Abstract
The ability to sensitively probe and modulate electrical signals at cellular length-scale is a key challenge in the field of electrophysiology. Electrical signals play an integral role in regulating cellular behavior and in controlling biological function. From cardiac arrhythmias to neurodegenerative disorder, maladaptive phenotypes in electrophysiology can result in serious and potentially deadly medical conditions. Understanding how to monitor and control this behavior in a precise and non-invasive fashion represents an important step in developing next-generation therapeutic devices. As we develop a deeper understanding of neural network formation, electrophysiology has the potential to offer fundamental insights into the inner working of the brain. In this perspective, we will first explore traditional methods for examining neural function, briefly touching on recent genetic advances in electrophysiology, before turning to latest innovations in optical sensing and stimulation of action potentials in neurons. We will primarily focus our exploration on nongenetic optical methods, as these provide a high spatiotemporal resolution and can be achieved in a minimally invasive fashion.
Introduction
Since the first intracellular measurements of actions potentials made by Hodgkin and Huxley using saline filled glass capillaries inserted into giant squid axons1, patch clamp has become the ‘gold standard’ in the field of electrophysiology. Patch clamp provides a precise and direct measurement of ionic current exchange between the cell’s plasma membrane and the surrounding media. Unfortunately, conventional patch clamp is a time intensive process, requiring the careful manipulation of a fine tipped electrode, the delicate fabrication, polishing and maintenance of glass pipettes, and the careful consideration of electrical grounding and apparatus design to allow for precise low-noise recordings2. While high-throughput automated patch clamp platforms have recently become more readily available in industrial settings3, this is still not the case in most academic laboratories. Additionally, patch clamp has typically been limited to whole cells, or surface bound ion channels. As a result, researchers have started to examine additional, more spatially precise and less invasive methods for monitoring and stimulating neuronal electrical activities.
Two such techniques include calcium imaging and voltage sensitive dyes (VSDs), where temporary increases in intracellular calcium ion (Ca2+) concentrations can serve as an important secondary messenger for action potential propagation, while VSDs probe changes in membrane potential more directly. These fluorescent microscopy techniques offer the benefit of being able to simultaneously monitor many cells in real time, while providing spatially precise measurements. As a result, both VSDs and Ca2+ imaging has played a valuable role in advancing our understanding of neuronal signaling. However, the use of extrinsic organic fluorescent dyes comes with some innate drawbacks, such as fluorescent bleaching and effluent pumps limiting exposure times, along with potential cytotoxic effects. Additionally, in the case of Ca2+, many of these probes also show an affinity for other divalent ionic species, such as zinc and magnesium4,5, making selectively probing Ca2+ difficult.
More recently, these techniques have been combined with advances in genetic engineering and synthetic biology, to provide genetic based solutions to neuron modulation6, calcium imaging7, and voltage profiling8,9. In the case of voltage and calcium indicators, this technique usually works by linking a fluorescent resonance energy transfer (FRET) reporter with a voltage or calcium sensitive domain, both of which are in turn coupled to a site specific protein, such as a sodium ion channel9. As membrane depolarization occurs, the sensing domain responds, transducing the action potential into a mechanical signal, causing a simultaneous conformation change in the FRET reporter, resulting in a distinct fluorescent signal. When employed alone or in combination with one another10, the use of genetic reporters and neuromodulators can overcome many of the challenges inherent to exogenous fluorescent probes, such as photobleaching, and cytotoxicity. Additionally, the use of genetic approaches allows for site specific targeting, enabling precise control over which cell types and locations are expressing reporters. As a result, these techniques have been used to provide more precise spatial measurements of electrical propagation across and within neurons11, even extending to whole brain functional imaging7. Therefore, it is understandable how there is a great deal of interest surrounding genetic markers for use in electrophysiology. For a more in-depth look at genetic approaches, we recommend Lin & Schnitzer’s recent review9. Despite these advances however, there are still some deep concerns when it comes to clinical applications of these techniques.
While gene therapies have seen clinical use in human somatic cells for more than two decades now, early studies were beset by some tragic setbacks, including immunological response, and off-target gene delivery, with one instance resulting in leukemia-like symptoms12, and with another disrupting regulator networks in tissue growth causing uncontrolled cellular proliferation13. Additionally, the potential to alter gamete cells raises concerns over impacting fetal developmental and presents an ethical dilemma when considering the idea of ‘informed consent’ in unborn children. Despite these challenges, recent successes14–16 and the introduction of new gene editing technologies, such as zinc finger nucleases, transcription activator-like effector nucleases (TALENs), and CRISPR/Cas9, has brought renewed interest in the field, offering great potential in reducing off target mutations12,17. However, researchers have also begun to examine nongenetic based routes for exploring electrophysiology, including label free and nanomaterials-based approaches for monitoring and modulating neuronal activity. The hope is that by moving to these platforms, we can extend the range of what is possible in electrophysiology.
As compared to molecular probes, nanostructured materials have some potential benefits for use in optical sensing or modulation in biological systems. First, nanostructured materials can act in a bioorthogonal fashion, responding to activated triggers independent of the body’s normal signaling pathways. One early example of this is using magnetic nanoparticles as an adjunctive hypothermal treatment in cancer therapies18,19, with magnetic fields providing independent control over particle activation. Second, in contrast to genetic modification, which can act as a ‘permanent solution to a temporary problem’, nanostructured materials have the potential to act in a similar fashion to traditional drugs, where a dosage is administered for a certain lifetime, before the body is able to clear it. This is desirable in situations where an ailment is only expected to last for a limited duration. Third, the distinct size of nanostructured materials makes them uniquely situated for interfacing with biological systems20. As nanostructured materials share common dimensions with protein complexes, they can both interact with cellular biology at its natural length scale but can also be distributed in a ‘drug-like’ manner. As result, it’s possible to establish a network of electronic point-like stimulators and reporters for use in electrophysiology.
Here we will highlight several recent nongenetic strategies for measuring and modulating neuronal response. We choose to focus here on optical methods due to their high spatial and temporal precision. When coupled with nanomaterials, which have the advantage of being relatively small compared to traditional electrodes, optical methods provide an exciting opportunity for non-invasive electrical probing and stimulation.
Label Free Imaging - Soliton Model of Axonal Swelling
Moving past electrodes, one potential way to measure neuronal signal in a completely label free manner is using small, nanometer scale shifts in the membranes position and thickness as a neurons fire. To understand this behavior, we must consider how signals are propagated along an axon. In the early 1950’s, Hodgkin and Huxley (HH) proposed a mode for action potential propagation21. Going on to win a Nobel Prize in Physiology in 1963, the HH uses electrical circuit diagrams to describe neuronal behavior, treating the cell membrane as a capacitor, and ion channels as electrical conductors. While HH’s seminal work has been foundational to the field of electrophysiology, it makes very few predications about the mechanical behavior of neurons, however, there is a growing body of evidence to support that in addition to electrical signals, neurons also propagate mechanical signals during firing events.
In the 1980s, Ichiji Tasaki, who is also credited with helping discover the insulating properties of myelin sheaths22,23, pioneered a model of neuron firing where mechanical waves could propagate signals along the cell membrane24. Using light scattering across a fiber optic cable, Tasaki showed that when stimulated nerve fibers produced a barely perceptible swelling, resulting in mechanical deformations25,26. In contrast to the HH model, in Tasaki’s model nerve pulses are treated as electro-mechanical soliton waves27,28 (Figure 1a&b). Briefly, depolarization induces a change in membrane tension, which in turn causes a deformation in the cell’s radius in an adiabatic fashion, to maintain a constant pressure across the membrane29,30. The soliton theory of neuron activation is based on a thermodynamic analysis of the cell membrane, including variables such as volume, area and enthalpy changes31. As a result, the soliton model predicts that neuronal firing is an adiabatic reversible process, where negligible heat is exchanged with the surroundings. Both the HH and soliton model predict the existence of a voltage pulse during propagation, where that pulse is the signal itself in HH, while in the soliton model, it’s simply a thermodynamic biproduct of a more generic process. As a result combining the two models directly can be challenging31, however there is a growing body of evidence to support at least a portion of the soliton model.
