Abstract
One-anastomosis gastric bypass is an attractive bariatric procedure. It is effective in weight loss and comorbidity resolution. It is a relatively simple and fast operation with low complication rates that make it a suitable option in super-obese individuals. Although not proven yet, there are some concerns about its long-term safety profile in terms of biliary reflux, marginal ulcer, and esophagogastric malignancy. In this article, we review the technique of this procedure and discuss about some practical surgical highlights. Furthermore, we overview studies performed about this procedure and compare it to some other well-established bariatric operations, while providing a detailed study about the facts related to its outcomes and complications.
Keywords: Bariatric surgery, morbid obesity, one-anastomosis gastric bypass
INTRODUCTION
Obesity and type 2 diabetes mellitus (T2DM) are among the most important health issues in the world. Based on the National Health and Nutrition Examination Survey (2013–2014), it is estimated that 32.7% of US adults are overweight, 37.9% are obese, and 7.7% are morbidly obese.[1] Different types of bariatric and metabolic surgeries have been developed to address this problem which are the most effective and long-lasting treatments for morbid obesity, metabolic syndrome, and their related comorbidities.[2,3]
One-anastomosis gastric bypass (OAGB), first described by Rutledge in 1997,[4] is also known as mini-gastric bypass (MGB) or omega-loop gastric bypass. In surgical technique point of view, it is basically a loop anastomosis creation between a long and vertical lesser curvature-based gastric pouch and jejunum in 150–200 cm distance from the Treitz ligament[4,5,6,7,8,9] that is different from the procedure performed by Mason and Ito.[10]
Although OAGB raised severe criticism after its introduction,[11] in some recent articles,[12,13] it has favorable results in weight loss and obesity-related comorbidities, with a low rate of mid- and long-term complications.[4,5,6,7,8,9,12,13,14,15,16]
In this article, we review this procedure as an effective bariatric operation and discuss about its advantages and complications. We also focused on specific technical details to perform this procedure safely.
PROCEDURE
We searched in PubMed and Google Scholar using keywords such as “One Anastomosis Gastric Bypass (OAGB),” “Mini Gastric Bypass (MGB),” “Omega Loop Gastric Bypass,” “surgical method,” “Dumping Syndrome,” “Stapler Line Complications,” “Severe Malnutrition,” “Gastro-Esophageal Reflux Disease (GERD),” “Reflux,” “Diarrhea,” “Excessive weight loss,” “Marginal Ulcer,” and “Complication” to find related articles to OAGB/MGB and complications that indexed till February 2018. These papers were selected based on their relevancy to this issue. Relevancy is evaluated by the authors in terms of the article content. The abstracts without full texts or non-English papers were excluded from the study. Full-text original articles including case reports and case series and review articles were included in the study. At the next step, 93 articles were studied and 59 of them were selected to be reviewed in this study [Figure 1]. Different aspects of this procedure such as techniques, complications, and controversies were extracted from these papers. Some other general topics such as stapler line complications were extracted from articles not specifically related to this operation but mainly in the field of bariatric surgery. The definition of variables such as gastroesophageal reflux disease (GERD) and marginal ulcer was based on their general concept among surgeons because these topics are beyond the scope of this article.
Figure 1.
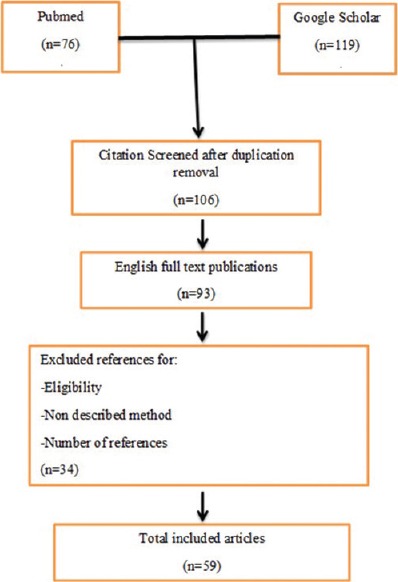
Flow diagram of article selection
RESULTS
Weight loss/comorbidity resolution
An overall about 70% of percentage excess body weight loss is reported in various literatures.[5,6,7,14,15,17,18,19] Lee et al. reported better results for OAGB in terms of weight loss in comparison to Roux-en-Y gastric bypass (RYGB).[15] This study also reported an excess weight loss (EWL) of 72% ± 19.3% in 10 years. In a meta-analysis of clinical trials about different methods of bariatric surgeries, a decrease of 11.3 kg/m2 from baseline body mass index (BMI) was reported after OAGB in 1 year[20] that was comparable to some powerful malabsorptive procedures such as biliopancreatic (BP) diversion and jejunoileal bypass.
