Abstract
Liver diseases constitute an important medical problem, and a number of these diseases, termed cholangiopathies, affect the biliary system of the liver. In this review, we describe the current understanding of the causes of cholangiopathies, which can be genetic, viral or environmental, and the few treatment options that are currently available beyond liver transplantation. We then discuss recent rapid progress in a number of areas relevant for decoding the disease mechanisms for cholangiopathies. This includes novel data from analysis of transgenic mouse models and organoid systems, and we outline how this information can be used for disease modeling and potential development of novel therapy concepts. We also describe recent advances in genomic and transcriptomic analyses and the importance of such studies for improving diagnosis and determining whether certain cholangiopathies should be viewed as distinct or overlapping disease entities.
Keywords: Liver, Transplant, Cholangiocyte, Hepatocyte, Bile duct, Organoid, Alagille syndrome, Biliary atresia, Primary sclerosing cholangitis (PSC), Primary biliary cholangitis (PBC), Cystic fibrosis
Highlights
-
•
Cholangiopathies constitute an important category of liver disease with few treatment options currently available.
-
•
Recent progress has been made in modelling cholangiopathies using transgenic mice and organoid systems.
-
•
The new models for cholangiopathies are important platforms for improving diagnosis and advancing therapy development.
1. Liver development and function
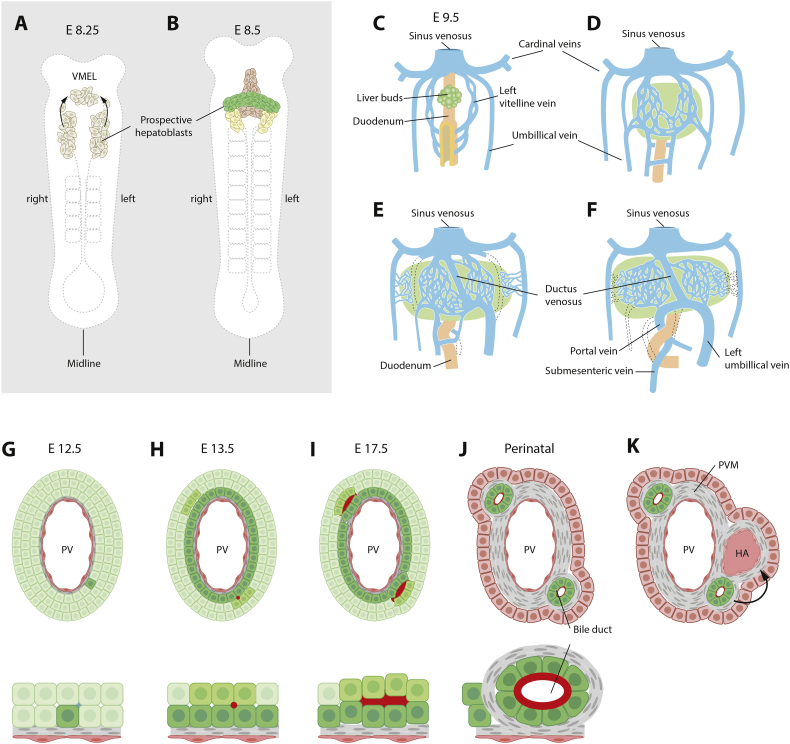
The liver originates from the ventral foregut endoderm and the hepatoblasts - cells that will give rise to cholangiocytes (a.k.a. biliary epithelial cells, BEC) and hepatocytes - emerge around embryonic day 8.5 in the mouse (Fig. 1A, B). The liver bud grows and at E9.5 envelops the vitelline, umbilical and posterior cardinal veins, leading to a close association between venous endothelial cells and hepatoblasts [1] (Fig. 1C–F). The veins undergo extensive branching and once surrounded by hepatoblasts, vasculogenesis creates a network of hepatic sinusoids. In humans, it is unclear whether the vitelline veins contribute to the hepatic venous system, and instead it has been suggested that the left umbilical vein is the origin of the human hepatic venous system [2]. Importantly, the vasculature plays a key role in biliary development, and portal mesenchyme surrounding the portal vein and portal sinus signals to hepatoblasts to initiate intrahepatic bile duct formation via transforming growth factor-β (Tgfb-2 and Tgfb-3) [3,4] and Notch signaling (via the ligand Jagged1) [5] (Fig. 1G–J). Next, bile ducts and hepatoblasts secrete angiogenic factors that induce hepatic artery formation, (Fig. 1K) [6], demonstrating a reciprocal relationship between the vascular and biliary systems in inducing one another's formation and maintenance.
Fig. 1.
Embryonic development of the intrahepatic biliary system. (A,B) At circa embryonic day (E) 8.25 in mouse, cells in the ventral foregut endoderm and ventral midline endodermal lip (VMEL) arise and contribute to the developing liver bud. (C) Next, the liver bud grows to engulf the vitelline veins, which form a vascular plexus that gives rise to hepatic sinusoids. The umbilical veins and cardinal veins also contribute to hepatic sinusoid formation. Portions of the vitelline veins anastomose and establish the portal vein – the scaffold for biliary system formation. (D) Portal vein mesenchyme surrounding the portal veins induces formation of the ductal plate, a layer of cholangiocytes surrounding the portal vein, in a process that initiates near the hilum and progresses towards the periphery. Small lumina form, with cholangiocytes on the portal side and hepatoblast-like cells on the parenchymal side that subsequently differentiate into cholangiocytes. In mice, bile ducts then induce formation of the hepatic artery, while in humans the inductive signal is thought to come from the ductal plate itself.
The mechanisms controlling hepatoblast differentiation to the hepatocyte or cholangiocyte lineages are incompletely understood, but a number of signaling pathways including Wnt, FGF, TGFβ and Notch have emerged as important regulators of cholangiocyte differentiation. The fact that dysregulated Notch signaling causes Alagille syndrome demonstrates the importance of these pathways for human health. Recently, the transcriptomic signature for the mouse hepatoblast lineage choice towards a hepatocyte or cholangiocyte fate was derived [7], showing that protein kinase C/mitogen-activated protein kinase (PKC/MAPK) signaling enhances cholangiocyte maturation. For early human hepatic differentiation, an analysis of in vitro differentiation of pluripotent cells to the hepatocytic lineage identified VEGF signaling as a driver of endothelial vascularization and hepatoblast differentiation [8].
The bile duct system is composed of intra- and extrahepatic ducts. The intrahepatic bile ducts are generated when cholangiocytes surrounding the portal vein first form the ductal plate, followed by the formation of small lumina between cholangiocytes next to the portal vein and hepatoblasts on the parenchymal side. The bile ducts then form by a discontinuous type of tubulogenesis known as cord hollowing [2,9] (Fig. 1G–J). The organization of the bile duct system is coupled with the acquisition of apical-basal polarization of both hepatocytes and cholangiocytes. The development of the extrahepatic biliary system follows a different trajectory and it is instead derived from the ventral pancreas.
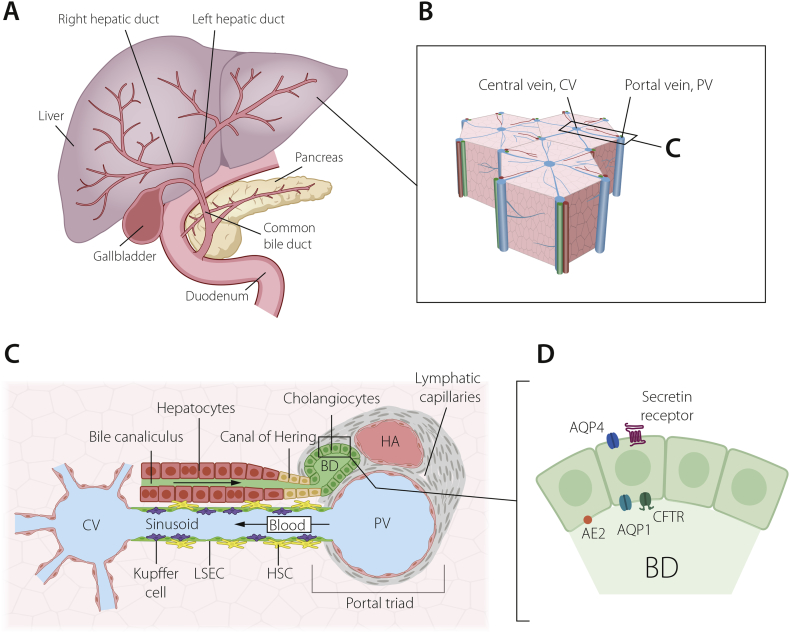
The bile duct system is important for transport of bile, which the liver produces to facilitate digestion of lipids and bilirubin excretion. Hepatocytes secrete bile into the canalicular space and further into the canals of Hering, which are lined jointly by hepatocytes and cholangiocytes (Fig. 2). Cholangiocytes contribute to the bile composition by secretion of fluids and electrolytes. The bile is then further transported via the bile ducts to the gall bladder for storage. The intrahepatic biliary tree is formed by convergence of small bile ductules into larger bile ducts towards the hilum, ending up in the left and right hepatic ducts. The extrahepatic biliary system resides outside the liver and includes the common hepatic duct, common bile duct and gallbladder.
Fig. 2.
The biliary system of the liver. (A) Schematic depiction of the extra- and intra-hepatic bile duct systems and links to the gall bladder. (B) The hexagonal lobular structure of the liver, with a central vein (CV) surrounded by six portal veins (PV), each paired with a bile duct and hepatic artery, a trio known as the portal triad, enlarged in (C). These three structures are embedded in portal vein mesenchyme, which also contains a lymphatic system.Blood flows centripetally from the portal veins and hepatic arteries to the central vein, along sinusoids lined by liver sinusoidal endothelial cells (LSECs), Kupffer cells and hepatic stellate cells (HSCs).Bile flows instead along bile canaliculi formed by hepatocytes, towards the canals of Hering and into the bile ducts. (D) Bile ducts are highly polarized structures, with an apical cilium (not pictured) and apicobasal distribution of channels and receptors, including anion exchange protein 2 (AE2), aquaporin 1 and 4 (AQP1, AQP4), the cystic fibrosis transmembrane receptor (CFTR) and the secretin receptor.