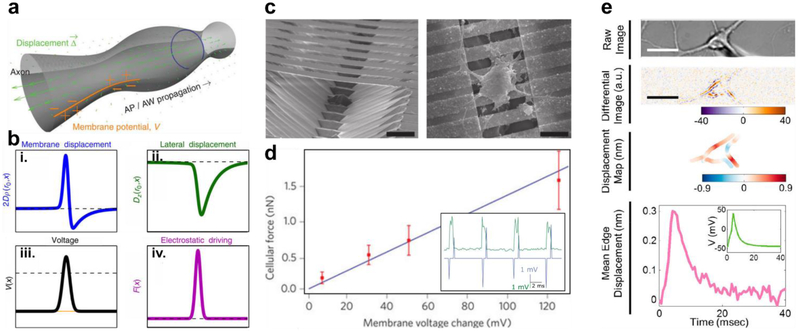
Figure 1. Soliton Waves Induce Membrane Displacement During Neuron Action Potential Firing.
(a) Illustration of a soliton action wave (AW) traveling through an axon during an action potential (AP), showing membrane displacement accompanying voltage propagation. (b) Predicted circumferential (i), 2Dρ (ro. x), and lateral (ii.), Dz(ro. x), membrane displacement as a function of axon radius, ro, and AP propagation speed, CAP such that x = z -CAPt, where z is the longitudinal direction and t is time. Corresponding voltage (iii), V(x), and mechanical force waves (iv), F(x), giving rise to the observed displacement. (c) Scanning electron micrograph of an experimental setup using piezoelectrical (PZT) nanoribbons to measure membrane displacement during action potential propagation, with (right) and without (left) neurons present (scale bars, 15 µm). (d) Calibrated PZT force response of neurons compared to membrane potential. Experimental data (red) is shown compared to predicted displacement (blue). Inset: Measured PZT nanoribbon response (green) showing lateral membrane displacement induced by spontaneous depolarization (blue). Inset: optical image showing the experimental patch clamp setup (scale bar, 12 µm). (e) DIC micrograph of a neuron (top) with corresponding differential imaging (upper middle) and calibrated displacement map (lower middle), indicating a shift in membrane position during axon firing (lower) (scale bars, 15 µm). Modified and reproduced with permission from A. Hady et al27 (a&b), T. Nguyen et al29 (c&d), and Y. Yang et al32(e). Copyright 2015 & 2012 Springer Nature, and 2018 American Chemical Society respectively.
In addition to Tasaki’s initial measurements showing tissue deformation, he also went on to investigate the soliton’s model claim about adiabatic heat exchange, showing a minimal amount of heat generated outside of the nodes of Ranvier33, at least within detectable limits. This suggested some validity to a thermodynamic approach for modeling action potential propagation. Further corroborating this is the discovery that many ion channels appear to be mechanically activable34. In a recent demonstration of this, K. Pool et al. used elastomeric pillar arrays as force transducers to stimulate dorsal root ganglia neurons35, showing that nanoindentation could evoke mechanosensitive current flow at both the neurite and the soma. Similarly, atomic force microscopy (AFM) measurements have also been used to corroborate the soliton model, with neurons showing a slight deformation during nerve firing36. As a more precise control, T. Nguyen et al. were able to carefully measure these mechanical deformations using a piezoelectric (PZT) probe29 (Figure 1c&d). In their setup, Nguyen et. al. fabricated suspended PZT nanoribbons, which can sensitively detect changes in their curvature, acting as electromechanical force transducers. To calibrate this device, they used an AFM to apply a known amount of force to the PZT nanoribbons, recording the resulting electrical signal. Next, culturing individual neurons on the device, there were able to show that during action potential propagation, axonal swelling on the order of a several nanometers occurs. Collectively, this work suggests that deformations in the cell membrane can be used as an alternative to electrical signals for monitoring neuron signaling.
More recently, Y. Yang et al. 32 were able to exploit this behavior, demonstrating that using differential mapping, these deformations can be observed optically (Figure 1e). In their setup, Y. Yang used differential interference contrast (DIC) microscopy to image neurons cultured in-vitro. Employing a spatial averaging filter to reduce shot noise, they scanned across regions of interest near the border of the cell, enhancing small changes in pixel intensity. Then, using whole cell patch clamp they evoked repeated action potentials, averaging neuronal response over a large number of cycles (~440 traces). Using a fast Fourier transform (FFT) filter, they were able to further reduce noise, enabling a detailed measurement of membrane deformation, claiming a sub-nanometer precision for their technique. This allowed them to match mechanical deformations with voltage potentials, demonstrating that both processes show a coupled behavior. To calibrate this technique, they showed that they could accurately predict nanometer scale movements in a PZT controlled stage. If repeated, this technique could serve as a substantial boon to the field of electrophysiology.
First, this technique allows for the label free imaging of action potentials. Requiring no fluorescent probes or markers, substantially reducing the potential for cytotoxic side effects. Second, the equipment needed for their technique is readily available, with many labs already having the requisite patch clamp and optical microscope setups available. Also, using no labeling reagents makes this technique further available, reducing the material cost associated with using antibodies. Additionally, as a primarily data analysis driven technique, the specialized scripts and filters needed for image processing, can be distributed quite readily. Despite these advantages however, it is difficult to imagine how this technique might be translated to an in-vivo model. They reported a minimum of ten repeated action protentional is needed to reduce shot noise to a level that yields measurable results. Although at 20 Hz, this only dictates half a second, maintaining complete stability over that time scale may prove challenging. Requiring minute measurements of membrane displacements, it would be difficult to decouple this from other mechanical perturbations present in an animal model such as those caused by respiration, or even a heartbeat. Even given these restrictions, this technique still represents an important advance for the field.
Plasmonics Enabled Optoporation for Intracellular Neural Recording
Optical methods can potentially help aid electrode design, enabling improved intimate contact with the intracellular environment for electrical recordings. To achieve better contact, researchers have continued to push smaller electrode arrays, with scaling laws playing a critical role in device design. Traditional transcranial and transdermal implants are made from rigid, bulky materials. This mismatch in material properties at the biotic-abiotic interface can lead to irritation and poor device integration37,38, creating long term limits on device performance. As a result, progress has been made in making softer, more conformal electrical devices using ultra-thin nanomaterial based fabrication techniques39–41. Such devices capitalize on distinct wave-like geometries42 and on the fact that a material’s bending stiffness is inversely proportional to that material’s characteristic length scale43, with thinner devices becoming more flexible. By moving to thin and deformable substrates, these devices are able to adapt to the soft curvilinear surface of biological tissues creating a better match in material properties.
In addition to reducing device dimensionality, researchers are also interested in increasing electrical signal, attenuating background noise and improving device performance. One way to achieve this is by transitioning from extracellular to intracellular recording devices. Initial approaches for probing intracellular components have included the use of nanoscale electrode arrays44–48, however one of the main challenge associated with this process is introducing these materials into cells in a non-invasive fashion. Current methods to achieve this include, chemical, mechanical and electroporation49, along with surface functionalization45,50,51 and some limited cases of spontaneous penetration52. However, the use of harsh methods to introduce these probes typically act in a non-site specific manner, can perturb cell activity, and introduces a ‘blind spot’ in recording time by creating interfering electrical signals.