Advantages
The OAGB does not have various limitations of the other operations and offers many features of an ideal bariatric operation.[4,5,17] OAGB has excellent results in weight loss, comorbidity treatment, and patient satisfaction in both short and long term.[18,19,21,22,23] It also offers the ease of revision or reversal procedures.[7,8,24] In fact, OAGB has the powerful potentials of a simple malabsorptive bariatric procedure. It has both gastric and intestinal components that induce hormonal alterations which can result in weight loss and resolution of comorbidities in morbidly obese patients.[8,14,15,21,22] Based on its relative simple operation technique, this procedure can be used easily in super-obese individuals.[25] In a comparative study, better results achieved by OAGB in comparison to RYGB in terms of weight loss in different time intervals. EWL in OAGB group at 6 months was similar to RYGB group in 12 months (76.3% vs. 71%). EWL in 12 months in OAGB group was 89% (71% in RYGB). Mean BMI was lower in OAGB group in 6 and 12 months. At 6 months, mean BMI was 28.8 kg/m2 in OAGB versus 32.3 in RYGB. In 12 months, this index was 26.8 in OAGB versus 30.4 in RYGB group.[23] It seems that OAGB has more efficacy in weight loss in comparison with RYGB.
Regarding RYGB as a gold standard bariatric procedure,[26] OAGB is an alternative for it in terms of efficacy and risk of complications. In operation technique, OAGB has two definite advantages. With a longer gastric pouch, creation of a tension-free gastrojejunostomy (GJ) is more possible without second anastomosis (jejunojejunostomy) with its complication such as internal hernia (IH) that makes the operation simpler and faster to perform. Although there are few case reports of internal herniation after OAGB,[27] it has less prevalence comparing to 9% risk of internal herniation after RYGB in long term.[28,29]
Technical details
Different techniques are being used by the authors. Regardless of general topics, creation of a long gastric pouch is an important part of this procedure. After dissection of the gastrohepatic omentum adjacent to lesser curve just below the crow's foot and entering retrogastric space, the first 45-mm stapler is fired in a relative perpendicular direction to the lesser curvature. It is a key point to emphasize that creating a long pouch is most desirable in order to keep bile stream away from the esophagus as much as possible. It has been shown that shorter pouch <9 cm is correlated with postoperative duodenogastroesophageal reflux.[24] Moreover, this will help to perform a tension-free anastomosis.[24] Then, next staplers are fired parallel to the lesser curve up to the His angle in the upper portion of the stomach 1–2-cm lateral to esophagogastric junction. Authors recommend the creation of a narrow pouch on a 36–40 Fr bougie. There are some variations to this procedure, in particular, “antireflux afferent limb” described by Carbajo et al.[6]
After visualization of Treitz ligament, GJ is created 150–200 cm distal to it. Although longer limb can induce greater weight loss, major complications such as EWL and malnutrition are more common in longer limb lengths,[8] especially longer than 250 cm.[30] GJ anastomosis usually is created with a 45-mm stapler, and competency of the anastomosis can be assured by a leak test.[4,5,8,15,18]
HOW TO PREVENT INTRAOPERATIVE COMPLICATIONS
Stapler line bleeding
Appropriate stapler size (height) for the tissue is recommended. Using longer stapler height (like green cartridges) for thin tissues may cause bleeding from stapler line or intraluminal bleeding at the site of. These may lead to early postoperative intra-abdominal or upper gastrointestinal (GI) hemorrhage. On the other hand, using short height cartridges (like white) on a thick tissue can be a risk factor for leakage from the anastomosis or stapler line.[31]
Shipping safety cover should not be removed until the stapler is loaded into the instrument. Stapler gun handle must be motion free while firing, especially while creating the anastomosis. Usually, waiting at least 15 s before firing allows adequate compression time before cutting the tissue by staplers.[30] After a few seconds of delay following jaws lock, the stapler gun should fire slowly.[3,31,32,33]
Complications and management
OAGB in comparison to RYGB has lower complication rate and equal efficacy in weight loss. Lee et al. reported that OAGB is a simpler operation in comparison to RYGB and had lower major complication rate (1.8% vs. 3.2%).[34] In another study, OAGB is reported at least equally effective in EWL comparing to RYGB (about 73% vs. 60%).[15]
Leak