Biliary Atresia (BA) (see below) is a cholangiopathy that mostly affects the extrahepatic biliary tree. The transcription factors Pdx1, Hes1 and Sox17 are important for development of the extrahepatic biliary tree [2] and Sox17 expression is downregulated in experimental models for BA. It has long been established that cholangiocytes are a heterogeneous cell population, and can, for example, be subdivided into large and small cholangiocytes, which differ in terms of expression of certain markers such as the secretin receptor and CFTR (for review see [10,11]). The extent of cholangiocyte heterogeneity is however not well understood. Recent studies provide evidence for at least two major cholangiocyte populations but how they relate to morphologically distinguishable cholangiocyte subtypes is not clear. Cholangiocytes immunoreactive for MIC1-IC3 and expressing high levels of ST14 (suppression of tumorigenicity 14), are far more clonogenic than ST14-low cells, but express similar leves of Sox9, Epcam, Krt19 and Hnf1β. On the other hand, ST14-high cholangiocytes express higher levels of Pkhd11, Bmp4, Vim and Rspo1 [12,13], and can engraft when transplanted into mice. Importantly, the MIC1-IC3 monoclonal antibody, from Novus Biologicals, is raised against nonparenchymal cells from DDC-treated mice, and is suggested to react with oval cells/hepatic proliferating duct cells, which means these experiments enrich for cells present or arising in ductular regenerative processes. The organization of possible subclasses of cholangiocytes along the biliary tree still needs to be established, and it will be interesting to learn whether there are for example hilar–peripheral zonation principles similar to the recently established portal-central zonation of hepatocytes [14].
Single cell RNA-sequencing has provided higher-resolution insight into liver cell populations, as well as into the various differentiation steps (Table 1). Sequencing of different organs during mouse embryonic development (E9.5-E11.5) confirmed a transient hybrid epithelialmesenchymal cell state [15] previously identified in a small subset of liver cells by single cell RNA-sequencing [16], and also suggested by experiments transplanting mesenchymal cells into liver via intrasplenic injection, wherein the mesenchymal cells adjacent to intraheptic vascular structures took on a hepatic fate [17]. Single cell RNA-sequencing of developing liver also suggests a self-regulating transcription factor network including Hnf4α, Hnf1β and Grhl2 [15], and both Hnf1β and Grhl2-regulated networks are enriched for target genes regulating tube development. Future work to dissect apart the regulatory networks controlling cholangiocyte differentiation and bile duct morphogenesis will improve our understanding of embryonic development, as well as providing crucial guidance to develop therapeutics or improve stem cell differentiation protocols for cell replacement therapy. As an example, when differentiated induced pluripotent stem (iPSC) cells, mesenchymal stem cells (MSCs) and human umbilical vein endothelial cells (HUVECs) were co-cultured, hypoxia was shown to regulate hepatic vs cholangiocyte differentiation via suppression of TGFβ signaling [18]. Single cell RNA-sequencing of developmental and disease models is likely to further yield interesting insight into mechanisms of cholangiopathies, and provide molecular targets for therapeutic intervention.
Table 1.
Single Cell RNA sequencing experiments of liver, or cells differentiated into liver cells.
| Species, stage | Number of cells sequenced | Method used and Read depth | Main findings related to cholangiocytes | Additional notes | Reference |
|---|---|---|---|---|---|
| Mouse Adult female liver 6–10 weeks and fetal liver E14.5 |
>50 mouse tissues 60,000 cells total; 3730 cells from fetal liver; 6426 cells from adult liver |
Microwell-Seq Proof of principle in cell lines shows saturated sequencing yields 6,500 genes from 55,000 transcripts per cell. Sequencing depth used for tissues not stated. |
Adult liver scRNA seq identified (in addition to several other cell types) 4 types of hepatocytes: pericentral, periportal, Fabp1-high, and mt-Nd4 high; and identified two types of epithelial (biliary) cells: undefined, and Spp1-high. |
Including cell lines/cultures, >400,000 cells were sequenced in this paper. Liver not explicitly discussed in main text, some data in supplementary figures and data available and explorable at http://bis.zju.edu.cn/MCA. Fetal liver is mainly immune cells, as well as AFP-high hepatocytes and stem/progenitor cells. |
[138] |
| Mouse E11.5, 12.5, 13.5, 14.5, 16.5, 18.5, P2.5 whole liver and P3.25 Epcam-sorted cells |
E11.5-P2.5 dissociated and randomly picked on a C1 RNA-Seq IFC (Fluidigm).P3.25 FACS sorted for Epcam 557 cells from dissociated liver, 52 from Epcam-sorted P3.25 |
C1 Fluidigm chip For dissociated liver: unique mapped reads 1.1 -3.8million per cell. 3000-6000 genes per cell with FPKM>1. For Epcam -sorted cells, 2000 genes per cell at same sequencing depth and mapping rate. |
Cholangiocytes isolated as Epcam positive cells showed high Spp1 expression, and higher expression of Jag1/Notch2 and Hes1 than hepatoblasts. Comparison of embryonic hepatoblasts with Epcam+ cholangiocytes at P3.25 showed that the two E11.5 hepatoblasts (but not later embryonic hepatoblasts) clustered with the cholangiocytes, suggesting hepatoblasts may commit to this fate earlier than previously thought. |
Hepatoblast/mesenchymal hybrid cells co-express Dlk1 and Vimentin. Cdh1 is proposed as a highly specific and sensitive marker for isolation of embryonic hepatoblasts. |
[16] |
| Mouse E9.5, E10.5 & E11.5 liver. |
Organs dissected and trypsinized, individual cells mouth pipetted to lysis buffer. 332 sequenced cells from liver, 320 used after QC for further analyses |
Modified STRT protocol An average of 6361 genes per cell from 0.43 million UMI transcripts. |
E9.5-E11.5 liver possibly contains multiple clusters of mesoderm-derived cells, one clear cluster of epithelial cells and possibly several clusters of hematopoietic cells. Epithelial cells with mesenchymal features: some Epcam/Cdh1 positive cells in liver also express Vimentin. Dlk1 expression not described. |
1916 cells in total sequenced. Cells with fewer than 2000 genes/cell removed –> 1819 were used in analyses, from embryonic mouse including forebrain, hindbrain, skin, heart, somite, lung, liver, and intestine. |
[15] |
| Human In vitro: 2D culture of iPSCs (TkDA3–4, University of Tokyo) undergoing hepatic differentiation and 3D culture of liver bud organoids derived from hepatic cells differentiated from the iPS cell line, cocultured with HUVECS (Lonza) and MSCs (Lonza) In vivo: Adult (three donors: donor 1, female, 55; donor 2, male, 65; donor 3, male, 21) and fetal (two donors, gestation weeks 10.5 and 17.5) Mouse E14.5, E15.5, and E16.5 |
Liver bud organoid cells: Liver bud organoids, different constellations of cells: 177 cells dissociated, no selection. Isolation of adult human liver cells: 256 cells from human adult liver. Protocol of hepatocyte or other cell isolation from adult liver published in [82]; liver is dissociated and cell types separated using centrifugation steps. Isolation of fetal human cells: 238 cells from fetal stages, dissociated and briefly cultured (12h) on laminin-coated plates to remove red blood cells, followed re-dissociation of cells. Isolation of mouse hepatoblasts: 92 cells from mouse liver, dissociated, erythrocytes were lysed, and magnetic bead sorted for Dlk1. |
C1 Fluidigm chip 1–5 million reads per cell. Cells were excluded from further analyses if they had < 100,000 reads, < 1,000 expressed genes or failed to express housekeeping genes ACTB or GAPDH |
This manuscript does not explicitly identify cholangiocytes, but provides valuable insight into which culture systems better support in vitro differentiation faithful to in vivo hepatoblast growth. |
iPSC-derived hepatoblasts undergoing culture in liver bud organoids more closely resemble fetal liver hepatic cells than do 2D cultured iPSC-derived hepatoblasts. Ligand-receptor pair analyses of co-cultured cells in organoids showed a KDR/VEGFA signaling pair in which VEGFA secreted by immature hepatocytes stimulates KDR on endothelial cells, which in turn support hepatoblast growth. |
[8] |
| Human Naïve-like H9 iPSCs, primed iPSCs, and embryoid bodies. |
Cells allowed to differentiate into embryoid bodies vitro and dissociated for analysis. 482 cells were identified as liver cells. 498 cells identified as epithelial. |
C1 Fluidigm chip 175,000 transcripts per cell, ca 5000 genes per cell |
Epithelial cell cluster is SOX9 and FOXP1 positive, and differentiation is regulated by Hippo and AMPK pathways. This could be a liver epithelial (biliary) population, or other epithelial cells. |
4822 cells sequenced in total that passed quality control, of which 2636 were embryoid body cells. Day 8 embryoid bodies included liver-like cells characterized by APOA1, TTR, FGB and AFP. |
[81] |
| Human Reanalysis of cells in [8] |
See [8] | See [8] | Hypoxia induces hepatic differentiation accompanied by TGFB1 and TGFB3 suppression. However, extensive hypoxia increases TGFBs and cholangiocyte marker expression. Single cell RNA seq suggests the source of TGFB, from previously published non-hypoxia experiments. No focus on biliary cells. |
TGFB2 is expressed in mesenchymal cells (MCs) while both TGFB1 and TGFB3 are expressed in ECs and MCs. TGFB receptor 1 (TGFBR1) is expressed in fetal hepatocytes and MCs. | [18] |
Despite the promise of single cell RNA sequencing, a few remaining challenges impede the widespread adoption of this technology [19]. The technology is still relatively expensive, and typically investigators must choose between sampling a greater number of cells at lower read depth, or a lower number of cells at greater read depth. Regardless of approach, it is estimated that only ca 10–20% of the transcriptome is actually sequenced. Depending on which cells are to be analysed, tissues must be dissociated and cells isolated to single cells, a process which may induce transcriptional changes in cells, or deplete sensitive cell types. The amount of time from animal death to cell lysis also affects results. Finally, data analysis is computationally demanding and requires in depth bioinformatical knowledge of a field with rapidly evolving computational methods.
