Abstract
The oral mucosae and draining lymph nodes are primary entry points for invading pathogens, particularly during immunosuppressive HIV/SIV infections. Innate immunity against oral stimuli, including natural killer (NK) cells, is understudied. Herein, we demonstrate functional NK cell responses to pathogen-associated molecular patterns (PAMPs) of potential oral pathogens in rhesus macaques.
Introduction
Gastrointestinal and oral mucosae are two of the most heavily immune-surveyed areas in the body. However, compared to gut mucosal studies, the network of lymph nodes and immune cells in the oral mucosae monitoring and maintaining homeostasis with the resident commensal microbiome while also responding to pathogenic microorganisms remains poorly understood 1, 2. Innate immune cells contribute significantly to the initial response towards invading pathogens, and specifically natural killer (NK) cells play crucial roles in preventing oral cancers 3–7 and oral thrush/candidiasis8. Interestingly, while systemic NK cell function diminishes with immunodeficiency resulting from HIV or SIV infections9–11, previous work from our laboratory showed little functional differences between oral NK cells from SIV-infected and uninfected animals when stimulated with mitogens12. Unfortunately artificial stimulation does not recapitulate toll-like receptors (TLR) activation resulting from pathogen-associated molecular patterns (PAMPs). TLR expression on NK cells has been a controversial topic, but a consensus in the field suggests that while NK cells may express some TLR they do not seem to rely directly on TLR engagement in order to respond to an infection or abnormal cell, but more on their environment 8, 9. Rather antigen-presenting cells such as dendritic cells (DC), macrophages (MØ) or B cells respond to these signals and indirectly activate the NK cell response.
Materials and Methods
Animals
Indian rhesus macaques (Macaca mulatta) were analyzed in this study. All animals were experimentally naïve and euthanized as normal animals for an unrelated research project. Matched oral and mesenteric lymph nodes were isolated from all animals following euthanasia. All samplings were reviewed and approved by the local Institutional Animal Care and Use Committee and were carried out in accordance with recommendations detailed in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health with recommendations of the Weatherall report; “The use of non-human primates in research”.
Indirect NK cell stimulation using TLR ligands
Mononuclear cells from OLN or MLN were rested in R5 media (RPMI + 5%FBS + 1% penstrep) at 37°C for 6h, then stimulated in the presence of Golgi Stop (BD Biosciences, concentrations as recommended by manufacturer) with either 1μg/mL CpG ODN 2395 (InvivoGen, CA) in DOTAP (ROCHE, Germany), 1μg/mL LPS-PG (InvivoGen, CA), 10μg/mL Zymosan (InvivoGen, CA) in R5, or were cultured in R5 only for 12h at 37°C.
Flow cytometry
All antibodies used were purchased from BD Biosciences unless specified otherwise. For surface staining antibodies against the following antigens were used: CD3 (SP34.2), CD8α (SK1), CD11c (3.9, Biolegend), CD14 (MϕP9), CD20 (L27), CD16 (3G8), CD45 (D058-1283), CD56 (NCAM16.2), CD80 (L307.4), CD86 (2311), CD159 (Z199, Beckman Coulter). Intracellular staining antibodies included TNF-α (MAb11), IFN-γ (B27), CD107a (H4A3) and MIP1β (24006, R&D Systems). Flow cytometry data was acquired on a LSRII (BD Biosciences, La Jolla, CA) and analyzed with FlowJo software (version 10.2, Tree Star, Ashland, OR).
Statistical analyses
Statistical and graphing analyses were performed with GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA). Wilcoxon Matched pairs test and Student’s t-test were used where indicated, and p-values of p < 0.05 were considered to be statistically significant.
Results
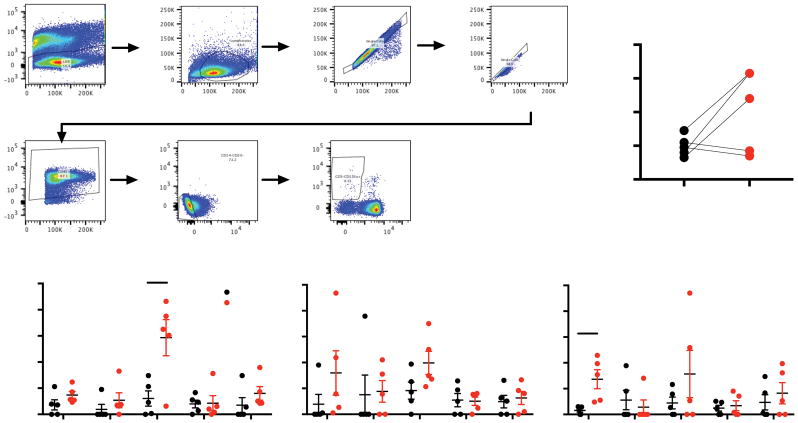
Strengths of using non-human primate models include investigation of the earliest responses at tissue sites of infection. Despite this, the initial responses to infection by NK cells, and the preceding mechanisms leading to their activation are still incompletely understood. Our group has previously shown that there are little differences in oral NK cell responses in normal and SIV-infected macaques 12 following mitogen stimulation, but it is unclear whether NK cells in mucosal-draining lymph nodes might respond differently against more typical pathogen agonists. In order to address this deficit we established a rapid TLR stimulation assay mimicking the earliest responses to infection in OLN and matched MLN, while decreasing feedback-signaling events from long term-stimulation. Total NK cells were identified as CD3-CD14-CD20-NKG2A/C+ (Fig. 1A) among total mononuclear cells using standard NK cell phenotyping for rhesus macaques optimized in our laboratory 12. Frequencies of NK cells in MLN were not statistically different compared to those found OLN, though there was a trend towards a greater NK Cell frequency in the MLN (Fig. 1B). Interestingly all three agonists tested elicited measurable responses from NK cells in both OLN and MLN (Fig. 1C) with Zymosan eliciting the most robust IFN-γ response in MLN (p = 0.015, Student’s t-test), and LPS-PG eliciting the strongest CD80 response in MLN over OLN (p = 0.012, Student’s t-test).
