Abstract
Hendra virus (HeV) and Nipah virus (NiV) are members of the genus Henipavirus, within the family Paramyxoviridae. Nipah virus has caused outbreaks of human disease in Bangladesh, Malaysia, Singapore, India and Philippines, in addition to a large outbreak in swine in Malaysia in 1998/1999. Recently, NiV was suspected to be a causative agent of an outbreak in horses in 2014 in the Philippines, while HeV has caused multiple human and equine outbreaks in Australia since 1994. A swine vaccine able to prevent shedding of infectious virus is of veterinary and human health importance, and correlates of protection against henipavirus infection in swine need to be better understood. In the present study, three groups of animals were employed. Pigs vaccinated with adjuvanted recombinant soluble HeV G protein (sGHEV) and challenged with HeV developed antibody levels considered to be protective prior to the challenge (titers of 320), however activation of the cell-mediated immune response was not detected, and the animals were only partially protected against challenge with 5x105 PFU of HeV per animal. In the second group, cross-neutralizing antibody levels against NiV in the sGHEV vaccinated animals did not reach protective levels, and with no activation of cellular immune memory, these animals were not protected against NiV. Only pigs orally infected with 5x104 PFU of NiV per animal were protected against nasal challenge with 5x105 PFU of NiV per animal. This group of pigs developed protective antibody levels, as well as cell-mediated immune memory. Peripheral blood mononuclear cells restimulated with UV-inactivated NiV upregulated IFN-gamma, IL-10 and the CD25 activation marker on CD4+CD8+ T memory helper cells and to lesser extent on CD4−CD8+ T cells. In conclusion, both humoral and cellular immune responses were required for protection of swine against henipaviruses.
Keywords: Nipah virus, Hendra virus, swine, neutralizing antibody, cell-mediated immune response
Introduction
Hendra virus (HeV) and Nipah virus (NiV) are members of the genus Henipavirus, within the family Paramyxoviridae. Nipah virus caused a relatively large outbreak in swine with transmission to humans in Malaysia and Singapore in 1998/1999 [1], and later human outbreaks in Bangladesh, India and Philippines. The virus was also suspected to be a causative agent of an outbreak in horses in 2014 in the Philippines with transmission to humans [2]. Domestic animals such as cats, dogs, goats and cows were reported to be infected with NiV beside the natural reservoir, mainly pteropus bats [3]. Following the first outbreak in 1994, outbreaks of HeV in horses were reported in Australia, and all cases of human HeV infections were associated with transmission of the virus from horses [4,5]. Natural infections were reported only in bats and dogs, although experimentally a number of mammalian species can be infected with HeV, including swine [6–8].
Due to the high case-fatality rates for henipavirus infections, and lack of human vaccines, both viruses are classified as biosafety level 4 (BSL4) agents. This renders studies on pathogenesis and immune response in vivo difficult, and consequently the current knowledge on virus-host interaction is rather limited. Our understanding of the host immune response to henipaviruses is essentially based on vaccination studies. The humoral immune response, specifically development of neutralizing antibodies, is considered to be a critical component of the protective immune response in henipavirus infected host [9–12]. A limited number of reports also suggested the importance of the cellular immune response, at least in swine, horses and hamsters [13–15]. Vaccine development, production and efficacy testing are similarly complex issues due to the requirement for biosafety and containment level 4 (CL4) for work with henipaviruses.
Despite promising results with the canarypox vectored vaccine candidate [13], a swine vaccine against NiV is still not available, even though there is a potential for new spill over into swine, considering the outbreak of NiV in horses in 2014 [2]. On the other hand, Equivac HeV® vaccine was licenced for use in horses in Australia in 2012. This vaccine, based on a recombinant HeV G protein (sGHEV), was found to be efficacious against both HeV and NiV in several species, including non-human primates [14, 16–22].
Vaccine efficacy testing and understanding protection against henipaviruses may represent a special challenge in swine: Firstly, the porcine host is able to mount an effective immune response in the natural settings [1]. Secondly, the existing experimental model is, despite relatively high challenge dose, not lethal [23]. Thirdly, unlike in other host species, NiV has the ability to infect a range of porcine immune cells, such as dendritic cells, monocytes, macrophages, NK cells and CD8+ T cells [24], with a highly probable negative impact on the early development of adaptive immune responses. There is indeed indication that the first veterinary vaccine candidate which demonstrated protection against NiV in swine, elicited both, humoral and cellular responses [13].
The aim of this study was to better understand an immune response against henipaviruses in swine, and to identify additional correlates of protection beside the development of neutralizing antibodies.
Material and Methods
Cells
Porcine peripheral blood mononuclear cells (PBMC) were isolated using cell collection tubes (CPT; Beckton-Dickinson) according to the manufacturer’s instructions. PBMC and Vero 76 cells (ATCC) were cultured as described previously [13,24].
Viruses, virus titration and isolation
Second passage of NiV re-isolated from lung of experimentally infected pig and human isolate of HeV, passage No. 6 in Vero 76 cells, were used in the animal infections and for the microtiter plaque reduction neutralization test (mPRNT). Viruses were titrated by plaque assay; virus isolation was performed in a plaque titration format as described previously [8,13].
Vaccine candidate
A vaccine candidate based on recombinant soluble HeV G protein (sGHEV) was provided in ready-to-use format by Zoetis, Inc. in a proprietary adjuvant formulation.
Animal experiments (See Table 1. and Fig. 1.)
Table 1.
Summary of the Experimental Groups
| Group | Pig No. | Immunization | Challenge | Shedding | Virus load | mPRNT Abs | Anti-G ELISA Abs | Recall antigen | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NiV | HeV | NiV | HeV | NiV | HeV | ||||||
| A | 30,31,33,34 | Infectious NiV | NiV | x | x | x | x | x | x | x | |
| 29 | Infectious NiV | none | x | x | |||||||
|
| |||||||||||
| B | 23,24,25,26 | sGHEV | HeV | x | x | x | x | x | x | x | |
|
| |||||||||||
| C | 18,19,20,21 | sGHEV | NiV | x | x | x | x | x | x | x | |
| 17 | sGHEV | none | x | x | x | x | x | ||||
|
| |||||||||||
| D | 22,32 | none | NiV | x | x | x | x | x | x | x | |
| 27 | none | HeV | x | x | x | x | x | x | x | ||
Note: X indicates that analysis was performed for the group
Please note that for discussion purposes, piglet No. 29 was not considered as part of the Group A, and piglet No. 17 was likewise not considered part of the Group C, because the two piglets were not challenged, and served as pre-challenge controls. Piglets 22, 32, and 27 were the challenge controls.