2. Cholangiopathies – an introduction
Dysfunction of cholangiocytes leads to cholangiopathies and both the intrahepatic and extrahepatic biliary trees can be affected; BA for example mostly affects the extrahepatic biliary tree. Cholangiopathies may be caused by genetic, viral, and environmental insults, as well as unknown stimuli. All cholangiopathies are associated with obstructed bile flow, immune responses and cholangiocyte proliferation. They are chronic diseases affecting the biliary epithelium which can proceed to biliary fibrosis, liver parenchymal damage, and further to endstage liver disease, requiring liver transplantation. Cholangiopathies can be classified into primary and secondary cholangiopathies, depending on whether the bile ducts are directly targeted in a disease (primary) or whether the bile ducts degrade as a consequence of injury or other pathological processes in the biliary tree (secondary). The salient features of the primary cholangiopathies, which are the main focus of this review, with regard to prevalence, genetics and current therapy possibilities, are summarized in Table 2 and Suppl File 1 (for a complete list of primary and secondary cholangiopathies, see [20]).
Table 2.
Classification of Primary Cholangiopathies.
| Cholangiopathy | Prevalence; Sex preponderance | Current therapy | Genetic cause | Ref. |
|---|---|---|---|---|
| Genetic | ||||
| Alagille syndrome (ALGS) | 2.2–3.3 in 100,000 live births; no sex preponderance | Medical: supportive | JAG1(majority), NOTCH2 | [83] |
| Surgical: liver transplantation | ||||
| Caroli disease (CD) and Caroli syndrome (CS) with congenital hepatic fibrosis | 0.1 in 100,000 live births; no sex preponderance | Medical: supportive | PKHD1 | [84] |
| Surgical: portosystemic shunting, liver transplantation | ||||
| Cystic fibrosis-associated liver disease | 12.5 in 100,000 live births | Medical: Ursodeoxycholic acid (UDCA), supportive | CFTR | [85]; [86] |
| Surgical: liver transplantation | ||||
| Polycystic liver disease (autosomal dominant polycystic liver disease ADPLD, autosomal dominant polycystic kidney disease ADPKD, autosomal recessive polycystic kidney disease ARPKD) | ADPLD: 1–9 in 100,000 live births | Medical: supportive | ADPLD: PRKCSH, SEC63; ADPKD: PKD1, PKD2, GANAB; ARPKD: PKHD1 | [84]; [87] |
| ADPKD: 100–250 in 100,000 live births; ARPKD: 5 in 100,000 live births | ||||
| Surgical: aspiration of cyst fluid, liver transplantation (uncommon indication) | ||||
| Idiopathic/multifactorial | ||||
| Biliary atresia | 5–14.3 in 100,000 live births; higher prevalence in Asia; female: male ratio 1.4:1 | Medical: post-operative systemic corticosteroids, choleretic (agent stimulating bile flow) | [88] | |
| Surgical: Kasai portoenterostomy, liver transplantation | ||||
| Primary biliary cholangitis (formerly, primary biliary cirrhosis) | 35 in 100,000; female: male ratio 9:1 | Medical: UDCA, supportive | [89] | |
| Surgical: liver transplantation | ||||
| Primary sclerosing cholangitis | 4 in 100,000; female: male ratio 1:2 | Medical: supportive | [49] | |
| Surgical: therapeutic endoscopic retrograde cholangiopancreatography (ERCP), biliary reconstruction, liver transplantation | ||||
| Autoimmune cholangitis | Not well-defined. Currently consideredas autoimmune hepatitis-PBC/PSC overlaps | [91] | ||
| Idiopathic childhood/ adulthood ductopenia | 0.5 in 100,000; male preponderance | Medical: supportive | [92] | |
| Surgical: liver transplantation | ||||
| IgG4-related sclerosing cholangitis | 4.6 in 100,000 (Japan); male preponderance | Medical: systemic corticosteroids | [93] | |
| Surgical: biliary stenting, liver transplantation | ||||
| Malignant | ||||
| Cholangio-carcinoma (de novo or malignant transformation from choledochal cysts, primary sclerosing cholangitis) | 1–2 in 100,000 live births (North America) | Non-surgical: transarterial chemoembolization, transarterial radioembolization, radiofrequency ablation (for unresectable tumors) | [94] | |
| Surgical: complete resection, liver transplantation | ||||
Briefly, biliary atresia (BA) is a devastating, progressive, inflammatory, fibro-obliterating cholangiopathy and the predominant surgical cause for prolonged neonatal jaundice. The standard treatment is timely diagnosis and performance of Kasai portoenterostomy: jaundice clearance is however achieved in only 60–70% of treated patients. Recurrent cholangitis, portal hypertension and cirrhosis remain life-long risks and 50% of patients eventually require liver transplantation. Alagille syndrome (ALGS) is a rare inherited genetic multi-organ disorder affecting the liver, heart, skeleton, kidneys and eyes. The most common symptom is prolonged neonatal jaundice caused by progressive ductal paucity. Currently, apart from liver transplantation, treatment modalities are supportive. Primary biliary cholangitis (PBC) is a chronic, progressive, immune-mediated cholestatic liver disease characterized by inflammatory damage of the intrahepatic bile ducts of small to intermediate sizes. Patients may present with fatigue and pruritis, and eventually develop cirrhosis and liver failure. Currently, the only FDA-approved medical treatment is ursodeoxycholic acid which improves liver function and delays disease progression. Some potential therapeutic agents from clinical trials are promising especially for non-responders to ursodeoxycholic acid. Primary sclerosing cholangitis (PSC) is a chronic, progressive cholestatic fibroinflammatory disease causing multifocal strictures and segmental dilatations of the intrahepatic and extrahepatic bile ducts. PSC is associated with inflammatory bowel disease (IBD), particularly ulcerative colitis (UC) in 80% of patients. Without a known cause, the only current curative treatment modality is liver transplantation. Caroli disease (CD) is a rare hereditary disorder characterized by saccular dilatations of the intrahepatic bile ducts. Treatment is expectant and depends on clinical features. Localized forms can be treated by hepatic resections but diffuse disease ultimately requires liver transplantation. Up to 30% of Cystic Fibrosis patients develop cystic fibrosis-associated liver disease (CFLD). Viscous and reduced bile flow result in cholangiocyte injury, periductal inflammation, abnormal bile duct proliferation and periportal fibrosis. Clinical features appear late and are related to damage of the hepatobiliary system. Current treatment is expectant. Improved understanding of the pathophysiology is the key to developing more disease-specific therapeutics. Polycystic liver diseases (PLD) are autosomal dominant disorders characterized by embryonic ductal plate malformation of the intrahepatic biliary tree. Initial treatment is conservative, with the use of somatostatin analogues to halt cyst growth. Surgical decompression and liver transplantation may eventually be required. Some primary cholangiopathies, including primary sclerosing cholangitis (PSC), choledochal cysts, Caroli disease and Caroli syndrome, and cirrhosis itself are risk factors for development of malignant cholangiocarcinoma, a liver cancer with poor prognosis [21].
The genetic contribution to cholangiocyte pathology differs extensively between the different disease forms, ranging from diseases with a clear-cut monogenic cause, to diseases which are largely idiopathic, with only susceptibility genes identified. Monogenic diseases include Notch pathway mutations in ALGS [4,22], and claudin mutations in neonatal sclerosing cholangitis [2]. While diseases which are largely idiopathic, with only susceptibility genes identified, include BA (which is associated with ADD3 mutations in a small fraction of patients [23,24], and with additional susceptibility loci defined), and PSC, in which 23 susceptibility loci have been reported [25,26].
3. Modelling cholangiopathies in vivo and in vitro
In vivo and in vitro modeling increasingly contribute to unraveling disease mechanisms and providing platforms for exploring new therapies. With regard to cholangiopathies, an important step was the development of protocols that direct stem or progenitor cells to differentiate into cholangiocytes. Protocols for deriving cholangiocytes from human embryonic stem cells (ES cells) and iPS cells have been established [[27], [28], [29], [30]], which open up new vistas for disease modeling, as iPS cells can be derived directly from cholangiopathic patients and retain the genetic configuration of the patient. The ability to develop organoids, i.e. mini-organs, from various organs is another important technological development, and this approach has recently been applied also to the liver. In one liver organoid system, EpCam+ ductal cells produce cholangiocytes, but can, upon R-spondin withdrawal, switch to produce hepatocytes [31]. This system, using cells directly derived from the patients as starting material, recapitulates disease phenotypes for A1AT-deficiency and, important from a cholangiopathy perspective, ALGS [31]. Organoids derived from iPS cells have also been used to model some cholangiopathies including ALGS, polycystic liver disease and cystic fibrosis [30]. More recently, organoids from the extrahepatic biliary tree have been developed, and, as discussed in further detail below, show promise in replacing failing or lost biliary tissue in a mouse model for biliary injury [32].
Animal models are increasingly important in disease research. Rodent-based models have yielded valuable insights into cholangiopathies, although it should be remembered that there are important differences between humans and rodents in terms of liver function, which may limit the extent to which rodent data can be extrapolated to humans. Bile duct ligation models have been available for half a century [33] and recapitulate important aspects of cholangiopathies, such as cholangiocyte proliferation and fibrosis, although at a much more rapid pace than in the human equivalent. To mimic xenobiotic-induced cholangiopathies, feeding rodents toxic substances such as 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) and alpha-naphtyl-isothiocyanate (ANIT) has been extensively deployed, and these models provide a more slowly developing fibrosis, accompanied by bile duct proliferation, inflammation and infiltration of immune cells (for review see [33]) To study BA, infection of mice by Rhesus rotavirus type A (RRV) immediately after birth has proven useful to mimic the disease process [34]. An interesting recent addition to BA modeling is the plant toxin biliatresone, which disrupts the extrahepatic biliary system in zebrafish and causes disrupted cell polarity in cholangiocyte organoids [35]. An intriguing aspect of biliatresone is its reduction of the transcription factor Sox17, which, as discussed above, is a critical factor for biliary development.