Overall incidence of leak is reported 0.1%–1.9% by Mahawar et al. in their systematic review.[35] This incidence is comparable with the leak rates in RYGB (0.1%–5.6%)[36] or is lower than laparoscopic sleeve gastrectomy (LSG, 2.4%).[37] Chevallier et al. showed a 0.6% leak rate which is similar to other studies.[6,7,15,18,19,30] This can be justified by a good blood supply to the gastric pouch due to a tension-free anastomosis in a narrow and thin wall stomach. Reports of leak rate in RYGB are from 0.19%[38] to 3.1%.[27] In a study by Genser et al., 35 cases of leak were reported from 2321 OAGB operations (about 1.5%).[39] Arterial hypertension and heavy smoking were identified as predicting factors for leak after OAGB. They explained three different types of leak. Type 1 (32%) was from the gastric pouch stapler line. Type 2 (11%) was from the GJ anastomosis. Type 3 included that 20 cases of 35 leaks (57%) had the evidence of postoperative peritonitis or intra-abdominal sepsis and abscess without any specific leakage site identified in postoperative imaging studies or during the reoperation. This 57% is undetermined leak site group. None of the leak cases were from the remnant stomach.[39] The most common clinical symptoms and signs were abdominal pain (77%), fever (71%), and tachycardia (66%). Fifteen patients had leak symptoms before hospital discharge and 9 of them (60%) had high levels of amylase in their drain fluids (>400 IU/L).[39]
Another study was performed by Masoomi et al. about predictive factors for leaks after gastric bypass surgeries.[40] They showed that age older than 50 years, male sex, open procedure technique, congestive heart failure, chronic lung disease, and chronic renal failure are factors associated with higher rate of leaks.[40]
Lee et al. showed that perioperative leak rate in OAGB was comparable with RYGB (1.3% vs. 1.4%).[15] Musella et al. reported 0.4% early leak rate in their series.[30] Noun et al. reported 5 leaks of 1000 OAGB performed in a study (0.5%) and all of their leaks were from the gastric pouch and none of them was from the anastomosis.[7] If the location of leak is identified, it must be suture repaired. Any intra-abdominal abscess must be drained promptly. Intra-abdominal irrigation using saline or lactated ringer is suggested in some papers.[39] In a chronic leak with a severely damaged gastric tissue that performing any anastomosis is not safe due to inflammation and fibrosis, a proximal gastrectomy and esophagojejunostomy can be considered. In any case, the least invasive option must be tried because the anatomy is deteriorated, and tissues are not very suitable for an extensive procedure.[32]
Most of the leaks happen after the patient discharge.[40] The same scenario is shown in SG literatures.[37] This will emphasize the importance of a close follow-up after different bariatric procedures.
In any suspicion for leakage after surgery, an aggressive management strategy must be employed by early operative re-exploration[36,39,40] that can be done by laparoscopy in most cases.
Inadequate weight loss
Inadequate weight loss is relatively uncommon in OAGB. In a study by Lee et al. on 1322 cases of OAGB, only 8 patients (1.7%) underwent revision surgery due to inadequate weight loss.[24] Some of these patients can be approached by diet modification. However, intractable cases can be managed easily by increasing the length of bypassed jejunum or reducing the size of gastric pouch. Revision of this operation to other more potent procedures such as BP Diversion-Duodenal Switch (BPD-DS) is another option which is a more complex operation with higher complication rate.
Malnutrition and excessive weight loss
This can be a more common indication for revision surgery after OAGB.[22] Some studies on RYGB showed that the BP limb length has more efficacy on weight loss in comparison to alimentary limb.[41] This can be justified by the greater effect on malabsorption. All the jejunum lengths bypassed in OAGB are accounted as BP limb, and this may rationalize the greater impact of OAGB on malnutrition at least in theory. This can be favorable in super-obese patients. In such cases, the decreased intake of calories and nutrients can lead to excessive weight loss or nutritional deficiencies. Hence, regular follow-up is necessary in patient's lifetime, and in the event of excessive weight loss or a specific deficiency, treatment such as extra supplements may be necessary. There are few reports of mortality due to severe malnutrition and hypoalbuminemia after OAGB.[42]
Due to stronger malabsorptive effects, OAGB has been reported to be accompanied with lower hemoglobin levels than RYGB[15] but the albumin levels were similar after both procedures. A report of severe hypoalbuminemia and steatohepatitis leading to death has been published in 2017.[42] In another study, two cases of excessive weight loss and hypoalbuminemia from 1000 cases of OAGB have been reported[43] who probably needed reversal operation. However, some of these complications are preventable by close cooperation between the patient and bariatric team. High-quality protein content diet and necessary supplements as well as iron, multivitamins, and trace elements must be recommended to the patients.