In contrast to this, M. Dipalo et al.53 has recently shown that optical methods can be used to aid device integration. Highlighting a previously described method for generating transient holes in the cell membrane via a plasmonicly created pressure waves54, they were able to optically introduce nanocylinder electrodes intracellularly in a non-invasive manner. This allowed them to cleanly transition from extracellular to intracellular recording, with a limited transition time between the two spaces (less than 2 seconds), simultaneously increasing signal intensity. Additionally, by using an optical method, or ‘optoporation’, they were able to address electrodes on an individual basis, allowing site-selective recording of intracellular signals. This has the potential benefit of allowing simultaneous extra and intracellular recordings, which can be difficult to achieve using other entry methods. Furthermore, this can be used to select precise electrode locations, to preferentially record from certain regions of the cell, such as the axon or dendrites, leading to a better understanding of spatial inhomogeneity in cellular signaling.
Optical Modulation of Neuronal Activities with Nanostructured Materials
In addition to recording neuronal activity, researchers are also interested in remotely activating signaling pathways. The need for this is readily apparent when we consider retinal degenerative disorders, such as Retinitis pigmentosa, which occurs in as many as 1 out of 4,000 individuals worldwide55. As a genetically heterogenous disorder, Retinitis pigmentosa is difficult to treat; slowly causing impairment in vision due to the degradation of rod and cone photoreceptors in the back of the eye56. This means that neuronal pathways are still competent to fire, but they have lost their ability to transduce light into electrical signals. While there is currently no cure available, there has been a great interest in using optical stimulation method to overcome some of these challenges56, for instance using photovoltaic retinal prosthetics57.
Nanostructured materials provide a potentially promising platform for achieving this, working as photoactive materials for use in stimulating neurons. In addition to their small length scale, and excellent temporal and spatial resolution, nanostructured materials synthesis and design can be selectively controlled to increase device performance and delivery. Similarly, the ability to be delivered in a drug-like manner makes nanostructured materials a promising candidate for use in potential clinical applications. To date, there have been primarily two pathways used to optically elicit membrane depolarization; photothermal and photoelectrochemical stimulation.
• Photothermal Stimulation
Infrared light alone is capable of inducing a capacitance change resulting in membrane depolarization58. However, this can lead to long term photothermal damage and eventually apoptosis. Additionally, IR radiation is efficiently absorbed by water, leading to spatially imprecise stimulation. Therefore, a more localized source is desired, one that can act as a light-to-heat transducer, transferring energy from an optical source to a desired point within the cell membrane (Figure 2a). Nanostructured materials can act as efficient heat generators, with recent attempts to photothermally stimulate cells including the use of such materials as carbon nanotube films59, biopolymers films60, and gold nanoparticles61,62.
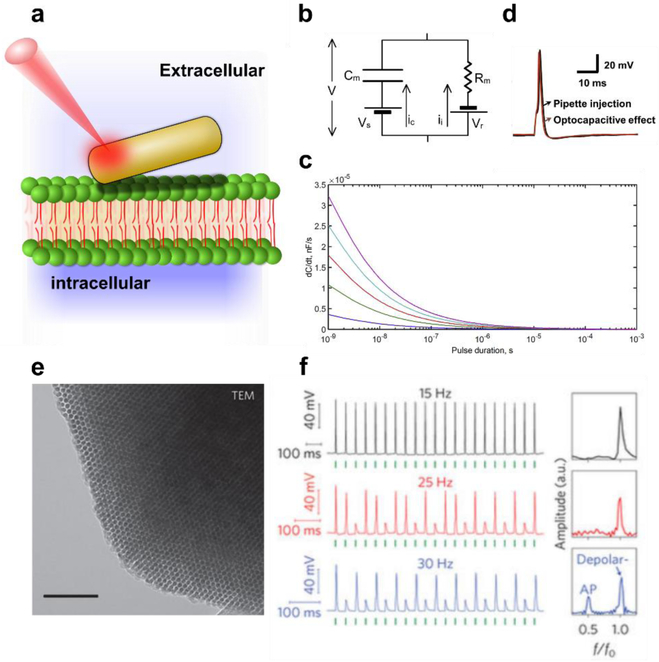
Figure 2. Nanomaterial Based Photothermal Stimulation of Neurons.
(a) Schematic diagram of a gold nanoparticle locally heating the cell membrane, changing the membrane’s capacitance, Cm, and inducing a depolarization event. (b) Equivalent circuit diagram, showing the net surface potential (Vs), capacitive current (Ic), membrane resistance (Rm), reversal potential (Vr) and ionic current (Ii) respectively. (c) Predicted time derivatives of capacitive current for varying laser pulse powers (high to low power, from top to bottom), indicating that Ic is maximized with higher intensity lasers at the time of pulse initiation (d) Corresponding experimentally measured photothermally induced neuronal action potential (25 × 95 gold nanorod, 785 nm, 5 mW, 1 ms laser pulse). (e) Transmission Electron Microscope (TEM) micrograph of hexagonally packed silicon nanowires, with the mesoporous structure enabling rapid localized heating (scale bar 100nm). (f) Membrane potential recordings of DRG neurons photothermally stimulated using mesoporous silicon, at different frequencies (Left, 5.32 µJ), with corresponding normalized Fast-Fourier Transforms (right). Green ticks indicate the time of delivery for the laser pulse. F and F0 are the output and input frequencies receptively. Modified and reproduced with permission from J. Carvalho-de-Souza et al.61 (b-d) and Y. Jiang et al.61 . (e-f). Copyright 2017 Elsevier and 2016 Springer Nature respectively.
To quantitatively understand how photothermal stimulation can give rise to membrane depolarization, we refer to Carvalho-de-Souza et al.61, where they represented the cell membrane as an equivalent circuit following the HH model (Figure 2b), such that Cm is the membrane capacitance. Modeling the cell membrane in this way gives rise to the governing relation:
| (1) |
Where γ is the efficiency of thermal transfer between a particle and the membrane (~1% in the case of gold nanorods), C0 is the initial capacitance, E is radiative pulse of energy, and F(t) is the equation for change in particle temperature as a function of time, t. While Carvalho-de-Souza et al.61 go on to model F(t) more precisely for a gold nanorod, this equation can be used to form some intuitive insights into photothermal stimulation. Namely, that larger laser powers, E, lead to higher capacitance changes (Figure 2c), and that materials which generate, F(t), and transfer heat, γ, most efficiently serve as the best devices to change membrane capacitance. Carvalho-de-Souza et al. also provide additional insight that photothermal stimulation is maximized when dCm/dt is highest61, suggesting that a laser pulse is most effective for stimulating neurons when it is initially turned on. To experimentally confirm these ideas, they showed that gold nanorods could be used to locally elicit action potentials that showed similar traces to those produced by current injection patch clamp (Figure 2d). This is exciting as gold nanorods are readily available in a laboratory, and can be labelled for site-specific targeting62. Also, as the researchers used a 785 nm laser, which is within the optical window for increased tissue penetration, this suggests that this approach has some potential for in-vivo use. Finally, we highlight this work as it offers some concise metrics for improving device performance.
