60] | 0·016 | 1·01 | 2·03 [1·14–3·60] | 0·015 | 1·01 | |||
| sfa/foc for adhesion | 1·65 [0·98–2·77] | 0·060 | 1·65 [0·95–2·87] | 0·074 | 1·03 | 1·63 [0·95–2·79] | 0·075 | 1·01 | ||||||||||||
| Treatment | ||||||||||||||||||||
| Delayed definitive therapy | 1·54 [0·96–2·48] | 0·073 | 1·58 [0·97–2·55] | 0·063 | 1·00 | |||||||||||||||
HR, hazard ratio; CI, confidence interval; VIF, variance inflation factor; ESBL, extended-spectrum beta-lactamase; ST, sequence type.
Multivariate analysis model 1 was constructed by a stepwise selection procedure and the other five from model two to model six were taken including fixed host factors, and flexible pathogen and treatment variables: phylogenetic group B2 and afa in model 2; ST131 sublineage H30Rx, fyuA and afa in model 3; MDR, and afa/foc in model 4, group 1 CTX-M ESBL production, fyuA and afa in model 5, and pathogen variables fyuA, and afa and a treatment variable. Bonferroni corrected significance level was considered as P < 0.008 and the significant P values are indicated with asterisks.
3.4.2. Host-pathogen-treatment tripartite risk factors for early mortality by six multivariate analysis models
In accordance with statistical specification, clinical importance, and multicollinearity between variables, six multivariate models were constructed (Table 4). The model 1 constructed by a stepwise selection method perceived host- and pathogen-associated risk factors, i.e., critically ill (5·85 [4·06–8·42]), primary infection of peritoneum (2·80 [1·10–7·15]) and chronic liver diseases including cirrhosis (2·09 [1·06–4·12]) for patient factors; and group 1 CTX-M ESBL (2·05 [1·40–3·01]), fyuA (1·53 [1·08–2·16]) and afa (2·21 [1·27–3·86]) for pathogen factors. Primary infection of urinary tract (0·56 [0·39–0·81]) was the one protective factor associated with patients. The multivariate models 2 to 6 constructed through clinical importance, and multicollinearity discerned risk factors associated with the tripartite component: critically ill, primary infection of peritoneum, and chronic liver disease including cirrhosis among host variables; and phylogenetic group B2, ST131-sublineage H30Rx, MDR, group 1 CTX-M ESBL, fyuA and afa among pathogen variables. A pathogen variable sfa/foc and a treatment variable delayed definitive therapy displayed a risk tendency.
4. Discussions
This prospective observational study for E. coli BSI cases was undertaken to evaluate the risk factors through an organic alliance of host-pathogen-treatment tripartite components. Many variables were difficult to place in an analysis panel for a single multivariate model since multicollinearity between variables could underestimate the risks of each variable. Through fine estimation of the multicollinearity, six multivariate models were constructed by taking into account the statistical specification, clinical importance, as well as intra- and inter-component multicollinearities. Those delicate statistical models for multivariate analysis allowed us to examine the risk factors in accordance to the host-pathogen-treatment tripartite components. However, the multiple comparisons could result in false discoveries in the statistical models and multiple corrections were needed through a conservative correction test. The Bonferroni correction test, which set the significance cut-off at P < 0.008, disclosed critically ill and primary infection of urinary tract for host-associated risk and protective factors, respectively; phylogenetic group B2, group 1 CTX-M ESBL, fyuA, and afa for pathogen-associated risk factors; and none for the treatment-associated factor (Table 4).
Prior reports constantly denoted that E. coli ST131 is a worrisome clone having an outstanding risk for extra-intestinal infections including BSI. However, those have mostly been limited to a single-centre study [2], a restricted origin of infection [27], collection of incidence occurring in a very limited period [28], and incomplete strain typing for E. coli isolates [28], and consequently, the results were debatable. This one-year multi-centre prospective study conducting balanced complete research allowed to highlight the ST131-sublineage H30Rx for more association with HO infection, primary UTI, and early mortality, along with its renowned MDR (Fig. 2). Higher early mortality marked in the H30Rx clone (15·6%) was in conflict with the higher proportion of primary UTI, a protective factor against early mortality. The peculiar proportion of risk factors, i.e., MDR (98·3%), and group 1 CTX-M production (92·2%) in H30Rx clone could explain the high early mortality rate. The high proportion of H30Rx (49·9% ST131) in this study should be noted. Getting greater identification of ST131-sublineage H30Rx causative for BSI is a global issue including Canada [29], and Turkey [30]. By reason of its weighty risk for early mortality, the dissemination of the clone needs to be extensively scrutinized.