In a study by Lee et al., about 40% of revisions after OAGB were due to malnutrition.[24] In some cases (0.5%–1% in Rutledge's series), significant excessive weight loss and deficiencies have been treated by reversal of the OAGB.[8,44] Fortunately, it is a very simple procedure involving division of the GJ and performing a gastrogastrostomy. This is one of the real advantages of the OAGB: it is an easily reversible procedure if there is any necessity to do.[4]
Revising the operation to a LSG is another valuable option here. After GJ takes down, distal part of the pouch is anastomosed to the antral part of the remnant stomach. After releasing the greater curvature from about 6 cm to pylorus up to the previously divided stomach, a vertical SG is performed by repeating stapler firing parallel to the lesser curve while a calibration tube or an endoscope has been advanced through the newly made gastrogastrostomy not only to protect this anastomosis but also to avoid a tight or a twisted SG. With this strategy, patients are offered improvement in their malnutrition without significant changes in their BMI.[26]
Bile reflux
About 1%–2% of patients complain of bilious vomiting once in 2 or 3 months. Musella et al. reported an overall 4% rate for bile reflux (BR) in OAGB and found a statistical correlation between postoperative reflux with pouches shorter than 9 cm.[30] They also found a correlation with preoperative GERD.[30] Regarding the BR issue, OAGB is sometimes compared to Mason's loop GJ which had a great risk of BR esophagitis.[10] However, it must be noticed that in Mason operation, anastomosis is constructed by a horizontally oriented gastric pouch that leads to a close contact between bilious gastric contents and the esophagus, but this is not the case in OAGB which has a long and vertical gastric pouch. Taking these into account, GERD is a rare problem after OAGB by performing the anastomosis to the lower part of the stomach. This is supported in a study performed by Chevallier et al.[45] They performed upper GI endoscopy and biopsy in 2 and 4 years after OAGB and found just foveolar hyperplasia (a sign of BR) in about 17% at 2 years and 4.6% at 4 years postoperatively without any dysplasia or metaplasia. In symptomatic, severe, and intractable BR, conversion of OAGB to RYGB is both simple and effective that can be done laparoscopically.
The first step is the addition of probiotic foods such as yogurt and avoiding inciting foods such high-fat or high-volume meals. At first, medical treatment for a marginal ulcer (i.e., proton-pump inhibitors [PPI]) is recommended. However, in severe and intractable cases, a revisional procedure should be considered. Revision to RYGB can be performed by anastomosing jejunum at least 50 cm distal to previous GJ to a distal part of BP limb and then cutting the jejunum between these two anastomoses.[28] In our experience, side-to-side Braun jejunojejunostomy had not good efficacy for controlling the BR.
Marginal ulcer
The incidence of marginal ulcer is 1%–6% in multiple studies which is similar to RYGB.[4,5,15,18,44] It is possible that this complication is underestimated because some asymptomatic cases can exist while its reported incidence is widely based on endoscopic ulcer detection in symptomatic patients. However, this can be true for RYGB too. A concern of marginal ulcer at the GJ site is always present in any gastric bypass procedure. This has been attributed to continuous acid secretion by the gastric pouch that causes erosions at the edges of GJ. Cigarette smoking and using nonsteroidal anti-inflammatory agents are strong risk factors.[29] GJ creation with permanent suture materials may lead to develop stitch ulcer at the anastomosis site. A large or dilated gastric pouch can result in excessive acid load in contact with the jejunum mucosa and inducing ulcer at the jejunal side of the anastomosis. Furthermore, a gastrogastric fistula can have an ulcerogenic effect by acid reflux from gastric remnant into the gastric pouch. Considering that gastric pouch produced acid load is a major etiologic factor, creating a narrow but long pouch and prescribing a PPI after the procedure should reduce its incidence. Smoking cessation and Helicobacter pylori eradication must be considered preoperatively in all patients.[29,46]
The presence of ischemic tissues near the anastomosis can also lead to these ulcers, but their exact importance is not studied yet. Kular et al.[18] reported very low incidence of postbariatric marginal ulcers in the state of Punjab in India, probably due to the fresh vegetarian diet and very minimal use of cigarette smoking.[21]
The most common symptom is abdominal pain. Nausea, vomiting, hematemesis, anemia, and signs related to anastomosis stricture are other clinical manifestations. Upper GI endoscopy is the best workup study. An upper GI contrast study is useful to document a gastrogastric fistula.[22]
When the diagnosis is established, medical management with a PPI plus sucralfate (1 g/oral liquid every 6 h) should be started. Most ulcers will respond to this therapy.
In rare cases of intractable marginal ulcers not responding to medical therapy, a revisional procedure can be considered. After separating the gastric pouch and GJ from gastric remnant, GJ is excised. If the gastric pouch is dilated, trimming the pouch from the lateral side parallel to the lesser curve will reduce its size. After excision of the anastomosis, a new anastomosis is reconstructed between gastric pouch and jejunum.