Figure 1. Experimental Design.
Schematic representation of each group is detailed to indicate sampling, vaccination/inoculation and euthanasia timelines. Group A, primary NiV infection is indicated as days post infection (dpi); while secondary NiV challenge is indicated by days post challenge (dpc). Group B and group C were both vaccinated, therefore dpv represents days post vaccination. Here, dpc represents subsequent challenge with either HeV or NiV as indicated. Group D, challenge control pigs have no pre-treatment, therefore no timeline is present prior to challenge. Sampling is indicated by downward facing arrows.
Four-week old Landrace pigs were acclimatized for one week. All animal care and handling protocols were approved by the institutional Animal Care Committee, and met the requirements of the Canadian Animal Care Council (CSCHAH AUD #: C-10-001). The animals were anesthetized with isoflurane in oxygen for all sampling and infection procedures. Components of the studies employing live virus work were conducted under BSL4 conditions at the NCFAD CL4 facility.
Group A
Five pigs were infected orally with 5 x 104 PFU of NiV in 3ml per pig, and one animal was euthanized at 7 days post infection (dpi) as an infection control. At 28 dpi, a challenge control pig was added to the group, and all animals were challenged intranasally (IN) with 5 x 105 PFU of NiV in 3ml per pig. Blood was collected prior to the infection and at 2 dpi; blood and nasal washes were collected at 7, 14, 21 and 28 dpi. Blood and nasal washes were collected at 3 days post challenge (dpc), and all pigs were sampled and euthanized at 5/6 dpc.
Group B
Four pigs were vaccinated intramuscularly (IM) with 2ml of pre-formulated adjuvanted sGHEV, and boosted IM at 21 days post vaccination (dpv) with the same dose. The animals were challenged IN at 35 dpv with 5 x 105 PFU of HeV in 3ml per pig. Blood was collected prior to vaccination, and at 7, 14, 21, 28 and 35 dpv. Post challenge sampling and euthanasia were performed as for group A.
Group C
Pigs were vaccinated as described for group B, followed by IN challenge at 35 dpv with 5 x 105 PFU of NiV in 3ml per pig. All the sampling and euthanasia were performed as for group B.
Group D
HeV or NiV challenge control animals were included in all three segments of the study, and are in the text distinguished as DHeV and DNiV. The animals were challenged at 8 (control for Group A) or 9 (controls for groups B and C) weeks of age.
Sample collection (See Table 1 and Fig. 1)
Nasal washes were collected by lavage of the nasal passages with PBS/0.5% bovine serum albumin (BSA)/1% PenStrep. Blood was collected from the right cranial vena cava directly into serum separator tubes or cell separator tubes. Selected tissues and fluids were collected for virus isolation and viral RNA detection at 5 or 6 dpc: brain (hind cerebrum), trigeminal ganglion, olfactory bulb, cerebrospinal uid (CSF), spleen, palatine tonsil, nasal turbinates, trachea, lung, cell pellet from the bronchoalveolar lavage (BALF), submandibular and lung associated lymph nodes. Attempts were made to distribute the necropsies representatively: one NiV challenge control pig (group DNiV) was euthanized at 5 dpc and one at 6dpc; two pigs from group A were euthanized at 5 dpc, two at 6dpc, and two pigs from group C were euthanized at 5 dpc and two at 6 dpc. Two pigs from group B and the HeV challenge control (group DHeV) were euthanized at 5 dpc and two pigs from group B were euthanized at 6dpc.
Virus neutralization assay
Serum antibody titers were determined by microtiter plaques reduction neutralization test (mPRNT) as described previously [8, 13]. Wells with reduction of plaques greater than 90% were considered positive for antibody presence.
IgG indirect ELISA
Nunc F flat bottom polystyrene plates were coated with 20 ng/100 μl/well of recombinant NiV G protein sGNIV [16] in 0.06 M carbonate/bicarbonate buffer (pH 9.6) overnight at 4°C. Plates were washed between steps five times with 0.01 M PBS(pH 7.2)/0.05% Tween 20. Plates blocked with 3% BSA/10% horse serum/0.1% Tween 20/0.01 M PBS(pH 7.2) for 1 hr at 37°C were incubated for 1 hr with 1/100 dilution of pig serum in blocking buffer. Rabbit horseradish peroxidase conjugated anti-porcine antibodies (100 μl/well) (Cedarlane) were added at 1/1000 dilution in blocking buffer for 1 hr at 37°C. The incubation was followed by the addition of 100 μl/well of enzyme substrate 2,2′-azino-bis[3-ethylbenzothiazoline-6-sulphonic acid] (Roche Diagnostics). OD405 values were measured using Spectra Max Plus. The indirect sGHEV ELISA was performed as outlined above.
Real time RT-PCR
Viral RNA loads in body fluids and tissue homogenates were determined as described previously for HeV[8] and NiV[24] in samples spiked with 0.5 μl/sample of Armored Enterovirus RNA (Asuragen). Probe 5′-TET-CGT GGC GGA ACC GAC TAC TTT GG-NFQ-3′ within the enterovirus 5′-untranslated region, forward CCTGTCGTAACGCGCAAGT and reverse CAGCCACAATAAAATAAAAGGAAACA primers were added to the RT-PCR reaction. Reaction products were semi-quantified based on standard curves generated employing plasmid DNA with cloned NiV N or HeV M genes respectively, and the quantities were expressed as log10 copy numbers/ml.
Restimulation
PBMC were washed twice with PBS, and 4x106 PBMCs were seeded per well in RPMI 1640/10% FBS/10mM HEPES/1% PenStrep. Cells were then treated with 0.5 μg/ml of Concanavalin A (control), or with 50–200 ng of sGHEV or sGNIV, or with UV inactivated NiV or HeV at an equivalent of MOI 0.01. Control cells were left unstimulated. Plates were incubated at 37 C with 5% CO2 for 5 days. PBMC were harvested by centrifugation at 300 g for 15 minutes and analyzed by flow cytometry. Interferon gamma (IFN-γ) (ABCAM, catalogue #ab113353) and Interleukin 10 (IL-10) (ThermoFisher, catalogue #KSC0101) were assayed in supernatants by ELISA, according to manufacturers’ instructions.