Transgenic mouse models have significantly contributed to an improved understanding of cholangiopathies, notably for diseases in which specific monogenic mutations are prevalent (Table 3). Mutations in the human MDR3 gene, which encodes a transport protein important for phosphatidylcholine excretion into bile, leads to cholestasis and biliary cirrhosis due to bile toxicity [36], and is also associated with cholelithiasis [37,38]. In keeping with this, the Mdr2 knockout (KO) mouse develops peribiliary inflammation as a result of breakdown of cholangiocytes in the biliary barrier [39]. An important but sometimes neglected aspect of cystic fibrosis, more generally considered a lung disorder, is the development of peribiliary fibrosis. While mice deficient for Cftr, encoding a transmembrane chloride channel, do not spontaneously develop cholangiopathies [40] until the age of 1 year [41], a liver phenotype can be provoked with oral dextran [33,42]. This work has more recently been extended to also define the proto-oncogene Src as an effector for a cholangiocyte phenotype in Cftr-deficiency [43,44]. Hepatic fibrosis and Caroli disease, which are caused by mutations in the PKHD1 gene [33], have also been assessed in transgenic mouse models, and disruption of the mouse Pkhd1 gene leads to aberrant bile duct development with cyst formation [45]. Mice heterozygous for the transcription factor gene Sox17 on specific genetic backgrounds recapitulate some aspects of BA [46], which is interesting in the light of the observed downstream effects of biliatresone, which includes downregulation of Sox17 levels (see above).
Table 3.
Transgenic mouse models for bile duct defects, cholestasis and cholangiopathies.
| Disease | Gene | Phenotype | Ref |
|---|---|---|---|
| Alagille syndrome | Jag1dDSL/+ | Jag1dDSL/+ pups were recovered at lower than expected frequencies (35% rather than 50%). No jaundice at any stage. Large decrease in Sox9+ ductal plate cells (>95%) at E18, a 75% reduction in bile ducts at P3-P7, and ductular reaction at P30, which is partially rescued in Jag1dDSL/+Rumi+/− (Poglut1) mice. |
[50] |
| Jag1dDSL/+Rumi+/−(back-crossed to C57BL/6 J background for >10 generations) | |||
| Jag1dDSL/+Lfng+/− | No phenotype at birth, though all double heterozygous mice and Jag1dDSL/+ alone were recoved at lower than expected frequencies. Massive bile duct proliferation in adult Jag1dDSL/+Lfng+/− and Jag1dDSLRfng+/− mice. |
[95] | |
| Small but significant increase in number of bile ducts in adult Jag1+/−Mfng+/−mice. | |||
| Jag1dDSL/+Rfng+/− | |||
| Jag1dDSL/+Mfng+/− (back-crossed to C57BL/6 J background) | |||
| Jag1dDSL/+Notch2del1/+ (mixed C57BL/6 J × 129S1/SvImJ background) | Half of Jag1dDSL/+Notch2del1/+mice die the first week after birth. Jaundice at P3. Absence of bile ducts. | [96] | |
| Jag1Ndr/Ndr (mixed C3H x C57bl6 background) | Ca 10% of Jag1Ndr/Ndrmice survive to postnatal day 10. Pups show delayed bile duct development, bile duct dysmorphology and cholestasis. 5% survive to adulthood, these show rescue of cholestasis with persistent bile duct dysmorphology. On a pure C3H background, Jag1Ndr/Ndr mice are embryonic lethal. | ([51]; [47]) | |
| Jag1loxP/dDSL; Alfp-Cre | Partially penetrant (50%) bile duct proliferation in conditional/null Jag1 mice. | [97] | |
| Jag1lox/lox;SM22-Cre | Jag1 is required in portal vein mesenchyme (Sm22-expressing) rather than endothelial cells or hepatoblasts. Absence of Jag1 from portal vein mesenchyme results in a failure to from bile ducts and postnatal jaundice. | ([5]; [97]) | |
| Notch2del1/del1 (mixed C57BL/6 J × 129S1/SvImJ background) | No bile ducts at p0. Later analyses precluded by kidney-related postnatal lethality. | [96] | |
| Notch2loxp/del2Alb1-Cre | Jaundice at P3, focal necrosis in liver. Scattered cholangiocytes but no bile ducts at P7. | [48] | |
| Notch2loxp/del3Alb1-Cre | |||
| Notch2lox/lox;AlbCre | Defective ductal plate remodeling, biliary cells present, but absence of bile ducts. Portal inflammation, fibrosis, bile duct dilation, and proliferation. | [98] | |
| RbpjloxP/Δ;Foxa3-Cre or | Fewer ductal plate cells at E16.5 and P0, and fewer bile ducts at P0 in RbpjloxP/Δ;Foxa3-Cre mice. When RBPj is deleted later, using AFP-Cre, there is a less severe reduction in peri-portal ductal cells, but similarly reduced number of bile ducts at postnatal stages. | [99] | |
| RbpjloxP/loxP;AFP-Cre | |||
| Rbpjloxp/loxpHnf6loxp/loxpR26ZG+/+Alb1-Cre | Bile ducts absent at postnatal stages, adult conversion of hepatocytes to cholangioytes driven by Tgfβ rescues the biliary tree. | [54] | |
| Sox9loxp/loxp;Alfp-cre | Delayed ductal plate remodeling. Normal bile ducts by the age of 5 weeks. | [100] | |
| Arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome | Alfp-Cre; Vps33bloxp/loxp | Cholestasis and fibrosis. | [101] |
| ARPKD | Pkhd1ex40 (Fibrocystin/polyductin) | Bile duct cysts | [102] |
| Autosomal recessive polycystic kidney disease & Caroli syndrome | |||
| Pkhd1del4/del4 | |||
| [45] | |||
| Bile duct proliferation, progressive bile duct enlargement and portal fibrosis. | |||
| Bilirubin clearance normal. | |||
| PLD-ADPKD: Polycystic liver disease associated with autosomal dominant polycystic kidney disease | Pkd1+/− | Late onset liver cysts (27% with liver cysts at 9–14 months, 87% in older mice) | [103] |
| Pkd1+/del17–21βgeo | |||
| pCx-Cre;Pkd1loxp/−or | |||
| pCx-Cre;Pkd2loxp/− | |||
| [104] | |||
| TPK1 and TPK3 mice (transgenic mice expressing ¨30 extra copies of human PKD1, as well as TSC2) | |||
| Liver cysts in aged heterozygous mice (>19 months). Homozygous mice are embryonic lethal. | |||
| [105] | |||
| Pkd2WS26/wS25 | |||
| Liver cysts by 4 weeks of age. | |||
| [107] | |||
| [108] | |||
| Inflammation, bile duct proliferation, and liver cysts. | |||
| Hypomorphic mice | |||
| ¨20% of Pkd2WS26/wS25 mice display liver cysts between 4 and 10 weeks of age. | |||
| Biliary atresia |
Sox17−/− SRY-related HMG-box 17 |
Smaller liver, inflammation, extraheptic bile duct stenosis and atresia. | [109] |
| Sox17 is required in gallbladder rather than hepatoblasts | |||
| Autosomal dominant polycystic liver disease | pCx-Cre;Prkcshloxp/loxp | Liver cysts. | [110] |
| pCx-Cre;Sec63loxp/loxp | |||
| Primary biliary cholangitis | Dominant negative TGF-βRII (driven by CD4 promoter lacking the CD8 silencer) | Liver fibrosis and bile duct destruction. | [111] |
| Onset is delayed by IL-12p35 deletion. | [113] | ||
| IL-12p40 deletion protects against liver inflammation in Dn TGF-βRII mice. | [114] | ||
| Dn TGF-βRII IL-12p35 −/− | |||
| Dn TGF-βRII IL-12p40 −/− | |||
| Primary biliary cholangitis | IL-2Rα−/− | Portal inflammation and biliary ductular damage. | [115] |
| IL-2Rα−/−IL12-p40−/− | |||
| Primary biliary cholangitis/ Sjögrens syndrome | |||
| Compared to IL-2Rα −/− mice alone, worsened portal inflammation and bile duct damage, but reduced colitis in IL-2Rα−/−IL12-p40−/−mice. | [116] | ||
| NOD.c3c4 mice | Autoimmune polycystic destructive cholangitis, granuloma formation, and eosinophilic infiltration in addition to extrahepatic bile duct effects. | ([117]; [118]) | |
| Ae2a,b−/− | Partially penetrant portal inflammation and bile ducts destruction (4/11 mice with severe or moderate inflammation). | [119] | |
| Cl(−)/HCO(3)(−) anion exchanger 2 (AE2) | |||
| Scurfy mice (Foxp3sf mutant) | Portal inflammation and bile duct destruction. | [120] | |
| Faslpr/lpr | Portal inflammation and cholangitis of small intrahepatic bile ducts. | [121] | |
| MRL (genetic background)/lpr (lymphoproliferation) mice | |||
| Primary sclerosing cholangitis | Mdr2−/− | Sex-dependent liver disease. Inflammation and ductular reaction in large portal tracts. Fibrosis and bile duct destruction. | ([122]; [123]; [124]) |
| (Abcb4 or ATP-binding cassette, sub-family B (MDR/TAP), member 4) | |||
| CDH1loxp/loxp; Alb-Cre (CDH1ΔL, Liver-specific E-cadherin knockout) | Periportal inflammation and periductal fibrosis leading to liver tumors. | [125] | |
| Krt19-Cre; CDH1loxp/loxp | E-cad is required primarily in bile ducts rather than hepatocytes to avoid cholestasis. | ||
| Adenovirus-Cre; CDH1loxp/loxp | |||
| Progressive Familial Intrahepatic Cholestasis (PFIC2) | Abcb11 (ATP-binding cassette, sub-family B (MDR/TAP), member 11, aka sister of P-glycoprotein (Spgp) or bile salt export pump (BSEP)) | Altered hepatocyte canalicular morphology and bile salt secretion defects, but mild/no cholestasis overall. | [126] |
| [127] | |||
| Cholic acid diet in these mice induces severe cholestasis, bile duct proliferation and cholangitis. | |||
| PFIC-like inherited cholestasis | Atp11c | Cholestasis which is worsened on a cholic acid diet. | [128] |
| ATPase Phospholipid Transporting 11C | |||
| Hyperbilirubinemia at postnatal stages that resolves with age. | |||
| Cystic fibrosis liver disease | Cftr−/− | Hepatosteatosis, focal cholangitis, and bile duct proliferation. Focal biliary cholangitis in aged (1 year) mice. | [130] |
| Cystic fibrosis transmembrane conductance regulator | |||
| [131] | |||
| Oral dextran induction of colitis induced greater bile duct injury with inflammation and bile duct proliferation. | |||
| Erythropoietic protoporphyria | fch/fch (ferrochelatase mutation) | Bile duct proliferation and biliary fibrosis. | [132] |
| General liver inflammation and liver fibrosis | Fra-1 overexpression driven byhistocompatibility complex class I antigen H2-Kb(H2) promoter (Fra-1tg) mice & Fra-1tgrag2−/− | Portal inflammation, ductular proliferation and biliary fibrosis. Fibrosis was attenuated but not completely rescued by Rag2 deletion. | [133] |
| Canaliculi and bile duct development defects | Lkb1loxp/loxp; Alb-Cre | Altered hepatocyte canalicular morphology and poorly formed/absent bile ducts | [134] |
| Ctnnb1loxp/loxp; Foxa3-Cre | Decrease in overall liver size and bile duct paucity | [135] | |
| Role of bile duct innervation | M3-R−/− (muscarinic 3 receptor) | Decreased bile flow but no liver injury or cholestasis. However, 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) feeding induced more severe liver injury with obstruction of bile ducts by porphyrin plugs. | [136] |
| Zellweger spectrum disorder (includes liver fibrosis) | Pex1G844D (peroxisomal biogenesis factor 1) | Bile deposits and bile duct proliferation (?) | [137] |
Research on the pathomechanisms for ALGS, which in the majority of cases is caused by mutations in the Notch ligand JAGGED1 (and with a minority of patients instead carrying NOTCH2 mutations), has benefitted hugely from analysis of transgenic mouse models. A conditional knock out of the Notch ligand Jag1 in portal vein mesenchyme [5] as well as Jag1/Notch2 double heterozygous mice [48] generate a bile duct phenotype resembling ALGS. Interestingly, a heterozygous Jag1 mouse model on a C57Bl6 genetic background generates an ALGS phenotype, and deletion of the Notch glucosyltransferase Poglut1 ameliorates the phenotype [50], arguing that the dosage of Notch signaling is important for development of ALGS. A recent transgenic model demonstrates that a missense mutation in Jag1 (Jag1H268Q), which lies in a hotspot for ALGS missense mutations,in homozygous form is sufficient to recapitulate most of the symptoms seen in patients including jaundice and ductopenia [51]. An interesting feature of these models is that cholestasis is generally transient in early postnatal mice, while adults display no cholestasis. This suggests that Notch-independent compensatory mechanisms can rescue ductopenia, and indeed, while in the majority of patients biliary breakdown continues [52,53], some patients with ALGS recover from cholestasis with time and even display regenerating liver nodules [51]. Recent work has taken ALGS mouse models one step further, identifying TGFβ signaling as a driver of adult Notch-independent regeneration of the biliary system, inducing hepatocyte transdifferentiation [54]. Collectively these studies suggest that there may be a therapeutic window for ALGS therapy and provide targets for intervention.