The first and second dominant ST131 and ST95 clones, both in part of the phylogenetic group B2, presented interesting contrasts. The ST131 was mostly MDR (93·3%), frequently produced groups 1 and 9 CTX-M ESBLs (80·2%), associated with HO infections (29·2%), and highly linked with secondary BSI from UTI (55·7%). Distinctly from the ST131 clone, the ST95 was mostly DS (53·6%), rarely produced beta-lactamases with extended-spectrum hydrolytic activities, associated infrequently with HO infection (11·4%), and even more linked with primary UTI (73·6%). The most curious trait of both clones was the resulting early mortality, 13·6% in ST131, and 5·7% in ST95 which could be led by notable differences in drug resistance, and beta-lactamase production.
As often reported not only for the E. coli BSI but also for the BSIs by other bacterial pathogens [15,31], the prompt definitive antimicrobial therapy ameliorated the prognosis of BSI and the combination antimicrobial therapy improved adequacy of antimicrobial treatment. Both calls attention to the antimicrobial stewardship for BSI treatment. It was impractical to point out the choice of specific antimicrobials associated with early mortality, because the antimicrobial choice was frequently dependent on the patients by their conditions. For instance, carbapenems, of which resistance was found in only three isolates, were adequate choices for all cases. However, a high rate of early mortality was noted at 17·0% (35/206) in patients taking carbapenems which must be associated with its more usage for critically ill and ICU inpatients (Table 3). Lack of significance in correlation between adequate antimicrobial treatment and early mortality could be derived from the habitual choice of antimicrobial regimen depending on the patient's condition.
This delicate prognosis assessment for patients with E. coli BSI accounting the host-pathogen-treatment tripartite component highlighted the importance of i) enough review of clinical information, ii) timely definitive therapy in accordance with the AST results, iii) strain typing and iv) virulence typing in addition to the routine laboratory testing. To be more informative for the E. coli BSI, an enriched panel of routine examination in clinical microbiology laboratories including strain typing and virulence typing for pathogens should be considered. For instance, the results from MLST and virulence typing come out with that of AST will allow more feasible antimicrobial regimen for definitive therapy. For sure, the current study itself cannot provide a clear guide to improve the prognosis of E. coli BSI however, it provided a fundamental idea to develop a prospective surveillance and concurrent intervention for the antimicrobial stewardship plans. Moreover, a national guidance for the management of E. coli BSI would be available out of overall efforts.
This study has several limitations. Primarily, limited races were included. The South Korean population is composed with relatively homogeneous East Asians. We did not even collect the human race data because all but few would be East Asians. Thus, the analyzed data could be restricted by human race. Secondly, only six out of 10 districts in South Korea were covered. Considering population difference by district, over 70% of South Korean population was able to be dealt with. However, the remaining 30% population was out of consideration and one of the four districts has an absolute climate difference. Thirdly, the participating hospitals had limited numbers of hospital beds. Numbers and ratio of in- and outpatients are varied by the number of hospital beds, and the participating hospitals had 500 to 1000 beds. Finally, the study was conducted for one year, which is too short to show sufficient dynamics of the BSI.
In a basis of the results, a scoring system for estimating the prognosis of E. coli BSI could be further schemed out. The SOFA scoring system is practical and trustful to predict the outcomes of patients. However, the system only manipulates host-associated factors. Further assessing the BSI cases caused by other bacterial species could impart systematic knowledge for bacterial BSI.
Funding sources
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2017E4400100#). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
All authors declare no competing interests.
Author contributions
EJY, and SHJ designed the study. EJY, MHC, HSL, and SHJ analyzed the data. EJY, and SHJ wrote the manuscript. YSP, and SHJ interpreted clinical importance. MHC, DK, HL, KSS, JHS [5], YU, YAK, JHS [8], and SHJ collected the clinical data and bacterial isolates. All authors have seen and approved the final version of the report.
Acknowledgements
We thank Hana Yu, and Yena Oh for technical support for microbiological experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.08.029.