Flow cytometry
Non-attached cells from the restimulated PBMCs were re-suspended in 1 ml of MSB (MACS Separation Buffer, Miltenyi Biotec) and labeled in the dark for 30 min at room temperature with a mix of FITC mouse anti-pig CD4α (BD Pharmingen™ Cat#559585), PE mouse anti-pig CD8α (BD Pharmingen™ Cat#559584) and mouse anti-pig CD25 primary antibody (AbD Serotec® Cat#MCA1736) in 100μl of chilled MSB, followed by anti-mouse Alexa Fluor® 647 antibody (Life Technologies Cat# MG121) in 100μl of chilled MSB. The cells were incubated again for 30 minutes in the dark, washed and fixed in 250 μl of a 4% paraformaldehyde to be removed from CL4 for analysis on FC500 flow cytometer (Beckman Coulter). Post analysis was performed using Kaluza 1.2 software (Beckman Coulter). The CD25 expression indices (EI) were calculated for CD4+CD8+, CD4−CD8+ and CD4+CD8− subpopulations in the PBMC population as follows: %CD25 positive cells x geometric mean fluorescence intensity for restimulated cells divided by %CD25 positive cells x geometric mean fluorescence intensity for unstimulated cells from each animal.
Statistics
SigmaPlot 13.0 software was used to generate the graphs, and SAS software (Version 9.4, SAS Institute Inc.) was used for the statistical analysis. Due to low number of animals in the experimental groups non-parametric tests were used for all comparisons. Comparison of the neutralizing antibody titers (expressed in log10) against NiV versus HeV in the serum samples was analyzed using Student’s t- test. The total CD4+ count in PBMC prior and post challenge was also analyzed using Student’s t- test. Comparison of ELISA OD values for anti-G antibodies in final (6dpc) serum samples was analyzed for each group independently using a paired t-test. Kruskal-Wallis test statistic was employed in comparison of groups (viral shedding and CMI analysis), first to determine whether the groups differed statistically, and in a second step to determine the statistical difference. Statistical significance is reported at P-value equal or less than 0.05.
Results
An overview of the experimental groups is presented in Table 1 and Fig. 1. Clinical signs (increased rectal temperature) developed only in the challenge control animals, and no adverse reactions were observed following the vaccination with sGHEV. No gross pathological changes which could be ascribed to NiV or HeV infections were observed in pigs during necropsies, including challenge control animals.
Antibody development
NiV infected pigs (group A) started to develop neutralizing antibodies by 14 dpi, and the serum antibodies reached protective levels [13] prior to challenge (Fig. 2.A). The serum samples where NiV was used as immunizing antigen (group A) showed significantly lower cross-neutralizing activity against HeV (P<0.001) compared to NiV neutralizing titers.
Figure 2. Neutralizing and anti-glycoprotein antibodies.
Titers of neutralizing antibodies as determined by mPRNT are plotted by group. Group A represents titers of neutralizing antibodies in serum of pigs infected and at 28dpi challenged with NiV (Fig. 2.A). Group B are pigs vaccinated with sGHEV and at 35dpv challenged with HeV (Fig. 2.B). Group C are pigs vaccinated with sGHEV and challenged with NiV (Fig. 2.C). White columns represent the mean (and standard deviation) of antibodies neutralizing NiV, and gray overlapping columns represent the mean (and standard deviation) of antibodies neutralizing HeV. Black arrows indicate boost for vaccinated animals (group B and C). Anti-G antibody development measured by ELISA is plotted in values of optical density at 405nm in Fig. 2.D for group A, Fig. 2.E. for group B, and Fig. 2.F for group C. Solid black line represents anti-NiV G (GNIV) antibody OD405 readings, and dashed line represents anti-HeV G (GHEV) antibodies.
Pigs vaccinated with sGHEV (groups B and C) required a boost at 21 dpv to develop neutralizing antibodies against HeV however the HeV neutralizing titers reached 320 at 35 dpv. By inference, neutralizing titers equal or above 320 would be considered protective (Fig. 2.B, 2.C) against HeV. The HeV antisera had significantly lower (P<0.001) cross-neutralizing titers against NiV (Fig. 2.B, 2.C) in comparison to the neutralizing titers against HeV. Consequently, with the highest NiV cross-neutralizing titers prior to the challenge reaching only 80, these antibody levels were not expected to be protective against NiV. All challenge control pigs failed to generate neutralizing antibodies to either HeV or NiV at 5–7 days post challenge (data not shown).
The sera were also tested by an indirect IgG ELISA against sGHEV and against sGNIV for development of antibodies against the G proteins. The levels of anti-G antibodies in NiV infected animals (group A) were notably lower compared to HeV antibodies in vaccinated animals (group B and C) prior to challenge, but did not require a boost for development (Fig. 2.D, 2.E and 2.F). There appeared to be only partial cross-reactivity of antibodies between HeV and NiV prior to the challenge, clearly noticeable for groups B and C. Due to overall low OD values for group A it was difficult to assess whether there was cross-reactivity with the sGHEV antigen prior to the challenge. However the OD values of antibodies against the sGHEV and the sGNIV antigen became very close post challenge. The observed cross-reactivity was not considered surprising for group C animals vaccinated with sGHEV and challenged with NiV(Fig. 2.F) (P=0.81). Interestingly, there was no statistical difference (P≥0.1) in OD450 values for anti-GNIV and anti-GHEV antibodies in post-challenge sera at 5/6dpc of pigs in group A (Fig. 2.D) exposed only to NiV based antigens (P=0.29) as well as for pigs in group B (Fig. 2.E) exposed only to HeV based antigens (P=0.24).
In general, the time course of development of anti-G antibodies corresponded with the development of neutralizing antibodies.