Risk of cancer
One of the most important criticisms about the OAGB is its potential risk for gastric or esophageal cancer. It has been mainly derived from the fact that exposure of gastroesophageal (GE) junction to the alkaline biliary reflux is a risk factor for Barrett esophagus (columnar dysplasia in distal esophageal epithelium) which is considered a premalignant lesion.[47] Some articles have shown that restrictive bariatric procedures, including gastric banding (AGB) and SG, can increase GE acid reflux leading to Barrett esophageal dysplasia and resultant GE adenocarcinoma.[48,49] In a systematic review about GE cancer after bariatric surgeries, a total of 33 cases have been reported, and 15 of them were after restrictive procedures.[50] In terms of cancer risk, OAGB has been considered conventionally related to Billroth II GJ although the increased risk of cancer is not proven after a partial gastrectomy in humans.[51] In another study, risk of cancer is slightly increased after 20–30 years in patients with gastric ulcer that operated with partial gastrectomy and Billroth II,[52] but it must be considered that these patients suffer from a gastric ulcer primarily and we know that a gastric ulcer at least doubles the risk of cancer. Besides, the effect of H. pylori was not considered in this study because it was not recognized well at that time.[53] However, it must be noted that there is an important difference between OAGB and Billroth II because there is a long and narrow gastric pouch in OAGB. Moreover, biliary secretions are diluted in about 200-cm distance from the Treitz ligament. Only four cases of gastric cancer have been reported after loop gastric bypass (not OAGB), but three of them were in the excluded stomach (remnant).[50] These remnant cancers may not basically be related to an OAGB operation. No gastric pouch cancer is reported after OAGB yet and the very few reports are all in the excluded part of stomach.[54] As a conclusion, gastric cancer due to OAGB has not been proved yet.
Dumping syndrome
Dumping syndrome is a phenomenon which is due to a fast entry of high-calorie and hyperosmolar food contents into the small intestine. High volume and rapid eating of simple sugars and carbohydrates can cause this phenomenon. In OAGB, the GJ can result in rapid transfer of hyperosmolar food material from the gastric pouch into the jejunum. In general, these patients find that sweets and liquid calories make them symptomatic. Sodas, ice cream, and candy are difficultly tolerated in OAGB patients except in small volumes, taken slowly. High-volume fatty foods are also very poorly tolerated and lead to bloating, diarrhea, and steatorrhea. The symptoms of dumping syndrome with OAGB can usually be controlled with simple dietary modifications. Medications such as somatostatin (octreotide) and acarbose have been used for dumping syndrome with a good success rate.[55]
Diarrhea
Most patients report increased frequency of defecation after OAGB. In Rutledge study, the rate of stool passage has been increased from 0.5 per day preoperatively to twice daily after the OAGB. Postbariatric surgery diarrhea has several etiologies. Besides, there is a relationship between obesity and diarrhea.[56] Short bowel syndrome, defined by lack of absorptive surface, occurs in about 4% of patients after bariatric surgery.[57] Treatment starts with supportive measures. In intractable cases, surgical options such as common limb lengthening or feeding gastrostomy in remnant can be considered.[43] Lactase enzyme deficiency in the intestinal mucosa leads to lactose malabsorption and intolerance with diarrhea. Treatment consists of decreasing or stopping the dairy products, using fermented dairy with low lactose content such as yogurt and/or giving lactase enzyme orally. Protein loosing enteropathy and resultant hypoalbuminemia is another complication mostly seen after malabsorptive bariatric surgeries such as BPD-DS or long-limb gastric bypass. Reduced gastric acid production, pancreatic atrophy, and small intestine bacterial overgrowth (SIBO) may play a role here. Daily protein intake must be 60–120 g in postbariatric patients.[58] Total parenteralnutrition (TPN), common limb lengthening, or reversal operations are other options in more serious cases.
T2DM, SIBO, celiac disease, bile acid malabsorption, inadvertent vagotomy during gastric bypass procedure, pancreatic exocrine insufficiency, dumping syndrome, and undiagnosed preoperative inflammatory bowel disease are other possible causes of diarrhea after any malabsorptive bariatric surgery, including OAGB.[58]
Internal hernia
The risk for IH is much lower in OAGB compared to RYGB. However, there are reports of IH after OAGB in the form of Petersen hernia.[59] Although the risk of IH in OAGB is significantly lower than RYGB, this is not zero, so in the presence of small bowel obstructive symptoms after OAGB, this diagnosis should be considered and proper diagnostic and therapeutic measures employed in an emergent manner.[42]
CONCLUSION
OAGB is a simple and effective procedure of bariatric surgery. It is highly efficient in managing morbid obesity and its comorbidities while having a reasonable complication rate in comparison to RYGB. On the other hand, it is a relatively simpler operation with a shorter learning curve to be completed by surgeons. It has been shown that this procedure has better results with less complication risk in comparison with RYGB. However, some long-term series in recent years showed that it is a promising procedure with good results and low complication rate while being technically a simpler operation comparing to RYGB. OAGB is a well-established procedure and can be one procedure of choice in morbidly obese and super-obese patients; however, it needs more long-term studies to evaluate all aspects.
Financial support and sponsorship
This study has been financially supported by Iran University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Prevention CfDCa Older Persons’ Health. Centers for Disease Control and Prevention. 2015. [Last accessed on 2015 Mar 19]. Available from: http://www.cdc.gov/nchs/fastats/older-american-health.htm .