4. The importance of cell polarity for bile duct integrity and function
The disease processes leading to cholangiopathies are complex and multifactorial. Biliary fibrosis is a cardinal feature of most cholangiopathies and an area of intense research. Progress has been made in a number of areas, including elucidating the role of integrins and prominin 1-positive progenitor cells in fibrosis [55,56] and how biliary tissue is remodeled during liver regeneration [57]. How different cell types, such as hepatic stellate cells, portal fibroblasts and so called reactive ductular cells (RDCs) contribute to fibrosis has, however, been subject to a number of excellent recent reviews [58,59] and will for space reasons not be further discussed in this review. Similarly, the importance of the immune system and infiltration of inflammatory cells has been the subject of recent reviews [60,61]. Here, we will instead focus on another important facet of the disease process, where considerable progress recently has been made: dysregulation of cholangiocyte cell polarity and barrier function in the bile ducts.
A hallmark of the bile duct system is epithelial cell polarization, and both hepatocytes and cholangiocytes display strong apical-basal polarity (Fig. 2). In cholangiocytes, a number of proteins are specifically localized to the apical (luminal) side, such as CFTR, aquaporin 1 (AQP1) and the anion exchange protein 2 (AE2). Conversely, AQP4 and the secretin receptor are specifically localized to the basal side [62] (Fig. 2). Lumen formation and cell polarization are, as discussed above, an integral part of early bile duct tubulogenesis and are disrupted in ALGS. A recent transcriptomic analysis of ALGS patients and an ALGS mouse model revealed that although cholangiocyte markers per se are not downregulated, instead genes encoding proteins with apical localization in cholangiocytes show reduced expression, including CFTR, SLC5A1 and CHST4 [51], suggesting morphogenesis defects rather than differentiation defects alone.
It will be interesting to explore how dysfunctional Notch signaling in ALGS links to the molecular programs setting up apical-basal polarity. Disruption of the primary cilia, a signaling center located at the apical side of cholangiocytes, leads to biliary fibrosis and macrophage infiltration in a mouse model for hepatorenal fibrocystic disease [63], and in line with this, reduction in the frequency of primary cilia has been observed in BA [64]. Similarly, a number of ciliopathies affect cholangiocyte and ductal plate differentiation [65]. Furthermore, BA is characterized by decreased levels of beta1-integrin, laminin b1 and nidogen [66], indicating that cell-matrix interactions at the basal side may also be important contributors to cholangiopathies.
An important part of the epithelial polarization process is the formation of tight junctions between cholangiocytes, necessary to maintain barrier function, to confine bile to the bile ducts and to avoid inflammatory cell invasion of the liver parenchyma, which may otherwise trigger or accelerate the fibrotic process [67]. Barrier integrity is disrupted in neonatal sclerosing cholangitis, which is caused by claudin mutations [2]. Claudin is a key protein in the tight junctions and perturbation of claudin function in zebrafish leads to aberrant bile duct development [68]. The transcription factor grainyhead-like 2 may be a key regulator of establishing the barrier function, as it regulates expression of claudins and Rab25, which is important for localizing claudins to the tight junctions [69].
5. Towards improved diagnosis and therapy development for cholangiopathies
Diagnosis is still far from perfect for a number of cholangiopathies, and this may result in failure to treat even when options are available (Table 2, Suppl File 1), or that an incorrect type of treatment is chosen. For example, the current treatment of BA (Kasai portoenterostomy (KPE)), in which all bile duct tissue up to the liver capsule is excised and a loop of jejunum is attached creating a portoenterostomy) relies on early diagnosis (within 60–100 days) and timely performance of KPE. Missed or late diagnosis of BA results in rapid progression to end-stage liver disease, rendering KPE futile and leaving liver transplantation as the only and last resort. Misdiagnosing ALGS as BA can lead to children erroneously receiving KPE, which in ALGS appears to result in higher rates of liver transplantations than when children with ALGS do not receive KPE [70,71]. From this, it is obvious that more precise biomarkers for BA and ALGS would be useful. Bulk transcriptomes (i.e. from a whole biopsy) from ALGS, PSC and progressive familial intrahepatic cholestasis type 2 biopsies have begun to reveal differentially expressed genes [51], which could provide biomarkers where genetic diagnosis is difficult, as well as provide mechanistic insight into disease processes and identify therapeutically amenable pathways. As bulk transcriptomes capture an average transcriptome for all cell types present in a biopsy, single cell RNAsequencing is however likely to be more successful for identifying cholangiocyte-specific markers, and in particular if this information can be transformed into new serum biomarkers, it is likely to become more clinically useful. An improved biomarker portfolio would allow us to address whether BA and ALGS may in fact represent extremes of a continuous disease spectrum that can pose ambiguity in the context of clinical diagnosis and management. Proteomics-based approaches may also be a valuable complement to improve diagnosis, and matrix metalloproteinase 7 (MMP7) was recently identified as a novel BA marker using this strategy [72].
Apart from understanding the causes of cholangiopathies, understanding the mechanisms of disease progression is equally important. As discussed above, there are currently limited curative options for cholangiopathies, other than liver transplantation, which is a high-risk procedure incurring high morbidity and post-transplantation issues with lifelong immunosuppression and post-transplant malignancies. The development of new therapies to ameliorate or reverse progressive cholangiocyte damage is therefore a prioritized research area. Success depends both on appropriate patient selection (with relevant and possibly new biomarkers) and availability of novel target therapies. Obeticholic acid (OCA) is a promising potential therapy for PBC patients with inadequate response to the FDA-approved first-line treatment ursodeoxycholic acid (UDCA) [73,74]. The efficacy and safety of OCA were demonstrated in two phase 2 studies and a phase 4 study is now under way (Supplementary File 2). Another potential therapeutic treatment for PSC is all-trans retinoic acid (ATRA), which demonstrated improvement in liver enzyme function in a phase 1 study, and a phase 2 study to evaluate its efficacy against fibrogenesis in PSC is currently ongoing (Supplementary File 2). A list of completed and current (May 2018) clinical trials for primary cholangiopathies (PBC, PSC and BA) is provided in Supplementary File 2.
In addition to pharmacological approaches, there is an increasing interest in cell-based therapeutic strategies and approaches harnessing the liver's own endogenous repair potential. For endogenous repair, an important question is which cells would be best suited to replace the lost or ailing cells. Research in liver disease has thus far mostly focused on replacing hepatocytes, and some research groups propose a cholangiocyte origin of cells taking part in the relevant repair processes in animal models [75,76], while other groups advocate hepatocytes as the cellular source [54,[77], [78], [79]]. A potential stem cell population expressing Lgr5, a hallmark for stem cells in different tissues, was observed in response to liver injury [80] and represents an interesting candidate cell type for endogenous repair. The replacement of cholangiocytes is yet less explored, but mouse models for ALGS, given their bile duct paucity, may be a suitable test platform to learn if new cholangiocytes can be generated in vivo. The report that new cholangiocytes are transdifferentiated from hepatocytes in an ALGS mouse model, in a TGFβ-dependant manner, is encouraging in this regard [54].
An alternative approach is to generate cells for transplantation in vitro. As discussed above, cholangiocytes can be in vitro differentiated by the organoid technology [31] or from pluripotent cells (ES and iPS cells) [[28], [29], [30]], and could be interesting sources of cells for transplantation. The recent report that the extrahepatic biliary tree can be partially reconstructed in animal models is a very exciting development [32].