Nasal shedding
Virus RNA shedding in nasal washes was detected in pigs inoculated with NiV (group A) 7 days post primary infection at 4 log10 copy number/ml, confirming that the animals were indeed infected using lower dose and oral route of infection (Fig. 3). Post challenge viral RNA shedding was significantly lower for group A compared to NiV challenge control (DNiV) with P=0.02 value at 6 dpc, while there was no reduction in RNA shedding in group C (P=1.0) compared to the challenge control animals (Fig. 3, NiV challenge panel DAC). Vaccination with sGHEV failed to reduce viral shedding post challenge, compared to respective (DNiV or DHeV) challenge controls, regardless of the challenge virus (Fig. 3, HeV challenge panel DB). No infectious virus was isolated from the nasal washes of group A post challenge. The higher amounts of viral RNA detected for nasal washes in the other groups corresponded with successful virus isolation, and the shedding was confirmed in titers of 1.6 – 2.5 log10 PFU/ml from the post challenge nasal washes. Regretfully, the full set of virus isolation data was not available due to bacterial contaminants in the samples.
Figure 3. Viral RNA shedding.
Detection of viral RNA in nasal washes collected at 7 dpi for the group A, and at 3 and 6 days post nasal challenge from all groups is given in log10 copy number per ml. Group A (NiV infected pigs challenged with NiV) is represented by black bars, and negative assays by black triangle. Nasal washes from NiV challenge control pigs are in gray (group DNiV), and striped gray bars represent sGHEV vaccinated pigs challenged with NiV (group C). RNA detection in nasal washes from pigs challenged with HeV is represented by white bars: plain bars represent challenge control (DHeV), and striped bars represent pigs vaccinated with sGHEV and challenged with HeV (group B).
Virus detection in tissues
HeV RNA and infectious virus detection are summarized in Fig. 4.A. Infectious HeV or viral RNA were not detected in samples from cerebrospinal fluid (CSF), olfactory bulb (OLF), trigeminal ganglion (TGG) or brain of HeV challenged pigs (group B and DHEV), consistent with a previous report, that HeV may have lower affinity for the CNS in pigs [8], and the data were omitted from the figure. There was no reduction in viral RNA load in the respiratory tract (nasal turbinates, traches, and lung lavage cell pellet) of vaccinated pigs post challenge. Reduction in viral RNA load was however detected to some extent in the respective draining lymphatic tissues and tonsils (Fig. 4.A). Interestingly, infectious virus was not detected in tissues of any of the sGHEV vaccinated animals post HeV challenge, in contrast to the HeV challenge control, where virus was isolated from cells pelleted from BALF (3.45 log10/PFU per pellet), lung associated lymph nodes (2.4 log10PFU/g), tonsils (4.05 log10PFU/g) and from submandibular lymph nodes (2.25 log10PFU/g) (Fig. 4.A, bottom panel). Thus the data indicated partial protection in the vaccinated animals against HeV challenge.
Fig. 4. Detection of viral RNA and infectious virus in samples collected post mortem.
The necropsies were performed either at 5 or 6 dpc. Data are presented only if the challenge control yielded positive result for a specific tissue. Detection of viral RNA (top panels) is given in log10 copy number per g of tissue (or per ml in case of cerebrospinal fluid); the infectious virus isolation titers (bottom panels) are given in log10 of PFU/ml or PFU/g of tissue. Triangles indicate no detection of virus or viral RNA. Fig. 4.A summarizes the HeV challenge results. Gray bars represent the challenge control (group DHeV), white bars represent results from pigs vaccinated with sGHEV and challenged with HeV (group B). Fig. 4.B summarizes the NiV challenge results. Top panel again indicates RNA detection, and bottom panel indicates detection of infectious virus. Gray bars represent the challenge controls (group DNiV), white bars represent results from pigs vaccinated with sGHEV and challenged with NiV (group C), black bars represent results for pigs infected and subsequently challenged with NiV (group A).
NiV RNA detection is summarized in Fig. 4.B. Nipah virus challenge control animals had RNA (top panel) and infectious virus (bottom panel) present in all tested samples (group DNiV). With no infectious virus and almost no viral RNA detected in the tissues after challenge (low levels of RNA detected in tonsils and submandibular lymph nodes), pigs infected with NiV (group A) were considered protected against NiV challenge.
In contrast to group A, NiV RNA was detected post challenge in pigs vaccinated with sGHEV and challenged with NiV (group C), with the exception of the cerebrospinal fluid and the spleen. Infectious virus was detected from BALF cell pellet, OLF and submandibular lymph nodes of three vaccinated pigs (group C) in titers ranging from 1.7 – 4.14 log10 PFU/g. Lower virus load was detected in lung associated lymph nodes, trachea, nasal turbinates and palatine tonsils (titers ranging from 1.4 – 1.9 log10 PFU/g) with some animals being negative on virus isolation. Based on this data, the vaccination of pigs with sGHEV did not confer protection against NiV challenge in three out of four animals, although there were noticeable differences in viral RNA load between challenge controls and the group C animals in the following samples: brain, CSF, lung associated lymph nodes and spleen, and no infectious virus was recovered from one pig (No. 19).
An interesting preliminary observation was made with the pig euthanized one week post oral infection, and not included in the challenge control group D. This infection route, combined with a somewhat lower dose of virus (2.5 x104 versus 5x105 used in the challenge) resulted in virus detection by RT-PCR only in tonsil, SLN, and nasal swab at 2 dpi (3.85 log10 copies/ml), and detection of infectious virus in tonsil (2.1 log10 PFU/g).