Using these insights, that increased heat generation and transfer can be used to improve photothermal stimulation, Jiang et al.63 recently showed that conformal mesoporous silicon scaffolds can be used in a similar fashion (Figure 2e). Due to the reduced thermal conductivity64, and the enhanced light absorption65 of amorphous mesoporous silicon, they hypothesized that these structures could provide an ideal material for photothermal stimulation. In this study they demonstrated that mesostrucutred silicon showed a marked reduction in the effective young’s modulus of the material as compared to bulk silicon (an ~2 orders of magnitude drop). This change in material mechanics allowed particles to form intimate contact with the cell membrane, giving them the potential for increased thermal transfer efficiency. Using this approach, they were able to demonstrate that trains of action potential could be elicited (Figure 2f), where cells were stimulated repeatedly with a reproducible neuronal response of up to 15 Hz. Collectively, this makes this class of material promising for future applications. We note however, that little is known about the long-term effects of chronic thermal exposure in the tissues. As a result, researchers have also turned to studying photovoltaic pathways.
• Photoelectrochemical Stimulation
Compared to photothermal stimulation relatively little work has been done in photoelectrochemical stimulation with freestanding materials, due to the difficulty in manufacturing nanoscale photovoltaics. However, photovoltaic devices offer the potential of being less damaging, and more sensitive to optical stimulation, with the work in this field, so far centering around organic polymers, quantum dots, and other semiconductor nanomaterials.
With regard to polymers, π-conjugated organic systems have been studied for more than two decades for use in optoelectronic applications66–68, however have only recently been explored for neuronal modulation. This has included efforts in producing synthetic ion pumps69, polymer based nanoparticles70 and films71–73, and standalone donor-acceptor linked molecules74,75. These compounds offer the potential benefit of being manufactured using traditional wet chemical synthesis techniques, allowing increased scaling of manufacture and low costs76. Additionally, their molecular length scale can enable spatially precise stimulation. However, there are still several challenges associated with these materials before they can begin to see wide spread use. First, π-conjugated networks are innately hydrophobic, creating complications in solubility when administering these polymers in a biological context. Second, the long-term stability of organic photovoltaics in open air and aqueous environments is still in question76. Finally, there are also concerns over the potential cytotoxicity of these devices, both from harmful breakdown products, but also from the harsh chemicals used in the initial polymer synthesis77. While these challenges still remain, there is fortunately a significant amount of research being done in this field for renewable energy applications. As a result, we expect that many of these obstacles will be addressable in the future.
Quantum dots have also seen some use as neuromodulators. In principle individual quantum dots embedded in the cell membrane can generate electron-hole pairs to initiate membrane depolarization78, however to date this behavior has only been observed using nanomaterial based films78,79. Also, if successful, the use of quantum dots in-vivo possess some additional technical challenges. As quantum dots are primarily composed of heavy metal compounds, such as cadmium and lead chalcogenides, they are typically cytotoxic and are difficult to embed into the cell membrane80. This means that surface coatings and shell deposition is needed to passivate the outer particle layer, however this also has the potential to interact with device performance, both in generating electron-hole pairs, and in transferring that potential to the cell membrane. While a great deal of research work has been done to improve quantum dot device performance using passivation layers81, more work is needed to address these challenges in a biological context.
More recently, researchers have turned to semiconducting nanowires for use as drug-like photoelectrochemical stimulators. Silicon nanowires in particle show promise, as they can be synthesized in a rationale manner, providing precise spatial control over nanowire morphology82–84, doping profiles85–87, and optical properties88–90. This allows for the fabrication of coaxial photovoltaic devices87 (Figure 3.a), which can be excited across a broad range of wavelengths, including within the near IR optical window for deep tissue penetration91. Additionally, silicon nanowires can be internalized spontaneously in phagocytic cells92, or can be surface modified to promote selective internalization into neurons51 (Figure 3.b). This increases their contact with cells while giving rise to potential intracellular applications.
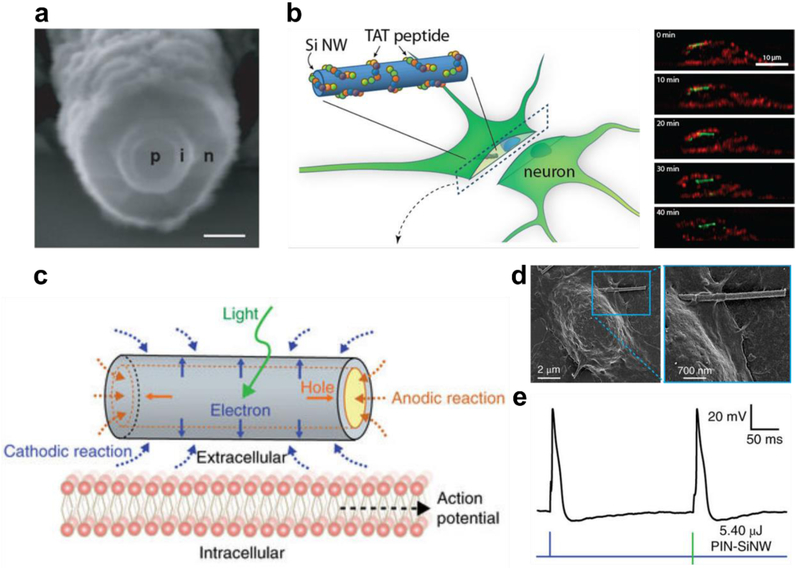
Figure 3. Silicon Nanowire Photoelectrochemical Stimulation of Neurons.
(a) Scanning electron micrograph of a coaxial photovoltaic PIN-SiNW (scale bar 100 nm) (b) Schematic diagram showing DRG uptake of surface modified silicon nanowires, with corresponding time-lapse confocal fluorescent micrograph cross-sections of the process (Red-plasma membrane, Alexa 594) (Green – SiNW, Alexa 555). (c) Schematic of photoelectrochemical stimulation of neurons upon light stimulation using PIN-SiNWs. (d) Scanning electron micrograph of a DRG neuron interfacing with a PIN-SiNW. (e) Current-clamp trace of membrane voltage in a DRG neuron stimulated by injected current (blue) and laser-pulsed PIN-SiNW (green), showing comparable action potentials. Modified and reproduced with permission from B. Tian et. al.87 (a), J. Lee, A. Zhang S. You & C. Lieber51 (b). and R. Parameswaran et. al.93 (c-e). Copyright 2007 Springer Nature, 2016 American Chemical Society, and 2018 Springer Nature respectively.
Using these materials, Parameswaran et al.93 recently demonstrated that free-standing coaxially doped p-type/intrinsic/n-type (PIN) silicon nanowires could be used to elicit neuronal action potentials (Figure 3.c). In this study Parameswaran et al. carefully studied the mechanism of neuronal activation, measuring photovoltaic potentials on an individual nanowire basis, simultaneously observing minimal local heating (a reported 0.36 K). PIN nanowires were then shown to be able to interface with the cell membrane, forming the intimate contact needed for stimulation (Figure 3.c). Finally, researchers also demonstrated that upon optical stimulation, PIN silicon nanowires were able to induce both trains and individual action potentials, which showed nearly identical traces to those observed using current injection patch clamp (Figure 3.d). We also note, that a similar study using gold titania (Au-TiO2) nanowires was published this year by J. Tang et al.94, where they showed that nanowire based stimulators that can be used to restore sight in retinal degenerate mice. They attribute the neuronal response in this report primarily to photocurrent generated at the Au-TiO2 interface. Overall, both studies are exciting as they show that nanowires can be used to photoelectrochemically modulate cell behavior in a non-invasive, nongenetic manner, with important implications for both fundamental studies and clinical therapeutics.