- 2.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 4.Rutledge R. The mini-gastric bypass: Experience with the first 1,274 cases. Obes Surg. 2001;11:276–80. doi: 10.1381/096089201321336584. [DOI] [PubMed] [Google Scholar]
- 5.Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: Six-year study in 2,410 patients. Obes Surg. 2005;15:1304–8. doi: 10.1381/096089205774512663. [DOI] [PubMed] [Google Scholar]
- 6.Carbajo M, García-Caballero M, Toledano M, Osorio D, García-Lanza C, Carmona JA, et al. One-anastomosis gastric bypass by laparoscopy: Results of the first 209 patients. Obes Surg. 2005;15:398–404. doi: 10.1381/0960892053576677. [DOI] [PubMed] [Google Scholar]
- 7.Noun R, Skaff J, Riachi E, Daher R, Antoun NA, Nasr M, et al. One thousand consecutive mini-gastric bypass: Short- and long-term outcome. Obes Surg. 2012;22:697–703. doi: 10.1007/s11695-012-0618-z. [DOI] [PubMed] [Google Scholar]
- 8.Lee WJ, Wang W, Lee YC, Huang MT, Ser KH, Chen JC, et al. Laparoscopic mini-gastric bypass: Experience with tailored bypass limb according to body weight. Obes Surg. 2008;18:294–9. doi: 10.1007/s11695-007-9367-9. [DOI] [PubMed] [Google Scholar]
- 9.Piazza L, Ferrara F, Leanza S, Coco D, Sarvà S, Bellia A, et al. Laparoscopic mini-gastric bypass: Short-term single-institute experience. Updates Surg. 2011;63:239–42. doi: 10.1007/s13304-011-0119-y. [DOI] [PubMed] [Google Scholar]
- 10.Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47:1345–51. doi: 10.1016/s0039-6109(16)38384-0. [DOI] [PubMed] [Google Scholar]
- 11.Fisher BL, Buchwald H, Clark W, Champion JK, Fox SR, MacDonald KG, et al. Mini-gastric bypass controversy. Obesity Surg. 2001;11:773–7. doi: 10.1381/09608920160558777. [DOI] [PubMed] [Google Scholar]
- 12.Collins BJ, Miyashita T, Schweitzer M, Magnuson T, Harmon JW. Gastric bypass: Why Roux-en-Y? A review of experimental data. Arch Surg. 2007;142:1000–3. doi: 10.1001/archsurg.142.10.1000. [DOI] [PubMed] [Google Scholar]
- 13.Johnson WH, Fernanadez AZ, Farrell TM, Macdonald KG, Grant JP, McMahon RL, et al. Surgical revision of loop (“mini”) gastric bypass procedure: Multicenter review of complications and conversions to Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3:37–41. doi: 10.1016/j.soard.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Chevallier JM, Chakhtoura G, Zinzindohoué F. Mini bypass gastrique. J Chir. 2009;146:60–4. doi: 10.1016/j.jchir.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Lee WJ, Ser KH, Lee YC, Tsou JJ, Chen SC, Chen JC, et al. Laparoscopic Roux-en-Y vs.mini-gastric bypass for the treatment of morbid obesity: A 10-year experience. Obes Surg. 2012;22:1827–34. doi: 10.1007/s11695-012-0726-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim Z, Hur KY. Laparoscopic mini-gastric bypass for type 2 diabetes: The preliminary report. World J Surg. 2011;35:631–6. doi: 10.1007/s00268-010-0909-2. [DOI] [PubMed] [Google Scholar]
- 17.Musella M, Milone M, Bellini M, Sosa Fernandez LM, Leongito M, Milone F, et al. Effect of bariatric surgery on obesity-related infertility. Surg Obes Relat Dis. 2012;8:445–9. doi: 10.1016/j.soard.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Kular KS, Manchanda N, Rutledge R. A 6-year experience with 1,054 mini-gastric bypasses- first study from indian subcontinent. Obes Surg. 2014;24:1430–5. doi: 10.1007/s11695-014-1220-3. [DOI] [PubMed] [Google Scholar]
- 19.Musella M, Susa A, Greco F, De Luca M, Manno E, Di Stefano C, et al. The laparoscopic mini-gastric bypass: The italian experience: Outcomes from 974 consecutive cases in a multicenter review. Surg Endosc. 2014;28:156–63. doi: 10.1007/s00464-013-3141-y. [DOI] [PubMed] [Google Scholar]
- 20.Padwal R, Klarenbach S, Wiebe N, Birch D, Karmali S, Manns B, et al. Bariatric surgery: A systematic review and network meta-analysis of randomized trials. Obes Rev. 2011;12:602–21. doi: 10.1111/j.1467-789X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 21.Milone M, Di Minno MN, Leongito M, Maietta P, Bianco P, Taffuri C, et al. Bariatric surgery and diabetes remission: Sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol. 2013;19:6590–7. doi: 10.3748/wjg.v19.i39.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moszkowicz D, Rau C, Guenzi M, Zinzindohoué F, Berger A, Chevallier JM, et al. Laparoscopic omega-loop gastric bypass for the conversion of failed sleeve gastrectomy: Early experience. J Visc Surg. 2013;150:373–8. doi: 10.1016/j.jviscsurg.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Disse E, Pasquer A, Espalieu P, Poncet G, Gouillat C, Robert M, et al. Greater weight loss with the omega loop bypass compared to the Roux-en-Y gastric bypass: A comparative study. Obes Surg. 2014;24:841–6. doi: 10.1007/s11695-014-1180-7. [DOI] [PubMed] [Google Scholar]