Detection of cytokines by indirect ELISA
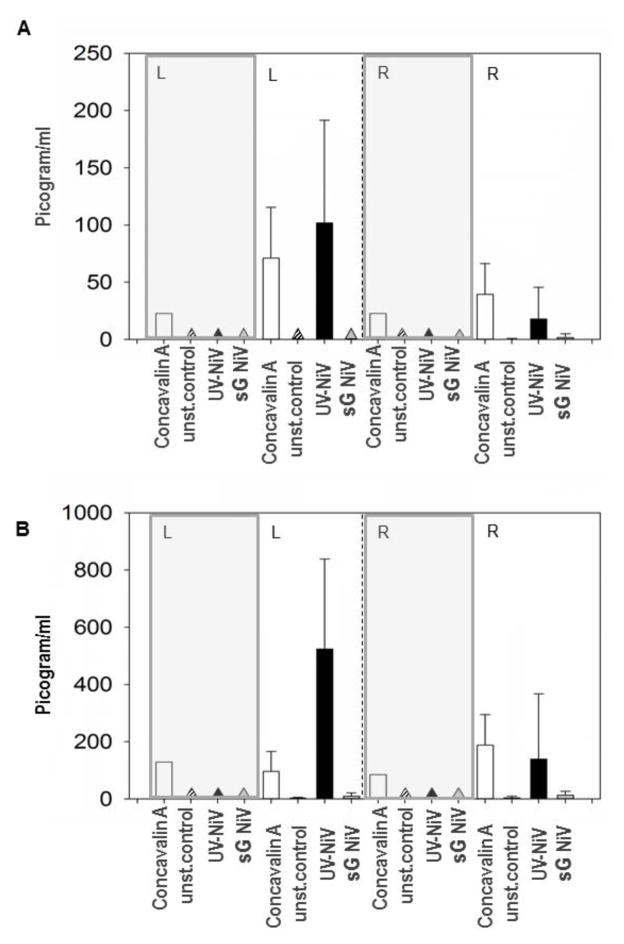
IFN-gamma and IL-10 production were assessed based on the observation that the two cytokines were upregulated in a previous vaccine efficacy study [13]. PBMC from all animals were re-stimulated prior to challenge: 28 dpi for NiV infected pigs (Group A) as well as for the sGHEV vaccinated pigs at 35 dpv (group B, C), and also at 5 – 7 days post challenge. Two doses (50 and 200 ng/well) of recombinant proteins, sGHEV or sGNIV, were employed in the restimulation assay, but failed to restimulate the PBMC even from the NiV infected pigs (data not shown). In addition, the PBMC restimulation was conducted using either UV inactivated NiV for animals to be challenged with NiV (groups A and C) or UV inactivated HeV for animals in group B (challenged with HeV), both prior to and post challenge. There was no production of IFN-gamma or IL-10 by restimulated PBMC from sGHEV vaccinated pigs or from challenge control pigs, except for the concanavalin A treated control cells. Both cytokines, IFN-gamma and IL-10, were upregulated in cells from pigs in group A (infected orally with NiV and challenged nasally 28 days later) prior to the challenge, and to a lower extent one week post challenge (Fig. 5).
Figure 5. Detection of IL-10 and IFN-gamma.
Production of IL-10 and IFN-gamma in restimulated PBMC was detected only in cells from pigs in Group A. Gray panels designate results from challenge control pigs. Triangles indicate lack of cytokine detection. Fig. 5.A Detection of IL-10 in picogram/ml in supernatants from restimulated PBMC: left section (L) prior to the challenge, right section (R) post challenge. White bars are cells restimulated with concavalin A, unstimulated cell controls (unst.control) are designated as stripe bars/triangles. Cells restimulated with UV inactivated NiV (UV-NiV) are indicated by black bars/triangles, and cells restimulated with sGNiV are designated as gray bars/triangles. Fig. 5.B illustrates production of IFN gamma by restimulated PBMC from group A pigs; designations are as in Fig. 5.A.
Since in general, Th1 cells secret IFN-gamma, and Th2 cells secrete IL-10, these data indicated that both Th1 and Th2 responses were activated in the restimulated cells from infected animals prior to challenge. However NiV infection appeared to downregulate IFN-gamma as well as IL-10 during the acute phase of the challenge (Fig. 5).
T-cell response
Activation of the T-cell response was assessed by labelling three cell markers: CD4, CD8, and the activation marker CD25. Current literature on swine immune cells, identifies the following T- cell populations based on these markers: CD4+CD8− cells are mostly T helper (Th) cells that do not respond to recall, however this population does contain regulatory CD4+CD8−CD25+ Treg cells (2 – 9%) which can respond to restimulation [25,26]. A second population, CD4+CD8+(lo)αα memory Th cells are the predominant CD4+ T cell subpopulation responding to restimulation [27,28]. A third cell population, CD4−CD8+ cytotoxic T cells upregulate CD25 upon antigenic stimulation along with the CD8 marker [29]. During asymmetric division, parental T cells yield both effector (CD4−CD8hi) and long lived memory (CD4−CD8lo) cytotoxic cells [30–32]. Due to the restricted number of markers labeled in this study, the CD4−CD8lo cytotoxic T memory cells could not be distinguished from the NK cells, which bear the CD4−CD8lo phenotype and upregulate CD25 as well [33].
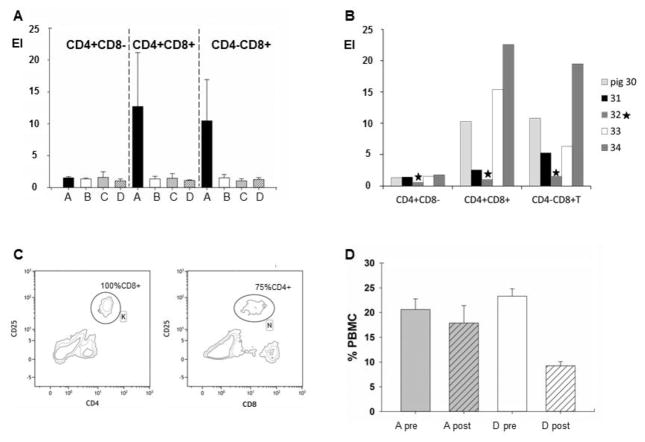
As anticipated, no CD25 upregulation was observed for the CD4+CD8− population (Fig. 6.A). Upregulation of the CD25 marker was detected only in PBMCs from pigs in the group A on CD4+CD8+ (predominantly T helper memory) cells and on CD4−CD8+ (predominantly cytotoxic T) cells (Fig. 6.A). The CD4−CD8+ cells (with both memory and effector T cells in this population, and likely containing also NK cells) were analyzed as one population when comparing all the experimental groups, as we were unable to distinguish cell populations based on intensity of staining for the CD8 marker in groups B and C. There was a statistically significant difference in the restimulation of CD4+CD8+ cells among the experimental groups (P=0.03), and the CD4+CD8+ restimulation for group A was significantly higher when compared to the challenge control group D (P=0.03). The upregulation of CD4−CD8+ cells indicated an even stronger difference among all the groups (P=0.01) with statistical difference in restimulation between groups A and D (P=0.3).