6. Outstanding questions
Cholangiopathies are rare diseases, but collectively they constitute a major clinical problem and a considerable burden for the healthcare system. Current challenges include the lack of functional therapies beyond liver transplantation as well as suboptimal methods for diagnosis. In this review, we have focused on describing recent progress especially in the molecular understanding of the diseases. Information from areas such as transgenic models, organoid technology and transcriptomics can now be used to make progress for diagnosis, and, in the long term, for therapy. An important outstanding question is how diagnosis can become more precise, and we envisage that the rapid technology development in the area of transcriptomics, and in particular in single cell RNA-sequencing, will contribute to identify new biomarkers for early and unambiguous diagnosis, and outcome prediction. This could lead to more timely and effective interventions, and improved outcomes. Currently, disease modeling using organoids and in vitro differentiation of iPS cells has mostly been used for monogenic cholangiopathies, notably ALGS, and it will be interesting to see if these technologies can also be applied to cholangiopathies with a more complex genetic makeup. Finally, novel organoid and in vitro culture systems open new vistas for accelerated testing of new drug candidates, which may help identify novel pharmacological principles that can be moved forward to animal experiments and clinical testing. Ultimately, it is hoped that a cellular and molecular understanding of biliary pathologies will enable accurate and rapid diagnosis, ensuring patients receive correct management and treatment.
Acknowledgments
PT is supported by Innovation and Technology Commission, Hong Kong, UICP project UIM/300, and Dr. Li Dak-Sum Research Centre, The University of Hong Kong – Karolinska Institutet Collaboration in Regenerative Medicine. E.R.A. acknowledges support from the Center of Innovative Medicine (CIMED) Grant, the Daniel Alagille Award, KI Funding, the Heart and Lung Foundation, and the Alex and Eva Wallström Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.08.024.
Contributor Information
Paul K.H. Tam, Email: paultam@hku.hk.
Emma R. Andersson, Email: Emma.Andersson@ki.se.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Swartley O.M., Foley J.F., Livingston D.P., Cullen J.M., Elmore S.A. Histology atlas of the developing mouse hepatobiliary hemolymphatic vascular system with emphasis on embryonic Days11.5-18.5 and early postnatal development. Toxicol Pathol. 2016;44:705–725. doi: 10.1177/0192623316630836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ober E.A. Development of the liver: Insights into organ and tissue morphogenesis. J Hepatol. 2018;68:1049–1062. doi: 10.1016/j.jhep.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Gérard C., Tys J., Lemaigre F.P. Presented at the seminars in cell & developmental biology. Elsevier; 2017. Gene regulatory networks in differentiation and direct reprogramming of hepatic cells; pp. 43–50. [DOI] [PubMed] [Google Scholar]
- 4.Siebel C., Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97:1235–1294. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann J.J., Zovein A.C., Koh H., Radtke F., Weinmaster G., Iruela-Arispe M.L. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabris L., Cadamuro M., Libbrecht L., Raynaud P., Spirli C., Fiorotto R. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology. 2008;47:719–728. doi: 10.1002/hep.22015. [DOI] [PubMed] [Google Scholar]
- 7.Yang L., Wang W.-H., Qiu W.-L., Guo Z., Bi E., Xu C.-R. A single-cell transcriptomic analysis reveals precise pathways and regulatory mechanisms underlying hepatoblast differentiation. Hepatology. 2017;66:1387–1401. doi: 10.1002/hep.29353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camp J.G., Sekine K., Gerber T., Loeffler-Wirth H., Binder H., Gac M. Multilineage communication regulates human liver bud development from pluripotency. Nature. 2017;546:533–538. doi: 10.1038/nature22796. [DOI] [PubMed] [Google Scholar]
- 9.Iruela-Arispe M.L., Beitel G.J. Tubulogenesis. Development. 2013;140:2851–2855. doi: 10.1242/dev.070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato K., Meng F., Giang T., Glaser S., Alpini G. Mechanisms of cholangiocyte responses toinjury. Biochim Biophys Acta. 2018;1864:1262–1269. doi: 10.1016/j.bbadis.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabibian J.H., Masyuk A.I., Masyuk T.V., O'Hara S.P., Larusso N.F. Physiology of cholangiocytes. Compr Physiol. 2013;3:541–565. doi: 10.1002/cphy.c120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung A.C., Lorenzo Pisarello M.J., LaRusso N.F. Pathobiology of biliary epithelia. Biochim Biophys Acta. 2018;1864:1220–1231. doi: 10.1016/j.bbadis.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B., Dorrell C., Canaday P.S., Pelz C., Haft A., Finegold M. Adult mouse liver contains two distinct populations of cholangiocytes. Stem Cell Rep. 2017;9:478–489. doi: 10.1016/j.stemcr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halpern K.B., Shenhav R., Matcovitch-Natan O., Toth B., Lemze D., Golan M. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542:352–356. doi: 10.1038/nature21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong J., Hu Y., Fan X., Wu X., Mao Y., Hu B. Single- cell RNA-seq analysis unveils a prevalent epithelial/mesenchymal hybrid state during mouse organogenesis. Genome Biol. 2018;19:31. doi: 10.1186/s13059-018-1416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su X., Shi Y., Zou X., Lu Z.-N., Xie G., Yang J.Y.H. Single-cell RNA-Seq analysis reveals dynamic trajectories duringmouse liver development. BMC Genomics. 2017;18:946. doi: 10.1186/s12864-017-4342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iansante V., Mitry R.R., Filippi C., Fitzpatrick E., Dhawan A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 2017;83:232–240. doi: 10.1038/pr.2017.284. [DOI] [PubMed] [Google Scholar]
- 18.Ayabe H., Anada T., Kamoya T., Sato T., Kimura M., Yoshizawa E. Optimal hypoxia regulates human iPSC-derived liver bud differentiation through intercellular TGFB signaling. Stem Cell Rep. 2018 Jul 12 doi: 10.1016/j.stemcr.2018.06.015. pii: S2213-6711(18)30278-9 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter S.S. Single-cell RNA sequencing for the study of development, physiology and disease. Nat Rev Nephrol. 2018;14:479–492. doi: 10.1038/s41581-018-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazaridis K.N., LaRusso N.F. Mayo Clinic Proceedings. Vol. 90. 2015. The cholangiopathies; pp. 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razumilava N., Gores G.J. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masek J., Andersson E.R. The developmental biology of genetic Notch disorders. Development. 2017;144:1743–1763. doi: 10.1242/dev.148007. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G., Tang C.S.-M., Wong E.H.-M., Cheng W.W.-C., So M.-T., Miao X. Common genetic variantsregulating ADD3 gene expression alter biliary atresia risk. J Hepatol. 2013;59:1285–1291. doi: 10.1016/j.jhep.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Barceló M.-M., Yeung M.-Y., Miao X.-P., Tang C.S.-M., Chen G., So M.-T. Genome-wide association study identifies a susceptibility locus for biliary atresia on 10q24.2. Hum Mol Genet. 2010;19:2917–2925. doi: 10.1093/hmg/ddq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung B.K., Karlsen T.H., Folseraas T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma. Biochim Biophys Acta. 2018;1864:1390–1400. doi: 10.1016/j.bbadis.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Ji S.-G., Juran B.D., Mucha S., Folseraas T., Jostins L., Melum E. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49:269–273. doi: 10.1038/ng.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Assuncao T.M., Sun Y., Jalan-Sakrikar N., Drinane M.C., Huang B.Q., Li Y. Development and characterization of human-induced pluripotent stem cell-derived cholangiocytes. Lab Invest. 2015;95:684–696. doi: 10.1038/labinvest.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa M., Ogawa S., Bear C.E., Ahmadi S., Chin S., Li B. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33:853–861. doi: 10.1038/nbt.3294. [DOI] [PubMed] [Google Scholar]
- 29.Sampaziotis F., de Brito M.C., Geti I., Bertero A., Hannan N.R. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat Protoc. 2017;12:814–827. doi: 10.1038/nprot.2017.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampaziotis F., de Brito M.C., Madrigal P., Bertero A., Saeb-Parsy K., Soares F.A.C. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33:845–852. doi: 10.1038/nbt.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M.A. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampaziotis F., Justin A.W., Tysoe O.C., Sawiak S., Godfrey E.M., Upponi S.S. Reconstruction of the mouse extrahepaticbiliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23:954. doi: 10.1038/nm.4360. [DOI] [PubMed] [Google Scholar]
- 33.Mariotti V., Strazzabosco M., Fabris L., Calvisi D.F. Animal models of biliary injury and altered bile acid metabolism. Biochim Biophys Acta (BBA) 2017;1864:1254–1261. doi: 10.1016/j.bbadis.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford S.E., Ramani S., Tate J.E., Parashar U.D., Svensson L., Hagbom M. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. doi: 10.1038/nrdp.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorent K., Gong W., Koo K.A., Waisbourd-Zinman O., Karjoo S., Zhao X. Identification of a plant isoflavonoid that causes biliary atresia. Sci Transl Med. 2015;7(286):286ra67. doi: 10.1126/scitranslmed.aaa1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X., Karlsen T.H. Genetics of primary sclerosing cholangitis and pathophysiological implications. Nat Rev Gastroenterol Hepatol. 2017;14:279–295. doi: 10.1038/nrgastro.2016.154. [DOI] [PubMed] [Google Scholar]
- 37.Poupon R., Rosmorduc O., Boelle P.Y., Chretien Y., Corpechot C., Chazouilleres O. Genotype-phenotype relationships in the low-phospholipid-associated cholelithiasis syndrome: a study of 156 consecutive patients. Hepatology. 2013;58:1105–1110. doi: 10.1002/hep.26424. [DOI] [PubMed] [Google Scholar]
- 38.Rosmorduc O., Hermelin B., Poupon R. MDR3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology. 2001;120:1459–1467. doi: 10.1053/gast.2001.23947. [DOI] [PubMed] [Google Scholar]