Appendix A. Supplementary data
Supplementary material
References
- 1.Laupland K.B. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect. 2013;19(6):492–500. doi: 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 2.Chung H.C., Lai C.H., Lin J.N. Bacteremia caused by extended-spectrum-beta-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob Agents Chemother. 2012;56(2):618–622. doi: 10.1128/AAC.05753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumbarello M., Spanu T., Di Bidino R. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother. 2010;54(10):4085–4091. doi: 10.1128/AAC.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC . Device-associated Module; 2018. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection) [Google Scholar]
- 5.Skvarc M., Stubljar D., Rogina P., Kaasch A.J. Non-culture-based methods to diagnose bloodstream infection: does it work? Eur J Microbiol Immunol (Bp) 2013;3(2):97–104. doi: 10.1556/EuJMI.3.2013.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opota O., Croxatto A., Prod'hom G., Greub G. Blood culture-based diagnosis of bacteraemia: state of the art. Clin Microbiol Infect. 2015;21(4):313–322. doi: 10.1016/j.cmi.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Russo T.A., Johnson J.R. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181(5):1753–1754. doi: 10.1086/315418. [DOI] [PubMed] [Google Scholar]
- 8.Wilson J., Elgohari S., Livermore D.M. Trends among pathogens reported as causing bacteraemia in England, 2004-2008. Clin Microbiol Infect. 2011;17(3):451–458. doi: 10.1111/j.1469-0691.2010.03262.x. [DOI] [PubMed] [Google Scholar]
- 9.Skogberg K., Lyytikainen O., Ollgren J., Nuorti J.P., Ruutu P. Population-based burden of bloodstream infections in Finland. Clin Microbiol Infect. 2012;18(6):E170–E176. doi: 10.1111/j.1469-0691.2012.03845.x. [DOI] [PubMed] [Google Scholar]
- 10.Nicolas-Chanoine M.H., Bertrand X., Madec J.Y. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27(3):543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abernethy J.K., Johnson A.P., Guy R., Hinton N., Sheridan E.A., Hope R.J. Thirty day all-cause mortality in patients with Escherichia coli bacteraemia in England. Clin Microbiol Infect. 2015;21(3):251–258. doi: 10.1016/j.cmi.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Jensen U.S., Knudsen J.D., Wehberg S., Gregson D.B., Laupland K.B. Risk factors for recurrence and death after bacteraemia: a population-based study. Clin Microbiol Infect. 2011;17(8):1148–1154. doi: 10.1111/j.1469-0691.2011.03587.x. [DOI] [PubMed] [Google Scholar]
- 13.Camins B.C., Marschall J., Devader S.R., Maker D.E., Hoffman M.W., Fraser V.J. The clinical impact of fluoroquinolone resistance in patients with E. coli bacteremia. J Hosp Med. 2011;6(6):344–349. doi: 10.1002/jhm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong-Beringer A., Hindler J., Loeloff M. Molecular correlation for the treatment outcomes in bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae with reduced susceptibility to ceftazidime. Clin Infect Dis. 2002;34(2):135–146. doi: 10.1086/324742. [DOI] [PubMed] [Google Scholar]
- 15.Kang C.I., Kim S.H., Park W.B. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother. 2004;48(12):4574–4581. doi: 10.1128/AAC.48.12.4574-4581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mora-Rillo M., Fernandez-Romero N., Francisco C.N. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence. 2015;6(1):93–100. doi: 10.4161/21505594.2014.991234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefort A., Panhard X., Clermont O. Host factors and portal of entry outweigh bacterial determinants to predict the severity of Escherichia coli bacteremia. J Clin Microbiol. 2011;49(3):777–783. doi: 10.1128/JCM.01902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H. Kor-GLASS system. Euro Surveill. 2018 [Google Scholar]
- 19.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Magiorakos A.P., Srinivasan A., Carey R.B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 22.Wirth T., Falush D., Lan R. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60(5):1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clermont O., Bonacorsi S., Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66(10):4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price L.B., Johnson J.R., Aziz M. The epidemic of extended-spectrum-beta-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. MBio. 2013;4(6):e00377–e00388. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2015. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. [Google Scholar]
- 26.Poirel L., Walsh T.R., Cuvillier V., Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Bano J., Picon E., Gijon P. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. J Clin Microbiol. 2010;48(5):1726–1731. doi: 10.1128/JCM.02353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee R., Johnston B., Lohse C. The clonal distribution and diversity of extraintestinal Escherichia coli isolates vary according to patient characteristics. Antimicrob Agents Chemother. 2013;57(12):5912–5917. doi: 10.1128/AAC.01065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peirano G., Pitout J.D. Fluoroquinolone-resistant Escherichia coli sequence type 131 isolates causing bloodstream infections in a Canadian region with a centralized laboratory system: rapid emergence of the H30-Rx sublineage. Antimicrob Agents Chemother. 2014;58(5):2699–2703. doi: 10.1128/AAC.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Can F., Kurt-Azap O., Nurtop E., Ispir P., Seref C., Ergonul O. Molecular epidemiology of bloodstream associated E. coli ST131 H30-Rx subclone infection in a region with high quinolone resistance. J Med Microbiol. 2016;65(4):306–310. doi: 10.1099/jmm.0.000224. [DOI] [PubMed] [Google Scholar]
- 31.Kang C.I., Kim S.H., Park W.B. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49(2):760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material