Figure 6. Cell mediated immune response.
PBMC were re-stimulated prior to the challenge: Fig. 6.A. Comparison of the CD25 upregulation in expression indices (EI) for CD4+CD8−, CD4+CD8+ and CD4−CD8+ T cell populations between the experimental groups. The groups are indicated by corresponding letters and bars: group A (black bar), group B (white bar), group C (gray bar), group D (striped bar). Fig. 6.B. Comparison of the CD25 upregulation in expression indices (EI) for CD4+CD8−, CD4+CD8+ and CD4−CD8+ T cell populations between individual pigs in group A (30, 31, 33 and 34) and the challenge control pig (No. 32 is indicated by star). Fig. 6.C. Example of the contour plots (pig No. 33, group A) for upregulation of CD25 marker in the CD4+ population (gate K) and the CD8+ population (gate N). The gates were further analysed for content of CD8+ cells (gate K) and CD4+ cells (gate N), indicating that while the CD4+ cells (gate K) were a homogeneous population of CD4+CD8+ cells, while the N gate was a mix of CD4+CD8+ population and CD4-CD8+ population. Fig. 6.D Comparison of total CD4+ cells frequency in unstimulated PBMC pre and post NiV challenge. Gray bars represent group A (pigs infected and 28 dpi challenged with NiV). White bars represent CD4+ frequency in the challenge control PBMCs. Plain bars labeled “pre” are values before the challenge and striped bars labeled “post” are values at 5 or 6 days after the challenge.
When comparing restimulation of PBMCs within group A on an individual basis (Fig. 6.B), pigs No. 30, 33 and 34 considered to be fully protected against NiV challenge had a higher upregulation of CD25 on Th (CD4+CD8+) memory cells as well as on the CD4−CD8+ cells in comparison to pig No. 31. This pig was the only one with residual viral RNA in some tissues, but would be nevertheless considered protected against challenge, based on all the other parameters. It was possible to distinguish between CD8hi and CD8lo cells in PBMCs from pigs in group A (example of a contour plot for pig No.33 in Fig. 6.C). Fig. 6.C illustrates based on upregulation of the CD25 marker that the pigs generated a memory CD4+CD8+ T cell response to NiV infection, as all the CD4+ cells with upregulated CD25 marker were also CD8+ positive (gate K). When CD8+ cells were analyzed for upregulation of CD25, three populations were revealed: CD8loCD25−, CD8+CD25+ (gate N) and CD8hiCD25−. Approximately 25% of cells in the gate N were CD4−CD8+ indicating restimulation of the cytotoxic T memory cells.
To assess the effect of the recombinant proteins on restimulation, three doses of either sGHEV or sGNIV, were administered (50, 100 and 200ng/well). Interestingly, both sGHEV and sGNIV failed to elicit any observable effect in PBMCs. Further, even in PBMCs generating a positive response to UV inactivated virus (group A), the recombinant sGHEV or sGNIV would not elicit a response (data not shown).
Difference in total CD4+ cell population in naïve (group D) and protected (group A) animals following NiV infection was observed in the unstimulated PBMC controls before and after challenge. The analysis indicated a drop in the total CD4+ population post challenge in NiV challenge control pigs (group D) (P<0.001), whereas there was no statistical difference in CD4+ population frequency pre and post challenge in unstimulated PBMC from pigs in group A (Fig. 6.D).
Discussion
The aim of this study was to elucidate factors involved in protective immune response in pigs against henipaviruses using experimental infection or immunization with sGHEV. Based on the requirement for henipavirus veterinary vaccines to interrupt transmission among pigs and importantly to humans, and based on previous experimental work [13], the following parameters were evaluated: infectious virus and viral RNA load in tissues and nasal washes (an indicator of shedding), development of antibodies, and development of adaptive cellular immune response. Disease prevention was not considered a reliable parameter of protection since in agreement with previous studies [34] clinical signs were observed post infection/challenge in only two of eight pigs (transient increase in rectal temperature).
Protection against nasal henipavirus challenge was evaluated in pigs vaccinated with adjuvanted sGHEV or orally infected with NiV(group A). Vaccinated pigs were challenged either with HeV (group B) or NiV (group C).
Animals in group A were considered protected against NiV challenge based on the significant reduction of viral RNA shedding and viral RNA load in tissues and body fluids at post mortem examination, and lack of detection of infectious virus.
As for groups B and C, based on the analysis of neutralizing antibodies, protective titers (160 – 320) were reached against HeV in sGHEV vaccinated animals, but not against NiV (titers of 40 – 80). As expected, sGHEV vaccinated animals challenged with NiV (group C) were not protected against NiV challenge. Surprisingly, the HeV vaccinated animals (Group B), expected to be protected against HeV challenge based on the neutralizing antibodies levels were only partially protected. Although there was some reduction in virus RNA load in tissues and no infectious virus was isolated from the tissues, there was no reduction of viral RNA in nasal washes and the pigs were shedding infectious virus early post infection.
Analysis of neutralizing activity of the sera revealed a low cross-neutralization of NiV by GHEV antibodies. In contrast, Mungall and colleagues [16] reported high cross-neutralization in serum from cats vaccinated with GHEV against NiV, while serum from cats vaccinated with GNIV had lower cross-neutralizing activity against HeV. Several animal models vaccinated with GHEV were cross-protected against NiV [16, 18, 21, 22]. Interestingly, attempts to cross-protect hamsters against HeV by vaccination with GNIV were only partially successful compared to protection against NiV [35]. Differences in cross-reactivity of anti-G antibodies determined by ELISA prior to the challenge were also apparent. Whether the observed lack of cross-reactivity between the anti-G antibodies generated against NiV or HeV is unique to swine, is difficult to assess at this time. Interestingly, anti-G antibody levels detected by ELISA in vaccinated animals were significantly higher than in the infected animals (group A) while exhibiting similar neutralizing activity against their respective viruses: a higher concentration of HeV anti-G antibodies were required to obtain the same neutralizing titers against HeV compared to the anti-G antibody levels in serum of NiV infected piglets required for NiV neutralization (Fig. 2). This underlines role of additional viral proteins in eliciting neutralizing antibodies, such as the fusion F protein [13].