- 39.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis–a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Rosen B.H., Chanson M., Gawenis L.R., Liu J., Sofoluwe A., Zoso A. Animal and model systems for studying cystic fibrosis. J Cyst Fibros. 2018;17:S28–S34. doi: 10.1016/j.jcf.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollheimer M.J., Fickert P. Animal models in primary biliary cirrhosis and primary sclerosing cholangitis. Clin Rev Allergy Immunol. 2015;48:207–217. doi: 10.1007/s12016-014-8442-y. [DOI] [PubMed] [Google Scholar]
- 42.Fiorotto R., Scirpo R., Trauner M., Fabris L., Hoque R., Spirli C. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. doi: 10.1053/j.gastro.2011.06.052. (1508.e1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiorotto R., Amenduni M., Mariotti V., Fabris L., Spirli C., Strazzabosco M. Src kinase inhibition reduces inflammatory and cytoskeletal changes in DeltaF508 human cholangiocytes and improves cystic fibrosis transmembrane conductance regulator correctors efficacy. Hepatology. 2018;67:972–988. doi: 10.1002/hep.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiorotto R., Villani A., Kourtidis A., Scirpo R., Amenduni M., Geibel P.J. The cystic fibrosis transmembrane conductance regulator controls biliary epithelial inflammation and permeability by regulating Src tyrosine kinase activity. Hepatology. 2016;64:2118–2134. doi: 10.1002/hep.28817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallagher A.-R., Esquivel E.L., Briere T.S., Tian X., Mitobe M., Menezes L.F. Biliary and pancreatic dysgenesis in mice harboring a mutation in Pkhd1. Am J Pathol. 2008;172:417–429. doi: 10.2353/ajpath.2008.070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uemura M., Ozawa A., Nagata T., Kurasawa K., Tsunekawa N., Nobuhisa I. Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice. Development. 2013;140:639–648. doi: 10.1242/dev.086702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansson E.M., Lanner F., Das D., Mutvei A., Marklund U., Ericson J. Control of notch-ligand endocytosis by ligand-receptor interaction. J Cell Sci. 2010;123:2931–2942. doi: 10.1242/jcs.073239. [DOI] [PubMed] [Google Scholar]
- 48.Lozier J., McCright B., Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toy E., Balasubramanian S., Selmi C., Li C.-S., Bowlus C.L. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterology. 2011;11:83. doi: 10.1186/1471-230X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thakurdas S.M., Lopez M.F., Kakuda S., Fernandez-Valdivia R., Zarrin-Khameh N., Haltiwanger R.S. Jagged1 heterozygosity in mice results in a congenital cholangiopathy which is reversed by concomitant deletion of one copy of Poglut1 (Rumi) Hepatology. 2016;63:550–565. doi: 10.1002/hep.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersson E.R., Chivukula I.V., Hankeova S., Sjöqvist M., Tsoi Y.L., Ramsköld D. Mouse model of Alagille syndrome and mechanisms of Jagged1 missense mutations. Gastroenterology. 2018;154 doi: 10.1053/j.gastro.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jinguji M., Tsuchimochi S., Nakajo M., Hamada H., Kamiyama T., Umanodan T. Scintigraphic progress of the liver in a patient with Alagille syndrome (arteriohepatic dysplasia) Ann Nucl Med. 2003;17:693–697. doi: 10.1007/BF02984977. [DOI] [PubMed] [Google Scholar]
- 53.Sparks E.E., Perrien D.S., Huppert K.A., Peterson T.E., Huppert S.S. Defects in hepatic Notchsignaling result in disruption of the communicating intrahepatic bile duct network in mice. Dis Model Mech. 2011;4:359–367. doi: 10.1242/dmm.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaub J.R., Huppert K.A., Kurial S.N.T., Hsu B.Y., Cast A.E., Donnelly B. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature. 2018;557:247–251. doi: 10.1038/s41586-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mavila N., James D., Shivakumar P., Nguyen M.V., Utley S., Mak K. Expansion of prominin-1-expressing cells in association with fibrosis of biliary atresia. Hepatology. 2014;60:941–953. doi: 10.1002/hep.27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng Z.-W., Ikenaga N., Liu S.B., Sverdlov D.Y., Vaid K.A., Dixit R. Integrin alphavbeta6 critically regulates hepatic progenitor cell function and promotes ductular reaction, fibrosis, and tumorigenesis. Hepatology. 2016;63:217–232. doi: 10.1002/hep.28274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamimoto K., Kaneko K., Kok C.Y.-Y., Okada H., Miyajima A., Itoh T. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. Elife. 2016;5 doi: 10.7554/eLife.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fabris L., Spirli C., Cadamuro M., Fiorotto R., Strazzabosco M. Emerging concepts in biliary repair and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2017;313:G102–G116. doi: 10.1152/ajpgi.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinzani M., Luong T.V. Pathogenesis of biliary fibrosis. Biochim Biophys Acta. 2018;1864:1279–1283. doi: 10.1016/j.bbadis.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 60.Crosby C.M., Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018 Jul 2 doi: 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shetty S., Lalor P.F., Adams D.H. Liver sinusoidal endothelial cells - gatekeepers of hepaticimmunity. Nat Rev Gastroenterol Hepatol. 2018;5:1751. doi: 10.1038/s41575-018-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villasenor A., Stainier D.Y.R. On the development of the hepatopancreatic ductal system. Semin Cell Dev Biol. 2017;66:69–80. doi: 10.1016/j.semcdb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Zimmerman K.A., Song C.J., Gonzalez-Mize N., Li Z., Yoder B.K. Primary cilia disruption differentially affects the infiltrating and resident macrophage compartment in the liver. Am J Physiol Gastrointest Liver Physiol. 2018;314:G677–G689. doi: 10.1152/ajpgi.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frassetto R., Parolini F., Marceddu S., Satta G., Papacciuoli V., Pinna M.A. Intrahepatic bile duct primary cilia in biliary atresia. Hepatol Res. 2018;48:664–674. doi: 10.1111/hepr.13060. [DOI] [PubMed] [Google Scholar]
- 65.Wills E.S., Roepman R., Drenth J.P.H. Polycystic liver disease: ductal plate malformation and the primary cilium. Trends Mol Med. 2014;20:261–270. doi: 10.1016/j.molmed.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Whitby T., Schroeder D., Kim H.S., Petersen C., Dirsch O., Baumann U. Modifications in integrin expression and extracellular matrix composition in children with biliary atresia. Klin Padiatr. 2015;227:15–22. doi: 10.1055/s-0034-1389906. [DOI] [PubMed] [Google Scholar]
- 67.Lazaro-Dieguez F., Musch A. Cell-cell adhesion accounts for the different orientation ofcolumnar and hepatocytic cell divisions. J Cell Biol. 2017;216:3847–3859. doi: 10.1083/jcb.201608065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheung I.D., Bagnat M., Ma T.P., Datta A., Evason K., Moore J.C. Regulation of intrahepatic biliary duct morphogenesis by Claudin 15-like b. Dev Biol. 2012;361:68–78. doi: 10.1016/j.ydbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Senga K., Mostov K.E., Mitaka T., Miyajima A., Tanimizu N. Grainyhead-like 2 regulatesepithelial morphogenesis by establishing functional tight junctions through the organization of amolecular network among claudin3, claudin4, and Rab25. Mol Biol Cell. 2012;23:2845–2855. doi: 10.1091/mbc.E12-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaye A.J., Rand E.B., Munoz P.S., Spinner N.B., Flake A.W., Kamath B.M. Effect of kasai procedure on hepatic outcome in alagille syndrome. J Pediatr Gastroenterol Nutr. 2010:1–3. doi: 10.1097/MPG.0b013e3181df5fd8. [DOI] [PubMed] [Google Scholar]
- 71.Lee H.P., Kang B., Choi S.Y., Lee S., Lee S.-K., Choe Y.H. Outcome of alagille syndrome patients who had previously received kasai operation during infancy: a single center study. Pediatr Gastroenterol Hepatol Nutr. 2015;18:175–179. doi: 10.5223/pghn.2015.18.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lertudomphonwanit C., Mourya R., Fei L., Zhang Y., Gutta S., Yang L. Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia. Sci Transl Med. 2017;9:eaan8462. doi: 10.1126/scitranslmed.aan8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gitto S., Guarneri V., Sartini A., Andreone P. The use of obeticholic acid for the management of non-viral liver disease: Current clinical practice and future perspectives. Expert Rev Gastroenterol Hepatol. 2018;12:165–171. doi: 10.1080/17474124.2018.1399060. [DOI] [PubMed] [Google Scholar]
- 74.Verbeke L., Farre R., Trebicka J., Komuta M., Roskams T., Klein S. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 75.Lu W.-Y., Bird T.G., Boulter L., Tsuchiya A., Cole A.M., Hay T. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raven A., Lu W.-Y., Man T.Y., Ferreira-Gonzalez S., O'Duibhir E., Dwyer B.J. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Font-Burgada J., Shalapour S., Ramaswamy S., Hsueh B., Rossell D., Umemura A. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaub J.R., Malato Y., Gormond C., Willenbring H. Evidence against a stem cell origin ofnew hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yanger K., Knigin D., Zong Y., Maggs L., Gu G., Akiyama H. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao W., Chen K., Bolkestein M., Yin Y., Verstegen M.M.A., Bijvelds M.J.C. Dynamics of proliferative and quiescent stem cells in liver homeostasis and injury. Gastroenterology. 2017;153:1133–1147. doi: 10.1053/j.gastro.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Han X., Chen H., Huang D., Chen H., Fei L., Cheng C. Mapping human pluripotent stem cell differentiation pathways using high throughput single-cell RNA-sequencing. Genome Biol. 2018;19:47. doi: 10.1186/s13059-018-1426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kegel V., Deharde D., Pfeiffer E., Zeilinger K., Seehofer D., Damm G. Protocol for isolation of primary human hepatocytes and corresponding major populations of non-parenchymal liver cells. J Vis Exp. 2016;109 doi: 10.3791/53069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gilbert M.A., Spinner N.B. Alagille syndrome: genetics and functional models. Curr Pathobiol Rep. 2017;5:233–241. doi: 10.1007/s40139-017-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cnossen W.R., Drenth J.P.H. Polycystic liver disease: an overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis. 2014;9:69. doi: 10.1186/1750-1172-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kobelska-Dubiel N., Klincewicz B., Cichy W. Liver disease in cystic fibrosis. Prz Gastroenterol. 2014;9:136–141. doi: 10.5114/pg.2014.43574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leeuwen L., Fitzgerald D.A., Gaskin K.J. Liver disease in cystic fibrosis. Paediatr Respir Rev. 2014;15:69–74. doi: 10.1016/j.prrv.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Srinath A., Shneider B.L. Congenital hepatic fibrosis and autosomal recessive polycystic kidney disease. J Pediatr Gastroenterol Nutr. 2012;54:580–587. doi: 10.1097/MPG.0b013e31824711b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchez-Valle A., Kassira N., Varela V.C., Radu S.C., Paidas C., Kirby R.S. Biliary atresia: epidemiology, genetics, clinical update, and public health perspective. Adv Pediatr. 2017;64:285–305. doi: 10.1016/j.yapd.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 89.Boberg K.M. Biliary Disease. Springer; 2017. The clinical burden of biliary disease: A global perspective; pp. 1–15. [Google Scholar]