- 24.Lee WJ, Lee YC, Ser KH, Chen SC, Chen JC, Su YH, et al. Revisional surgery for laparoscopic minigastric bypass. Surg Obes Relat Dis. 2011;7:486–91. doi: 10.1016/j.soard.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Peraglie C. Laparoscopic mini-gastric bypass (LMGB) in the super-super obese: Outcomes in 16 patients. Obes Surg. 2008;18:1126–9. doi: 10.1007/s11695-008-9574-z. [DOI] [PubMed] [Google Scholar]
- 26.Chen CY, Lee WJ, Lee HM, Chen JC, Ser KH, Lee YC, et al. Laparoscopic conversion of gastric bypass complication to sleeve gastrectomy: Technique and early results. Obes Surg. 2016;26:2014–21. doi: 10.1007/s11695-016-2066-7. [DOI] [PubMed] [Google Scholar]
- 27.Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–29. doi: 10.1097/00000658-200010000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amor IB, Petrucciani N, Kassir R, Al Munifi A, Piche T, Debs T, et al. Laparoscopic conversion of one anastomosis gastric bypass to a standard Roux-en-Y gastric bypass. Obes Surg. 2017;27:1398. doi: 10.1007/s11695-017-2646-1. [DOI] [PubMed] [Google Scholar]
- 29.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA, et al. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musella M, Susa A, Manno E, De Luca M, Greco F, Raffaelli M, et al. Complications following the mini/one anastomosis gastric bypass (MGB/OAGB): A multi-institutional survey on 2678 patients with a mid-term (5 Years) follow-up. Obes Surg. 2017;27:2956–67. doi: 10.1007/s11695-017-2726-2. [DOI] [PubMed] [Google Scholar]
- 31.Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M, et al. The science of stapling and leaks. Obes Surg. 2004;14:1290–8. doi: 10.1381/0960892042583888. [DOI] [PubMed] [Google Scholar]
- 32.Silecchia G, Iossa A. Complications of staple line and anastomoses following laparoscopic bariatric surgery. Ann Gastroenterol. 2018;31:56–64. doi: 10.20524/aog.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, Gagner M, et al. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: A systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231–7. doi: 10.1097/SLA.0b013e31826cc714. [DOI] [PubMed] [Google Scholar]
- 34.Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT, et al. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: A prospective randomized controlled clinical trial. Ann Surg. 2005;242:20–8. doi: 10.1097/01.sla.0000167762.46568.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahawar KK, Jennings N, Brown J, Gupta A, Balupuri S, Small PK, et al. “Mini” gastric bypass: Systematic review of a controversial procedure. Obes Surg. 2013;23:1890–8. doi: 10.1007/s11695-013-1026-8. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen HJ, Nergard BJ, Leifsson BG, Frederiksen SG, Agajahni E, Ekelund M, et al. Management of suspected anastomotic leak after bariatric laparoscopic roux-en-y gastric bypass. Br J Surg. 2014;101:417–23. doi: 10.1002/bjs.9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: A systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509–15. doi: 10.1007/s00464-011-2085-3. [DOI] [PubMed] [Google Scholar]
- 38.Dillemans B, Sakran N, Van Cauwenberge S, Sablon T, Defoort B, Van Dessel E, et al. Standardization of the fully stapled laparoscopic roux-en-Y gastric bypass for obesity reduces early immediate postoperative morbidity and mortality: A single center study on 2606 patients. Obes Surg. 2009;19:1355–64. doi: 10.1007/s11695-009-9933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genser L, Carandina S, Tabbara M, Torcivia A, Soprani A, Siksik JM, et al. Presentation and surgical management of leaks after mini-gastric bypass for morbid obesity. Surg Obes Relat Dis. 2016;12:305–12. doi: 10.1016/j.soard.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Masoomi H, Kim H, Reavis KM, Mills S, Stamos MJ, Nguyen NT, et al. Analysis of factors predictive of gastrointestinal tract leak in laparoscopic and open gastric bypass. Arch Surg. 2011;146:1048–51. doi: 10.1001/archsurg.2011.203. [DOI] [PubMed] [Google Scholar]