Analysis of the CMI response prior to challenge revealed that there were no detectable immune memory cells generated in response to immunization with GHEV, while pigs infected with NiV (group A) developed antigen-specific immune memory cells (Th and Tc). Interestingly, the CD4+ cell population remained stable following the challenge compared to the control pigs. This is a potential indicator of protection that has been suggested previously [24] and requires further studies.
Upregulation of IFN-gamma (typically secreted by Th1 cells) and IL-10 (typically secreted by Th2 cells) prior to challenge suggested that the oral infection with NiV lead to development of cellular memory with both Th1 and Th2 responses activated. The observed down-regulation of IL-10 and IFN-gamma in PBMC restimulated post-challenge is in agreement with the proposed immune-modulating characteristics of NiV during the acute phase of the infection [36–38]. Expression of IFN-gamma and IL-10 was downregulated along with IL-6, IL-4 and IL-1beta in the brain of NiV infected hamsters early post infection [37]. However, since in cell culture, NiV infected human dendritic cells were able to up-regulate IL-10 [39], the IL-10 block in vivo may be connected to IL-12 production.
Despite the fact that NiV infects multiple types of porcine immune cells leading to the proposed downregulation of Th1 and Th2 responses, and the fact that NiV non-structural proteins interfere with a number of regulatory pathways in the infected immune cells [40,41] resulting in skewed cytokine responses, the orally infected pigs developed humoral and cellular immune memory, and were able to mount an efficient protective immune response against NiV challenge. Studies elucidating the complex NiV virus - host interactions in swine may facilitate better understanding and development of treatment and vaccination strategies in humans and other species, albeit indirectly.
In addition, the present study confirmed the susceptibility of pigs to HeV even using much lower dose of 2.5x105 PFU/animal. The observed difference between HeV and NiV invasion of CNS in this study indicates that there may be a difference between the two viruses in their propensity to invade CNS. This would be in agreement with the previous pilot study employing a higher dose of HeV 107PFU/animal [8].
As a number of publications demonstrated efficacy of the sGHEV vaccine candidates against both HeV and NiV in several species [14, 16–18, 20–22], consideration was given to the sGHEV as a potential vaccine candidate in pigs as well. By demonstrating partial protection in sGHEV vaccinated animals challenged with HeV, the current study indicates, that vaccine formulation inducing cellular immunity may be efficacious in pigs, and warrants further work on developing sGHEV or more likely sGNIV as a vaccine against NiV in swine.
In conclusion, this work demonstrated that both arms of the immune response are critical for the protection of swine against henipaviruses. The study clearly underlined the importance of an adaptive cell-mediated immune response in protection against Hendra and Nipah virus. The sGHEV combined with an adjuvant stimulating cellular immune response should be again evaluated as a vaccine candidate against NiV in swine.
Acknowledgments
The authors would like to thank Kurtis Swekla, DVM and Cory Nakamura for their assistance with the animal experiments in CL4. The authors would like to acknowledge Zoetis, Inc. for providing the formulated vaccines. Funding for this study was provided from Canadian Food Inspection Agency technical development funds [grant number TDW1303]; from the National Institutes of Health [grant number AI054715-06] to C. C. Broder, and by the United States Department of Homeland Security, Science and Technology Directorate (Contract No. HSHQDC-13-0-00135) to J. A. Roth, Transboundary Animal Biologics Inc.
Footnotes
Conflict of Interest
The authors: John M. Hardham, Paul J. Dominowski, Dennis L. Foss, and Duncan Mwangi are employees of Zoetis. Brad S. Pickering, Greg Smith, Eva T. Weingartl, Christopher C. Broder, James A. Roth, and Hana M. Weingartl have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohd Nor MN, Gan CH, Ong BL. Nipah virus infection of pigs in peninsular Malaysia. Rev Sci Tech Off Int Epiz. 2000;19:160–165. doi: 10.20506/rst.19.1.1202. [DOI] [PubMed] [Google Scholar]
- 2.Ching PKG, de los Reyes VC, Sucaldito MN, et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg Infect Dis (Internet) 2015;21:328–331. doi: 10.3201/eid2102.141433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luby SP, Gurley ES. Epidemiology of henipavirus disease in humans. In: Lee B, Rota PA, editors. Henipavirus. Ecology, Molecular Virology and Pathogenesis. Springer; [Google Scholar]; Curr Top Microbiol Immunol. 2012;359:25–40. doi: 10.1007/82_2012_207. [DOI] [PubMed] [Google Scholar]
- 4.Murray K, Selleck P, Hooper P, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 5.Field H, Schaaf K, Kung N, et al. Hendra virus outbreak with novel clinical features, Australia. Emerg Infect Dis. 2010;16:338–340. doi: 10.3201/eid1602.090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field HE, Crameri G, Kung NY, Wang L. Ecological aspects of Hendra virus. In: Lee B, Rota PA, editors. Henipavirus. Ecology, Molecular Virology and Pathogenesis. Springer; [Google Scholar]; Curr Top Microbiol Immunol. 2012;359:11–23. doi: 10.1007/82_2012_214. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. Hendra virus, equine - Australia (09): New South Wales dog affected Pro-med: International Society for Infectious Diseases. 2013. [Google Scholar]
- 8.Li M, Embury-Hyatt C, Weingartl HM. Experimental infection study indicates swine as a potential host for Hendra virus. Vet Res. 2010;41:33. doi: 10.1051/vetres/2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, McEachern JA, Green D, Hancock TJ, Chan YP, Hickey AC, Dimitrov DS, Wang LF, Broder CC. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 2009;5:e1000642. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisbert TW, Mire CE, Geisbert JB, et al. Therapeutic Treatment of Nipah Virus Infection in Nonhuman Primates with a Neutralizing Human Monoclonal Antibody. Sci Transl Med. 2014;6:242ra82. doi: 10.1126/scitranslmed.3008929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillaume V, Contamin H, Loth P, et al. Nipah virus: vaccination and passive protection studies in a hamster model. J Virol. 2004;78:834–840. doi: 10.1128/JVI.78.2.834-840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillaume V, Wong KT, Looi RY, et al. Acute Hendra virus infection: Analysis of the pathogenesis and passive antibody protection in the hamster model. Virology. 