- 91.Rojas C.P., Bodicharla R., Campuzano-Zuluaga G., Hernandez L., Rodriguez M.M. Autoimmune hepatitis and primary sclerosing cholangitis in children and adolescents. Fetal Pediatr Pathol. 2014;33:202–209. doi: 10.3109/15513815.2014.898721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khanlou H., Sass D., Rothstein K., Manzarbeitia C., Reich D., Jacobson L. Idiopathic adulthood ductopenia: case report and review of the literature. Arch Intern Med. 2000;160:1033–1036. doi: 10.1001/archinte.160.7.1033. [DOI] [PubMed] [Google Scholar]
- 93.Culver E.L., Barnes E. IgG4-related sclerosing cholangitis. Clin Liver Dis (Hoboken) 2017;10:9–16. doi: 10.1002/cld.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bergquist A., von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 95.Ryan M.J., Bales C., Nelson A., Gonzalez D.M., Underkoffler L., Segalov M. Bile duct proliferation in Jag1/fringe heterozygous mice identifies candidate modifiers of the alagille syndrome hepatic phenotype. Hepatology. 2008;48:1989–1997. doi: 10.1002/hep.22538. [DOI] [PubMed] [Google Scholar]
- 96.McCright B., Lozier J., Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 97.Loomes K.M., Russo P., Ryan M., Nelson A., Underkoffler L., Glover C. Bile duct proliferation in liver-specific Jag1 conditional knockout mice: effects of gene dosage. Hepatology. 2007;45:323–330. doi: 10.1002/hep.21460. [DOI] [PubMed] [Google Scholar]
- 98.Geisler F., Nagl F., Mazur P.K., Lee M., Zimber-Strobl U., Strobl L.J. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 99.Zong Y., Panikkar A., Xu J., Antoniou A., Raynaud P., Lemaigre F. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Antoniou A., Raynaud P., Cordi S., Zong Y., Tronche F., Stanger B.Z. Intrahepatic bile ducts develop according to a new mode of Tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanley J., Dhar D.K., Mazzacuva F., Fiadeiro R., Burden J.J., Lyne A.-M. Vps33b is crucial for structural and functional hepatocyte polarity. J Hepatol. 2017;66:1001–1011. doi: 10.1016/j.jhep.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moser M., Matthiesen S., Kirfel J., Schorle H., Bergmann C., Senderek J. A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2005;41:1113–1121. doi: 10.1002/hep.20655. [DOI] [PubMed] [Google Scholar]
- 103.Lu W., Fan X., Basora N., Babakhanlou H., Law T., Rifai N. Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nat Genet. 1999;21:160–161. doi: 10.1038/5944. [DOI] [PubMed] [Google Scholar]
- 104.Boulter C., Mulroy S., Webb S., Fleming S., Brindle K., Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci. 2001;98:12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S. ERK1/2-dependent vascular endothelial growth factor signaling sustains cystgrowth in polycystin-2 defective mice. Gastroenterology. 2010;138 doi: 10.1053/j.gastro.2009.09.005. (360–371.e7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pritchard L., Sloane-Stanley J.A., Sharpe J.A., Aspinwall R., Lu W., Buckle V. A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum Mol Genet. 2000;9:2617–2627. doi: 10.1093/hmg/9.18.2617. [DOI] [PubMed] [Google Scholar]
- 108.Wu G., D'Agati V., Cai Y., Markowitz G., Park J.H., Reynolds D.M. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 109.Higashiyama H., Ozawa A., Sumitomo H., Uemura M., Fujino K., Igarashi H. Embryonic cholecystitis and defective gallbladder contraction in the Sox17-haploinsufficient mouse model of biliary atresia. Development. 2017;144:1906–1917. doi: 10.1242/dev.147512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fedeles S.V., Tian X., Gallagher A.-R., Mitobe M., Nishio S., Lee S.H. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet. 2011;43:639–647. doi: 10.1038/ng.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oertelt S., Lian Z.-X., Cheng C.-M., Chuang Y.-H., Padgett K.A., He X.-S. Antimitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 113.Tsuda M., Zhang W., Yang G.-X., Tsuneyama K., Ando Y., Kawata K. Deletion of interleukin (IL)-12p35 induces liver fibrosis in dominant-negative TGFβ receptor type II mice. Hepatology. 2013;57:806–816. doi: 10.1002/hep.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yoshida K., Yang G.-X., Zhang W., Tsuda M., Tsuneyama K., Moritoki Y. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wakabayashi K., Lian Z.-X., Moritoki Y., Lan R.Y., Tsuneyama K., Chuang Y.-H. IL-2 receptor α−/− mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 116.Yao Y., Yang W., Yang Y.-Q., Ma H.-D., Lu F.-T., Li L. Distinct from its canonical effects, deletion of IL-12p40 induces cholangitis and fibrosis in interleukin-2Rα−/− mice. J Autoimmun. 2014;51:99–108. doi: 10.1016/j.jaut.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Irie J., Wu Y., Wicker L.S., Rainbow D., Nalesnik M.A., Hirsch R. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koarada S., Wu Y., Fertig N., Sass D.A., Nalesnik M., Todd J.A. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–2323. doi: 10.4049/jimmunol.173.4.2315. [DOI] [PubMed] [Google Scholar]
- 119.Salas J.T., Banales J.M., Sarvide S., Recalde S., Ferrer A., Uriarte I. Ae2a,b-Deficient Mice Develop Antimitochondrial Antibodies and Other Features Resembling Primary Biliary Cirrhosis. Gastroenterology. 2008;134:1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 120.Zhang W., Sharma R., Ju S.-T., He X.-S., Tao Y., Tsuneyama K. Deficiency in regulatory T cells results in development of antimitochondrial antibodies and autoimmune cholangitis. Hepatology. 2009;49:545–552. doi: 10.1002/hep.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsuneyama K., Nose M., Nisihara M., Katayanagi K., Harada K., Nakanuma Y. Spontaneous occurrence of chronic non-suppurative destructive cholangitis and antimitochondrial autoantibodies in MRL/lpr mice: possible animal model for primary biliary cirrhosis. Pathol Int. 2001;51:418–424. doi: 10.1046/j.1440-1827.2001.01223.x. [DOI] [PubMed] [Google Scholar]
- 122.Fickert P., Zollner G., Fuchsbichler A., Stumptner C., Weiglein A.H., Lammert F. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 123.Smit J.J., Schinkel A.H., Oude Elferink R.P., Groen A.K., Wagenaar E., van Deemter L. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 124.van Nieuwerk C.M., Groen A.K., Ottenhoff R., van Wijland M., van den Bergh Weerman M.A., Tytgat G.N. The role of bile salt composition in liver pathology of mdr2 (−/−) mice: differences between males and females. J Hepatol. 1997;26:138–145. doi: 10.1016/s0168-8278(97)80020-7. [DOI] [PubMed] [Google Scholar]
- 125.Nakagawa H., Hikiba Y., Hirata Y., Font-Burgada J., Sakamoto K., Hayakawa Y. Loss of liver E-cadherin induces sclerosing cholangitis and promotes carcinogenesis. Proc Natl Acad Sci. 2014;111:1090–1095. doi: 10.1073/pnas.1322731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang R., Salem M., Yousef I.M., Tuchweber B., Lam P., Childs S.J. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang R., Lam P., Liu L., Forrest D., Yousef I.M., Mignault D. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38:1489–1499. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 128.Siggs O.M., Schnabl B., Webb B., Beutler B. X-linked cholestasis in mouse due to mutationsof the P4-ATPase ATP11C. Proc Natl Acad Sci. 2011;108:7890–7895. doi: 10.1073/pnas.1104631108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Durie P.R., Kent G., Phillips M.J., Ackerley C.A. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blanco P.G., Zaman M.M., Junaidi O., Sheth S., Yantiss R.K., Nasser I.A. Induction of colitis in cftr−/− mice results in bile duct injury. Am J Physiol Liver Physiol. 2004;287:G491–G496. doi: 10.1152/ajpgi.00452.2003. [DOI] [PubMed] [Google Scholar]
- 132.Meerman L., Koopen N.R., Bloks V., Van Goor H., Havinga R., Wolthers B.G. Biliary fibrosis associated with altered bile composition in a mouse model of erythropoietic protoporphyria. Gastroenterology. 1999;117:696–705. doi: 10.1016/s0016-5085(99)70464-6. [DOI] [PubMed] [Google Scholar]
- 133.Kireva T., Erhardt A., Tiegs G., Tilg H., Denk H., Haybaeck J. Transcription factor Fra-1 induces cholangitis and liver fibrosis. Hepatology. 2011;53:1287–1297. doi: 10.1002/hep.24175. [DOI] [PubMed] [Google Scholar]
- 134.Woods A., Heslegrave A.J., Muckett P.J., Levene A.P., Clements M., Mobberley M. LKB1 is required for hepatic bile acid transport and canalicular membrane integrity in mice. Biochem J. 2011;434:49–60. doi: 10.1042/BJ20101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan X., Yuan Y., Zeng G., Apte U., Thompson M.D., Cieply B. β-Catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Durchschein F., Krones E., Pollheimer M.J., Zollner G., Wagner M., Raufman J.-P. Genetic loss of the muscarinic M 3 receptor markedly alters bile formation and cholestatic liver injury in mice. Hepatol Res. 2018;48:E68–E77. doi: 10.1111/hepr.12928. [DOI] [PubMed] [Google Scholar]
- 137.Hiebler S., Masuda T., Hacia J.G., Moser A.B., Faust P.L., Liu A. The Pex1-G844D mouse: A model for mild human Zellweger spectrum disorder. Mol Genet Metab. 2014;111:522–532. doi: 10.1016/j.ymgme.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Han X., Wang R., Zhou Y., Fei L., Sun H., Lai S. Mapping the Mouse Cell Atlas by Microwell-Seq. Cell. 2018;172:1091–1107. doi: 10.1016/j.cell.2018.02.001. (e17) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2