Conclusions and Outlook
As more techniques become available for integrating non-genetic optical sensing and modulation into a single platform, many potential opportunities present themselves. First, researchers can consider how these devices can begin to be used for semiconductor-enabled synthetic and cellular biology. The ability for inorganic nanomaterials to be integrated not only at the cellular level, but also at subcellular length scales, means that these devices can potentially be used to probe both organelles and protein-protein interactions. Using surface modification, it’s possible for these nanoparticles to be used in a targeted, spatially and temporal precise fashion to interrogate subcellular species in a similar way to current synthetic biology approaches. These ‘cyborg cells’ would have the added benefit of containing an additional repertoire of biorthogonal cues and signals that are currently difficult to achieve in synthetic biology, such as localized photothermal and photoelectric responses. This gives rise to new opportunities for not only studying protein-protein interactions, but also exploring subcellular electrophysiology. In this way, it may become possible to expand on the existing body of knowledge on how cells regulate their internal signaling pathways, or their ‘interactome’. As more tools become available to study organelle level bioelectrics and biomechanics, it may become more apparent how these mechanical and electrical intracellular systems interact with traditional transcription and protein signaling pathways.
On top of fundamental cell biology, stimulators and probes also serve an important role in the clinic. From cardiac arrhythmias to neurodegenerative disorders, maladaptive phenotypes in electrophysiology can result in serious and potentially deadly medical conditions95,96. In the case of neural dysfunction, deep brain stimulation (DBS) is the FDA approved standard of care for treating many of these disorders, including Parkinson’s disease, dystonia and essential tremor97. DBS involves the insertion of multiple electrodes into select regions of the brain, however, the success rate of these studies so far has been limited98, in part due to bulky nature of these electrodes. Additionally, the therapeutic mechanism of DBS remains largely unknown97. Moving to wireless, nanoparticle based systems has been seen as an important step in improving this standard of care99, both by providing reporters that can be used to study the distributed impact of DBS, but also in providing less invasive stimulators that could potentially be used to replace their larger, bulkier counter parts. As a result, the use of label free optical techniques and nanoparticle-based stimulation may play an important role in treating neurodegenerative disorders in the future.
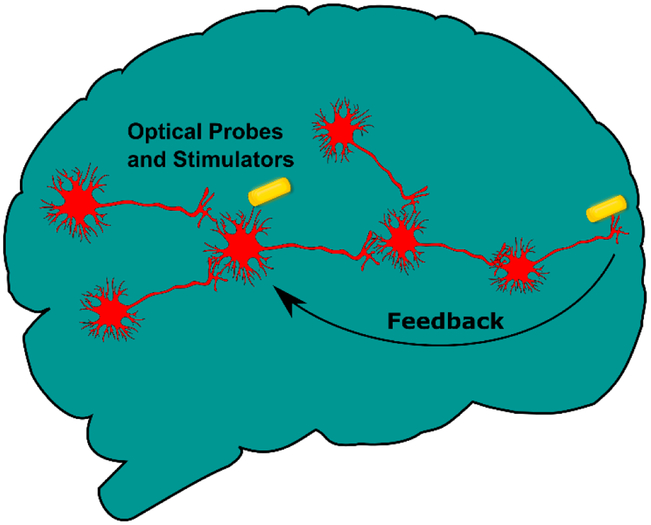
Additionally, by using both sensors and activators simultaneously it may be possible to establish a self-contained feedback loop. Such a system would allow for a study of pattern recognition and emergent behaviors in cellular signaling networks. Furthermore, this feedback system could be used to mimic three-dimensional brain mapping behavior in two dimensions (Figure 4.), where spatially defuse neurons are artificially linked using coordinated optical mapping and stimulation. Such a mapping could be used to recapitulate the complex networks formed across three-dimensions, while retaining the ease of two-dimensional culture (e.g. lack of vascularization, and access for cellular imaging). This ‘two-dimensional brain on a chip’ could help establish a bridge between in-vivo and in-vitro neuronal drug testing, with the potential to improve outcomes in clinical trials. Finally, incorporating feedback loops into neuronal systems has further implications in memory, learning and consciousness formation. Being able to address these emergent phenomena in a rational manner, provides an exciting prospect moving forward.
Figure 4. Optically Enabled Feedback Networks in 2D ‘Brain’ Culture.
Human brains form complex interactions across three dimensional interconnected networks which are difficult to replicate in two dimensional in-vitro cultures. As optical probes and stimulators become more stable, researchers can imagine using these devices to mimic complex spatial interconnects, using self-contained feedback loops to artificially link disparate regions in space.
Acknowledgements
JZ acknowledges support from Harvard University and by the Organ Design and Engineering Postdoctoral Training (ODET) program through Brigham and Women’s Hospital, National Institute of Biomedical Imaging and Bioengineering (NBIB) and the National Institute of Health under award number 5-T32-EB016652-04. BT acknowledges support from the Air Force Office of Scientific Research (AFOSR FA9550-15-1-0285) and the National Institutes of Health (NIH NS101488).
Footnotes
Notes
The authors declare no competing financial interest.
References
- (1).HODGKIN AL; HUXLEY AF Action Potentials Recorded from Inside a Nerve Fibre. Nature 1939, 144 (3651), 710–711. [Google Scholar]
- (2).Zhao Y; Inayat S; Dikin D. a; Singer JH; Ruoff RS; Troy JB Patch Clamp Technique: Review of the Current State of the Art and Potential Contributions from Nanoengineering. Proc. Inst. Mech. Eng. Part N J. Nanoeng. Nanosyst 2008, 222 (1), 1–11. [Google Scholar]
- (3).Dunlop J; Bowlby M; Peri R; Vasilyev D; Arias R High-Throughput Electrophysiology: An Emerging Paradigm for Ion-Channel Screening and Physiology. Nat. Rev. Drug Discov 2008, 7 (4), 358–368. [DOI] [PubMed] [Google Scholar]
- (4).Landero Figueroa JA; Subramanian Vignesh K; S. Deepe G; Caruso J Selectivity and Specificity of Small Molecule Fluorescent Dyes/probes Used for the Detection of Zn 2+ and Ca 2+ in Cells. Metallomics 2014, 6 (2), 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Tsien RY New Calcium Indicators and Buffers with High Selectivity against Magnesium and Protons: Design, Synthesis, and Properties of Prototype Structures. Biochemistry 1980, 19 (11), 2396–2404. [DOI] [PubMed] [Google Scholar]
- (6).Boyden ES; Zhang F; Bamberg E; Nagel G; Deisseroth K Millisecond-Timescale, Genetically Targeted Optical Control of Neural Activity. Nat. Neurosci 2005, 8 (9), 1263–1268. [DOI] [PubMed] [Google Scholar]
- (7).Ahrens MB; Orger MB; Robson DN; Li JM; Keller PJ Whole-Brain Functional Imaging at Cellular Resolution Using Light-Sheet Microscopy. Nat. Methods 2013, 10 (5), 413–420. [DOI] [PubMed] [Google Scholar]