- 41.Nergaard BJ, Leifsson BG, Hedenbro J, Gislason H. Gastric bypass with long alimentary limb or long pancreato-biliary limb – Long-term results on weight loss, resolution of co-morbidities and metabolic parameters. Obes Surg. 2014;24:1595–602. doi: 10.1007/s11695-014-1245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kermansaravi M, Abdolhosseini MR, Kabir A, Pazouki A. Severe hypoalbuminemia and steatohepatitis leading to death in a young vegetarian female, 8 months after mini gastric bypass: A case report. Int J Surg Case Rep. 2017;31:17–9. doi: 10.1016/j.ijscr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chevallier JM, Arman GA, Guenzi M, Rau C, Bruzzi M, Beaupel N, et al. One thousand single anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year period: Outcomes show few complications and good efficacy. Obes Surg. 2015;25:951–8. doi: 10.1007/s11695-014-1552-z. [DOI] [PubMed] [Google Scholar]
- 44.Rutledge R, Kular K, Marchanda N. A comparison of the outcomes of revision of the Roux-en-Y (RNY) and mini-gastric bypass (MGB); hard vs. easy? Eur J Endosc Laparosc Surg. 2014;1:1–6. DOI: 10.14744/less.2017.69188. [Google Scholar]
- 45.Chevallier JM, Trelles N, Arienzo R, Zinzindohoue F. Obesity Surgery. New York: Springer; 2011. Endoscopic findings after laparoscopic omega gastric bypass. [Google Scholar]
- 46.Rieu PN, Jansen JB, Biemond I, Offerhaus GJ, Joosten HJ, Lamers CB, et al. Short-term results of gastrectomy with Roux-en-Y or billroth II anastomosis for peptic ulcer. A prospective comparative study. Hepatogastroenterology. 1992;39:22–6. [PubMed] [Google Scholar]
- 47.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 48.Korswagen LA, Schrama JG, Bruins Slot W, Hunfeld MA. Adenocarcinoma of the lower esophagus after placement of a gastric band. Obes Surg. 2009;19:389–92. doi: 10.1007/s11695-008-9718-1. [DOI] [PubMed] [Google Scholar]
- 49.Howard DD, Caban AM, Cendan JC, Ben-David K. Gastroesophageal reflux after sleeve gastrectomy in morbidly obese patients. Surg Obes Relat Dis. 2011;7:709–13. doi: 10.1016/j.soard.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Scozzari G, Trapani R, Toppino M, Morino M. Esophagogastric cancer after bariatric surgery: Systematic review of the literature. Surg Obes Relat Dis. 2013;9:133–42. doi: 10.1016/j.soard.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Bassily R, Smallwood RA, Crotty B. Risk of gastric cancer is not increased after partial gastrectomy. J Gastroenterol Hepatol. 2000;15:762–5. doi: 10.1046/j.1440-1746.2000.02203.x. [DOI] [PubMed] [Google Scholar]
- 52.Csendes A, Burgos AM, Smok G, Burdiles P, Braghetto I, Díaz JC, et al. Latest results (12-21 years) of a prospective randomized study comparing billroth II and Roux-en-Y anastomosis after a partial gastrectomy plus vagotomy in patients with duodenal ulcers. Ann Surg. 2009;249:189–94. doi: 10.1097/SLA.0b013e3181921aa1. [DOI] [PubMed] [Google Scholar]
- 53.Ito M, Takata S, Tatsugami M, Wada Y, Imagawa S, Matsumoto Y, et al. Clinical prevention of gastric cancer by Helicobacter pylori eradication therapy: A systematic review. J Gastroenterol. 2009;44:365–71. doi: 10.1007/s00535-009-0036-8. [DOI] [PubMed] [Google Scholar]
- 54.Wu CC, Lee WJ, Ser KH, Chen JC, Tsou JJ, Chen SC, et al. Gastric cancer after mini-gastric bypass surgery: A case report and literature review. Asian J Endosc Surg. 2013;6:303–6. doi: 10.1111/ases.12052. [DOI] [PubMed] [Google Scholar]
- 55.van Beek AP, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: Pathophysiology, diagnosis, and management. Obes Rev. 2017;18:68–85. doi: 10.1111/obr.12467. [DOI] [PubMed] [Google Scholar]
- 56.Ho W, Spiegel BM. The relationship between obesity and functional gastrointestinal disorders: Causation, association, or neither? Gastroenterol Hepatol (N Y) 2008;4:572–8. [PMC free article] [PubMed] [Google Scholar]
- 57.McBride CL, Petersen A, Sudan D, Thompson J. Short bowel syndrome following bariatric surgical procedures. Am J Surg. 2006;192:828–32. doi: 10.1016/j.amjsurg.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 58.Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544–56. doi: 10.1038/nrendo.2012.48. [DOI] [PubMed] [Google Scholar]
- 59.Kermansaravi M, Kazazi M, Pazouki A. Petersen's Space Internal Hernia after Laparoscopic One Anastomosis (Mini) Gastric Bypass. Case reports in surgery. 2018;2018 doi: 10.1155/2018/9576120. [DOI] [PMC free article] [PubMed] [Google Scholar]