2009;387(2):459–465. doi: 10.1016/j.virol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Weingartl HM, Berhane Y, Caswell JL, et al. Recombinant Nipah virus vaccines protect pigs against challenge. J Virol. 2006;80(16):7929–7938. doi: 10.1128/JVI.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton D, Pallister J, Klein R, et al. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg Infect Dis. 2014;20:372–379. doi: 10.3201/eid2003.131159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBuysscher BL, Scott D, Marzi A, Prescott J, Feldmann H. Single-dose live-attenuated Nipah virus vaccines confer complete protection by eliciting antibodies directed against surface glycoproteins. Vaccine. 2014;32:2637–2644. doi: 10.1016/j.vaccine.2014.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mungall BA, Middleton D, Crameri G, et al. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J Virol. 2006;80:12293–12302. doi: 10.1128/JVI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallister J, Middleton D, Wang LF, et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29:5623–30. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallister JA, Klein R, Arkinstall R, et al. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol J. 2013;10:237. doi: 10.1186/1743-422X-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisbert TW, Daddario-DiCaprio KM, Hickey AC, et al. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS ONE. 2010;5:e10690. doi: 10.1371/journal.pone.0010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mire CE, Geisbert JB, Agans KN, et al. A recombinant Hendra virus G glycoprotein subunit vaccine protects nonhuman primates against Hendra virus challenge. J Virol. 2014;88:4624–31. doi: 10.1128/JVI.00005-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bossart KN, Rockx B, Feldmann F, et al. A Hendra Virus G Glycoprotein Subunit Vaccine Protects African Green Monkeys from Nipah Virus Challenge. Sci Transl Med. 2012;4(146):146ra107. doi: 10.1126/scitranslmed.3004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEachern JA, Bingham J, Crameri G, et al. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine. 2008;26:3842–52. doi: 10.1016/j.Vaccine.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middleton DJ, Weingartl HM. Henipaviruses in their natural animal hosts. In: Lee B, Rota PA, editors. Henipavirus. Ecology, Molecular Virology and Pathogenesis. Springer; [DOI] [PubMed] [Google Scholar]; Curr Top Microbiol Immunol. 2012;359:105–121. doi: 10.1007/82_2012_210. [DOI] [PubMed] [Google Scholar]
- 24.Stachowiak B, Weingartl HM. Nipah virus infects specific subsets of porcine peripheral blood mononuclear cells. PLoS ONE. 2012;7:e30855. doi: 10.1371/journal.pone.0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Käser T, Gerner W, Hammer SE, Patzl M, Saalmüller A. Phenotypic and functional characterisation of porcine CD4(+)CD25(high) regulatory T cells. Vet Immunol Immunopathol. 2008;15:122, 153–158. doi: 10.1016/j.vetimm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez AM, Zhu J, Huang X, Yang Y. The development and function of memory regulatory T cells after acute viral infections. J Immunol. 2012;189:2805–2814. doi: 10.4049/jimmunol.1200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saalmüller A, Werner T, Fachinger V. T-helper cells from naive to committed. Vet Immunol Immunopathol. 2002;10:87, 137–145. doi: 10.1016/s0165-2427(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 28.Denyer MS, Wileman TE, Stirling CM, Zuber B, Takamatsu HH. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet Immunol Immunopathol. 2006;110:279–292. doi: 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Charerntantanakul W, Roth JA. Biology of porcine T lymphocytes. Anim Health Res Rev. 2007;7:81–96. doi: 10.1017/S1466252307001235. [DOI] [PubMed] [Google Scholar]
- 30.Littman DR, Singh H. Asymmetry and immune memory. Science. 2007;315:1673–1674. doi: 10.1126/science.1141184. [DOI] [PubMed] [Google Scholar]
- 31.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefrançois L, Obar JJ. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol Rev. 2010;235:206–18. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jost S, Quillay H, Reardon J, Peterson E, Simmons RP, Parry BA, Bryant NN, Binder WD, Altfeld M. Changes in cytokine levels and NK cell activation associated with influenza. PLoS ONE. 2011;6:e25060. doi: 10.1371/journal.pone.0025060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middleton DJ, Westbury HA, Morrissy CJ, van der Heide BM, Russell GM, Braun MA, Hyatt AD. Experimental Nipah virus infection in pigs and cats. J Comp Pathol. 2002;126:124–136. doi: 10.1053/jcpa.2001.0532. [DOI] [PubMed] [Google Scholar]
- 35.Ploquin A, Szécsi J, Mathieu C, Guillaume V, Barateau V, Ong KC, Wong KT, Cosset FL, Horvat B, Salvetti A. Protection against henipavirus infection by use of recombinant adeno-associated virus-vector vaccines. J Infect Dis. 2013 Feb 1;207(3):469–478. doi: 10.1093/infdis/jis699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satterfield BA, Cross RW, Fenton KA, Agans KN, Basler CF, Geisbert TW, Mire CE. The immunomodulating V and W proteins of Nipah virus determine disease course. Nat Commun. 2015;6:7483. doi: 10.1038/ncomms8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockx B, Brining D, Kramer J, Callison J, Ebihara H, Mansfield K, Feldmann H. Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. J Virol. 2011;85:7658–7671. doi: 10.1128/JVI.00473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berhane Y, Weingartl HM, Lopez J, et al. Bacterial Infections in Pigs Experimentally Infected with Nipah Virus. Transbound Emerg Dis. 2008;55:165–174. doi: 10.1111/j.1865-1682.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 39.Gupta M, Lo MK, Spiropoulou CF. Activation and cell death in human dendritic cells infected with Nipah virus. Virology. 2013;441:49–56. doi: 10.1016/j.virol.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Rota PA, Lo MK. Molecular virology of the henipaviruses. In: Lee B, Rota PA, editors. Henipavirus. Ecology, Molecular Virology and Pathogenesis. Springer; [Google Scholar]; Curr Top Microbiol Immunol. 2012;359:41–58. doi: 10.1007/82_2012_211. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi M, Kitagawa Y, Zhou M, Itoh M, Bin Gotoh B. An anti-interferon activity shared by paramyxovirus C proteins: Inhibition of Toll-like receptor 7/9-dependent alpha interferon induction. FEBS Lett. 2014;588(1):28–34. doi: 10.1016/j.febslet.2013.11.015. [DOI] [PubMed] [Google Scholar]