- (8).Akemann W; Mutoh H; Perron A; Rossier J; Knöpfel T Imaging Brain Electric Signals with Genetically Targeted Voltage-Sensitive Fluorescent Proteins. Nat. Methods 2010, 7 (8), 643–649. [DOI] [PubMed] [Google Scholar]
- (9).Lin MZ; Schnitzer MJ Genetically Encoded Indicators of Neuronal Activity. Nat. Neurosci 2016, 19 (9), 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hochbaum DR; Zhao Y; Farhi SL; Klapoetke N; Werley CA; Kapoor V; Zou P; Kralj JM; MacLaurin D; Smedemark-Margulies N; et al. All-Optical Electrophysiology in Mammalian Neurons Using Engineered Microbial Rhodopsins. Nat. Methods 2014, 11 (8), 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hou JH; Kralj JM; Douglass AD; Engert F; Cohen AE Simultaneous Mapping of Membrane Voltage and Calcium in Zebrafish Heart in Vivo Reveals Chamber-Specific Developmental Transitions in Ionic Currents. Front. Physiol 2014, 5 AUG (September), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).LaFountaine JS; Fathe K; Smyth HDC Delivery and Therapeutic Applications of Gene Editing Technologies ZFNs, TALENs, and CRISPR/Cas9. Int. J. Pharm 2015, 494 (1), 180–194. [DOI] [PubMed] [Google Scholar]
- (13).Check E Gene Therapy Set Back. Nature 2002, 420 (April 2000), 116–118. [Google Scholar]
- (14).Ribeil J-A; Hacein-Bey-Abina S; Payen E; Magnani A; Semeraro M; Magrin E; Caccavelli L; Neven B; Bourget P; El Nemer W; et al. Gene Therapy in a Patient with Sickle Cell Disease. N. Engl. J. Med 2017, 376 (9), 848–855. [DOI] [PubMed] [Google Scholar]
- (15).Wilson JM Interview with Bennett Jean. Hum. Gene Ther. Clin. Dev 2018, 29 (1), 7–9. [DOI] [PubMed] [Google Scholar]
- (16).Rangarajan S; Walsh L; Lester W; Perry D; Madan B; Laffan M; Yu H; Vettermann C; Pierce GF; Wong WY; et al. AAV5–Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med 2017, 377 (26), 2519–2530. [DOI] [PubMed] [Google Scholar]
- (17).Maeder ML; Gersbach CA Genome-Editing Technologies for Gene and Cell Therapy. Mol. Ther 2016, 24 (3), 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Jordan A; Scholz R; Wust P; Fähling H; Felix Roland. Magnetic Fluid Hyperthermia (MFH): Cancer Treatment with AC Magnetic Field Induced Excitation of Biocompatible Superparamagnetic Nanoparticles. J. Magn. Magn. Mater 1999, 201 (1–3), 413–419. [Google Scholar]
- (19).Zhang E; Kircher MF; Koch M; Eliasson L; Goldberg SN; Renström E Dynamic Magnetic Fields Remote-Control Apoptosis via Nanoparticle Rotation. ACS Nano 2014, 8 (4), 3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Whitesides G The’right’size in Nanobiotechnology. Nat. Biotechnol 2003, 21 (10), 1161–1165. [DOI] [PubMed] [Google Scholar]
- (21).HODGKIN AL; HUXLEY AF A Quantitative Description of Membrane Current and Its Application to Conduction and Excitation in Nerve. J. Physiol 1952, 117 (4), 500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Tasaki I THE ELECTRO-SALTATORY TRANSMISSION OF THE NERVE IMPULSE AND THE EFFECT OF NARCOSIS UPON THE NERVE FIBER. Am. J. Physiol. Content 1939, 127 (2), 211–227. [Google Scholar]
- (23).Boullerne AI The History of Myelin. Exp. Neurol 2016, 283, 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tasaki I Physiology and Electrochemistry of Nerve Fibers; Academic Press, 1982. [Google Scholar]
- (25).Iwasa K; Tasaki I; Gibbons R Swelling of Nerve Fibers Associated with Action Potentials. Science (80-. ). 1980, 210 (4467), 338–339. [DOI] [PubMed] [Google Scholar]
- (26).Tasaki I; Iwasa K Further Studies of Rapid Mechanical Changes in Squid Giant Axon Associated with Action Potential Production. Jpn. J. Physiol 1982, 32, 505–518. [DOI] [PubMed] [Google Scholar]
- (27).El Hady A; Machta BB Mechanical Surface Waves Accompany Action Potential Propagation. Nat. Commun 2015, 6, 1–7. [DOI] [PubMed] [Google Scholar]
- (28).Villagran Vargas E; Ludu A; Hustert R; Gumrich P; Jackson AD; Heimburg T Periodic Solutions and Refractory Periods in the Soliton Theory for Nerves and the Locust Femoral Nerve. Biophys. Chem 2011, 153 (2–3), 159–167. [DOI] [PubMed] [Google Scholar]
- (29).Nguyen TD; Deshmukh N; Nagarah JM; Kramer T; Purohit PK; Berry MJ; McAlpine MC Piezoelectric Nanoribbons for Monitoring Cellular Deformations. Nat. Nanotechnol 2012, 7 (9), 587–593. [DOI] [PubMed] [Google Scholar]
- (30).Zhang PC; Keleshian AM; Sachs F Voltage-Induced Membrane Movement. Nature 2001, 413 (6854), 428–432. [DOI] [PubMed] [Google Scholar]
- (31).Appali R; Van Rienen U; Heimburg T A Comparison of the Hodgkin-Huxley Model and the Soliton Theory for the Action Potential in Nerves. Adv. Planar Lipid Bilayers Liposomes 2012, 16, 275–299. [Google Scholar]
- (32).Yang Y; Liu X-W; Wang H; Yu H; Guan Y; Wang S; Tao N Imaging Action Potential in Single Mammalian Neurons by Tracking the Accompanying Sub-Nanometer Mechanical Motion. ACS Nano 2018, acsnano.8b00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Abbott BC; Hill AV; Howarth JV The Positive and Negative Heat Production Associated with a Nerve Impulse. Proc. R. Soc. B Biol. Sci 1958, 148 (931), 149–187. [DOI] [PubMed] [Google Scholar]
- (34).Ranade SS; Syeda R; Patapoutian A Mechanically Activated Ion Channels. Neuron 2015, 87 (6), 1162–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Poole K; Herget R; Lapatsina L; Ngo HD; Lewin GR Tuning Piezo Ion Channels to Detect Molecular-Scale Movements Relevant for Fine Touch. Nat. Commun 2014, 5, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kim GH; Kosterin P; Obaid AL; Salzberg BM A Mechanical Spike Accompanies the Action Potential in Mammalian Nerve Terminals. Biophys. J 2007, 92 (9), 3122–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kim D-H; Song J; Choi WM; Kim H-S; Kim R-H; Liu Z; Huang YY; Hwang K-C; Zhang Y.-w.; Rogers J. a. Materials and Noncoplanar Mesh Designs for Integrated Circuits with Linear Elastic Responses to Extreme Mechanical Deformations. Proc. Natl. Acad. Sci 2008, 105 (48), 18675–18680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kim D-H; Ahn J-H; Choi WM; Kim H-S; Kim T-H; Song J; Huang YY; Liu Z; Lu C; Rogers JA Stretchable and Foldable Silicon Integrated Circuits. Science 2008, 320 (5875), 507–511. [DOI] [PubMed] [Google Scholar]
- (39).Liu J; Fu T-M; Cheng Z; Hong G; Zhou T; Jin L; Duvvuri M; Jiang Z; Kruskal P; Xie C; et al. Syringe-Injectable Electronics. Nat. Nanotechnol 2015, 10 (7), 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kim D-H; Ghaffari R; Lu N; Rogers J. a. Flexible and Stretchable Electronics for Biointegrated Devices. Annu. Rev. Biomed. Eng 2012, 14 (1), 113–128. [DOI] [PubMed] [Google Scholar]
- (41).Wang S; Xu J; Wang W; Wang GJN; Rastak R; Molina-Lopez F; Chung JW; Niu S; Feig VR; Lopez J; et al. Skin Electronics from Scalable Fabrication of an Intrinsically Stretchable Transistor Array. Nature 2018, 555 (7694), 83–88. [DOI] [PubMed] [Google Scholar]
- (42).Rogers JA; Someya T; Huang Y Materials and Mechanics for Stretchable Electronics. Science (80-. ). 2010, 327 (5973), 1603–1607. [DOI] [PubMed] [Google Scholar]
- (43).Rogers JA; Lagally MG; Nuzzo RG Synthesis, Assembly and Applications of Semiconductor Nanomembranes. Nature 2011, 477 (7362), 45–53. [DOI] [PubMed] [Google Scholar]
- (44).Robinson JT; Jorgolli M; Shalek AK; Yoon M-H; Gertner RS; Park H Vertical Nanowire Electrode Arrays as a Scalable Platform for Intracellular Interfacing to Neuronal Circuits. Nat. Nanotechnol 2012, 7 (3), 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Tian B; Cohen-Karni T; Qing Q; Duan X; Xie P; Lieber CM Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes. Science 2010, 329 (5993), 830–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Qing Q; Jiang Z; Xu L; Gao R; Mai L; Lieber CM Free-Standing Kinked Nanowire Transistor Probes for Targeted Intracellular Recording in Three Dimensions. Nat. Nanotechnol 2014, 9 (2), 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Duan X; Gao R; Xie P; Cohen-Karni T; Qing Q; Choe HS; Tian B; Jiang X; Lieber CM Intracellular Recordings of Action Potentials by an Extracellular Nanoscale Field-Effect Transistor. Nat. Nanotechnol 2012, 7 (3), 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Abbott J; Ye T; Qin L; Jorgolli M; Gertner RS; Ham D; Park H CMOS Nanoelectrode Array for All-Electrical Intracellular Electrophysiological Imaging. Nat. Nanotechnol 2017, No. February, 1–8. [DOI] [PubMed] [Google Scholar]
- (49).Abbott J; Ye T; Ham D; Park H Optimizing Nanoelectrode Arrays for Scalable Intracellular Electrophysiology. Acc. Chem. Res 2018, acs.accounts.7b00519. [DOI] [PubMed] [Google Scholar]
- (50).Almquist BD; Melosh NA Fusion of Biomimetic Stealth Probes into Lipid Bilayer Cores. Proc. Natl. Acad. Sci 2010, 107 (13), 5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Lee J-H; Zhang A; You SS; Lieber CM Spontaneous Internalization of Cell Penetrating Peptide-Modified Nanowires into Primary Neurons. Nano Lett 2016, 16 (2), 1509–1513. [DOI] [PubMed] [Google Scholar]
- (52).Zimmerman JF; Parameswaran R; Murray G; Wang Y; Burke M; Tian B Cellular Uptake and Dynamics of Unlabeled Freestanding Silicon Nanowires. Sci. Adv 2016, 2 (12), e1601039–e1601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Dipalo M; Amin H; Lovato L; Moia F; Caprettini V; Messina GC; Tantussi F; Berdondini L; De Angelis F Intracellular and Extracellular Recording of Spontaneous Action Potentials in Mammalian Neurons and Cardiac Cells with 3D Plasmonic Nanoelectrodes. Nano Lett 2017, 17 (6), 3932–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Messina GC; Dipalo M; La Rocca R; Zilio P; Caprettini V; Proietti Zaccaria R; Toma A; Tantussi F; Berdondini L; De Angelis F Spatially, Temporally, and Quantitatively Controlled Delivery of Broad Range of Molecules into Selected Cells through Plasmonic Nanotubes. Adv. Mater 2015, 27 (44), 7145–7149. [DOI] [PubMed] [Google Scholar]
- (55).Hartong DT; Berson EL; Dryja TP Retinitis Pigmentosa. Lancet 2006, 368 (9549), 1795–1809. [DOI] [PubMed] [Google Scholar]
- (56).Ghezzi D Retinal Prostheses: Progress towards the next Generation Implants. Front. Neurosci 2015, 9 (July), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Mathieson K; Loudin J; Goetz G; Huie P; Wang L; Kamins TI; Galambos L; Smith R; Harris JS; Sher A; et al. Photovoltaic Retinal Prosthesis with High Pixel Density. Nat. Photonics 2012, 6 (6), 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Shapiro MG; Homma K; Villarreal S; Richter CP; Bezanilla F Infrared Light Excites Cells by Changing Their Electrical Capacitance. Nat. Commun 2012, 3, 310–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Bareket L; Waiskopf N; Rand D; Lubin G; David-Pur M; Ben-Dov J; Roy S; Eleftheriou C; Sernagor E; Cheshnovsky O; et al. Semiconductor Nanorod-Carbon Nanotube Biomimetic Films for Wire-Free Photostimulation of Blind Retinas. Nano Lett 2014, 14 (11), 6685–6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Martino N; Feyen P; Porro M; Bossio C; Zucchetti E; Ghezzi D; Benfenati F; Lanzani G; Antognazza MR Photothermal Cellular Stimulation in Functional Bio-Polymer Interfaces. Sci. Rep 2015, 5, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Carvalho-de-Souza JL; Pinto BI; Pepperberg DR; Bezanilla F Optocapacitive Generation of Action Potentials by Microsecond Laser Pulses of Nanojoule Energy. Biophys. J 2017, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Carvalho-de-Souza JL; Treger JS; Dang B; Kent SBH; Pepperberg DR; Bezanilla F Photosensitivity of Neurons Enabled by Cell-Targeted Gold Nanoparticles. Neuron 2015, 86 (1), 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jiang Y; Carvalho-De-Souza JL; Wong RCS; Luo Z; Isheim D; Zuo X; Nicholls AW; Jung IW; Yue J; Liu DJ; et al. Heterogeneous Silicon Mesostructures for Lipid-Supported Bioelectric Interfaces. Nat. Mater 2016, 15 (9), 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Tanaka, Maruyama Kazunobu, Shimada Eiichi, Toshikazu Okamoto H Amorphous Silicon, 1st ed.; Wiley, 1999. [Google Scholar]
- (65).Handbook of Porous Silicon; Canham L, Ed.; Springer International Publishing: Cham, 2014. [Google Scholar]
- (66).Semiconducting Polymers; Hadziioannou G, van Hutten PF, Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, FRG, 1999. [Google Scholar]
- (67).Ardona HAM; Tovar JD Peptide π-Electron Conjugates: Organic Electronics for Biology? Bioconjug. Chem 2015, 26 (12), 2290–2302. [DOI] [PubMed] [Google Scholar]
- (68).Lu L; Kelly MA; You W; Yu L Status and Prospects for Ternary Organic Photovoltaics. Nat. Photonics 2015, 9 (8), 491–500. [Google Scholar]
- (69).Isaksson J; Kjäll P; Nilsson D; Robinson N; Berggren M; Richter-Dahlfors A Electronic Control of Ca2+ Signalling in Neuronal Cells Using an Organic Electronic Ion Pump. Nat. Mater 2007, 6 (9), 673–679. [DOI] [PubMed] [Google Scholar]
- (70).Tortiglione C; Antognazza MR; Tino A; Bossio C; Marchesano V; Bauduin A; Zangoli M; Morata SV; Lanzani G Semiconducting Polymers Are Light Nanotransducers in Eyeless Animals. Sci. Adv 2017, 3 (1), e1601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Antognazza MR; Ghezzi D; Musitelli D; Garbugli M; Lanzani G A Hybrid Solid-Liquid Polymer Photodiode for the Bioenvironment. Appl. Phys. Lett 2009, 94 (24), 1–4. [Google Scholar]
- (72).Ghezzi D; Antognazza MR; Dal Maschio M; Lanzarini E; Benfenati F; Lanzani G A Hybrid Bioorganic Interface for Neuronal Photoactivation. Nat. Commun 2011, 2 (1), 166. [DOI] [PubMed] [Google Scholar]
- (73).Ghezzi D; Antognazza MR; MacCarone R; Bellani S; Lanzarini E; Martino N; Mete M; Pertile G; Bisti S; Lanzani G; et al. A Polymer Optoelectronic Interface Restores Light Sensitivity in Blind Rat Retinas. Nat. Photonics 2013, 7 (5), 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Numata T; Murakami T; Kawashima F; Morone N; Heuser JE; Takano Y; Ohkubo K; Fukuzumi S; Mori Y; Imahori H Utilization of Photoinduced Charge-Separated State of Donor-Acceptor-Linked Molecules for Regulation of Cell Membrane Potential and Ion Transport. J. Am. Chem. Soc 2012, 134, 6092–6095. [DOI] [PubMed] [Google Scholar]
- (75).Rohan JG; Citron YR; Durrell AC; Cheruzel LE; Gray HB; Grubbs RH; Humayun M; Engisch KL; Pikov V; Chow RH Light-Triggered Modulation of Cellular Electrical Activity by Ruthenium Diimine Nanoswitches. ACS Chem. Neurosci 2013, 4, 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Cheng P; Zhan X Stability of Organic Solar Cells: Challenges and Strategies. Chem. Soc. Rev 2016, 45 (9), 2544–2582. [DOI] [PubMed] [Google Scholar]
- (77).Venkatesan S; Chen Q; Ngo EC; Adhikari N; Nelson K; Dubey A; Sun J; Bommisetty V; Zhang C; Galipeau D; et al. Polymer Solar Cells Processed Using Anisole as a Relatively Nontoxic Solvent. Energy Technol 2014, 2 (3), 269–274. [Google Scholar]
- (78).Lugo K; Miao X; Rieke F; Lin LY Remote Switching of Cellular Activity and Cell Signaling Using Light in Conjunction with Quantum Dots. Biomed. Opt. Express 2012, 3 (3), 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Pappas TC; Wickramanyake WMS; Jan E; Motamedi M; Brodwick M; Kotov NA Nanoscale Engineering of a Cellular Interface with Semiconductor Nanoparticle Films for Photoelectric Stimulation of Neurons. Nano Lett 2007, 7 (2), 513–519. [DOI] [PubMed] [Google Scholar]
- (80).Derfus AM; Chan WCW; Bhatia SN Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett 2004, 4 (1), 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Carey GH; Abdelhady AL; Ning Z; Thon SM; Bakr OM; Sargent EH Colloidal Quantum Dot Solar Cells. Chem. Rev 2015, 115 (23), 12732–12763. [DOI] [PubMed] [Google Scholar]
- (82).Luo Z; Jiang Y; Myers BD; Isheim D; Wu J; Zimmerman JF; Wang Z; Li Q; Wang Y; Chen X; et al. Atomic Gold-Enabled Three-Dimensional Lithography for Silicon Mesostructures. Science 2015, 348 (6242), 1451–1455. [DOI] [PubMed] [Google Scholar]
- (83).Tian B; Xie P; Kempa TJ; Bell DC; Lieber CM Single-Crystalline Kinked Semiconductor Nanowire Superstructures. Nat. Nanotechnol 2009, 4 (12), 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Gaboriau D; Aradilla D; Brachet M; Le Bideau J; Brousse T; Bidan G; Gentile P; Sadki S Silicon Nanowires and Nanotrees: Elaboration and Optimization of New 3D Architectures for High Performance on-Chip Supercapacitors. RSC Adv 2016, 6 (84), 81017–81027. [Google Scholar]
- (85).Garnett EC; Yang P Silicon Nanowire Radial P-N Junction Solar Cells. J. Am. Chem. Soc 2008, 130 (29), 9224–9225. [DOI] [PubMed] [Google Scholar]
- (86).Christesen JD; Pinion CW; Zhang X; Mcbride JR; Cahoon JF Encoding Abrupt and Uniform Dopant Pro Fi Les in Vapor À Liquid À Solid Nanowires by Suppressing the Reservoir E Ff Ect of the Liquid Catalyst 2014, No. 11, 11790–11798. [DOI] [PubMed] [Google Scholar]
- (87).Tian B; Zheng X; Kempa TJ; Fang Y; Yu N; Yu G; Huang J; Lieber CM Coaxial Silicon Nanowires as Solar Cells and Nanoelectronic Power Sources. Nature 2007, 449 (7164), 885–889. [DOI] [PubMed] [Google Scholar]
- (88).Priolo F; Gregorkiewicz T; Galli M; Krauss TF Silicon Nanostructures for Photonics and Photovoltaics. Nat. Nanotechnol 2014, 9 (1), 19–32. [DOI] [PubMed] [Google Scholar]
- (89).Kempa TJ; Kim S-K; Day RW; Park H-G; Nocera DG; Lieber CM Facet-Selective Growth on Nanowires Yields Multi-Component Nanostructures and Photonic Devices. J. Am. Chem. Soc 2013, 3–6. [DOI] [PubMed] [Google Scholar]
- (90).Brönstrup G; Jahr N; Leiterer C; Csáki A; Fritzsche W; Christiansen S Optical Properties of Individual Silicon Nanowires for Photonic Devices. ACS Nano 2010, 4 (12), 7113–7122. [DOI] [PubMed] [Google Scholar]
- (91).Kempa TJ; Cahoon JF; Kim S-K; Day RW; Bell DC; Park H-G; Lieber CM Coaxial Multishell Nanowires with High-Quality Electronic Interfaces and Tunable Optical Cavities for Ultrathin Photovoltaics. Proc. Natl. Acad. Sci 2012, 109 (5), 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Zimmerman JF; Murray GF; Wang Y; Jumper JM; Austin JR; Tian B Free-Standing Kinked Silicon Nanowires for Probing Inter- and Intracellular Force Dynamics. Nano Lett 2015, 15 (8), 5492–5498. [DOI] [PubMed] [Google Scholar]
- (93).Parameswaran R; Carvalho-de-Souza JL; Jiang Y; Burke MJ; Zimmerman JF; Koehler K; Phillips AW; Yi J; Adams EJ; Bezanilla F; et al. Photoelectrochemical Modulation of Neuronal Activity with Free-Standing Coaxial Silicon Nanowires. Nat. Nanotechnol 2018, 393a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Tang J; Qin N; Chong Y; Diao Y; Yiliguma; Wang Z; Xue T; Jiang M; Zhang J; Zheng G Nanowire Arrays Restore Vision in Blind Mice. Nat. Commun 2018, 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Ashkan K; Rogers P; Bergman H; Ughratdar I Insights into the Mechanisms of Deep Brain Stimulation. Nat. Rev. Neurol 2017, 13 (9), 548–554. [DOI] [PubMed] [Google Scholar]
- (96).Feigin VL; Abajobir AA; Abate KH; Abd-Allah F; Abdulle AM; Abera SF; Abyu GY; Ahmed MB; Aichour AN; Aichour I; et al. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017, 16 (11), 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Creed M; Pascoli VJ; Luscher C Refining Deep Brain Stimulation to Emulate Optogenetic Treatment of Synaptic Pathology. Science (80-. ). 2015, 347 (6222), 659–664. [DOI] [PubMed] [Google Scholar]
- (98).Perlmutter JS; Mink JW Deep Brain Stimulation. 2006, 447–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Birmingham K; Gradinaru V; Anikeeva P; Grill WM; Pikov V; McLaughlin B; Pasricha P; Weber D; Ludwig K; Famm K Bioelectronic Medicines: A Research Roadmap. Nat. Rev. Drug Discov 2014, 13 (6), 399–400. [DOI] [PubMed] [Google Scholar]