Figure 1. Activation of mucosal draining lymph node NK cells by TLR ligands.
(A) Representative gating strategy used to identify NK cells in lymph nodes. (B) Frequencies of NK cells found in OLN and MLN samples. (C) Background subtracted NK cell responses to ligands for TLR2/Dectin-1 (Zymosan), TLR9 (CpG) and TLR4/2 (LPS-PG) using CD107a, IFNγ, MIP1β, TNFα, CD80 as indicators of NK cell activation. Values from unstimulated controls were subtracted from stimulated values and any negative values were zeroed for readability. Wilcoxon Matched pairs test was used to compare samples between OLN and MLN groups, and Student’s t test was used for in vitro analyses; *, P < 0.05.
Discussion
Many works attributing NK responses following TLR stimulation have long incubation times (≥18h), and in some cases do not use pure NK populations 16–18. As a result, our indirect assay maybe a better approximation of in vivo NK cell stimulation during infection. Here we show that NK cells are stimulated using TLR and Dectin-1 agonists, but due to the abbreviated time of our experiment, we posit that the observed NK cell activation is indirect, rather than a direct activation via NK-cell specific TLR. These agonists include the TLR2/Dectin-1 agonist Zymosan – a proxy for fungal infections such as candidiasis 19, TLR2/4 agonist LPS-PG from Porphyromonas gingivalis to mimic common infections in the mouth, and TLR9 agonist CpG ODN to mimic pathogenic bacteria as well as KSHV infection which may lead to Kaposi sarcoma20, 21. We hypothesized that stimulation with these agonists will allow us to better elucidate the role of NK cells in microbial infections in the oral mucosae, especially in the context of immunocompromised diseases/conditions, and indeed show here that OLN NK cells are activated by these agonists. We propose that using rhesus macaques will provide a valuable model system with which to examine the NK response during the earliest activation events where antigen-presenting cells initiate a response via TLR activation.
Acknowledgments
Funding information:This work was supported by National Institutes of Health grants R01 DE026327 and R01 DE026014 (to R.K.R.), and the Harvard Center for AIDS Research grant P30 AI060354.
The authors thank Michelle Lifton of the BIDMC flow cytometry core for technical assistance, and Cordelia Manickam for helpful comments during the preparation of this manuscript.
References
- 1.Durudas A, Chen HL, Gasper MA, et al. Differential Innate Immune Responses to Low or High Dose Oral SIV Challenge in Rhesus Macaques. Curr HIV Res. 2011 Aug 24; doi: 10.2174/157016211797635928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel K, Pahar B, Van Rompay KK, et al. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J Virol. 2006 Jul;80(13):6357–6367. doi: 10.1128/JVI.02240-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews NC, Goodier MR, Robey RC, Bower M, Gotch FM. Killing of Kaposi’s sarcoma-associated herpesvirus-infected fibroblasts during latent infection by activated natural killer cells. Eur J Immunol. 2011 Jul;41(7):1958–1968. doi: 10.1002/eji.201040661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirianni MC, Vincenzi L, Topino S, et al. NK cell activity controls human herpesvirus 8 latent infection and is restored upon highly active antiretroviral therapy in AIDS patients with regressing Kaposi’s sarcoma. Eur J Immunol. 2002 Oct;32(10):2711–2720. doi: 10.1002/1521-4141(2002010)32:10<2711::AID-IMMU2711>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Kaur K, Chang HH, Cook J, Eibl G, Jewett A. Suppression of Gingival NK Cells in Precancerous and Cancerous Stages of Pancreatic Cancer in KC and BLT-Humanized Mice. Front Immunol. 2017;8:1606. doi: 10.3389/fimmu.2017.01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal R, Chaudhary M, Bohra S, Bajaj S. Evaluation of natural killer cell (CD57) as a prognostic marker in oral squamous cell carcinoma: An immunohistochemistry study. J Oral Maxillofac Pathol. 2016 May-Aug;20(2):173–177. doi: 10.4103/0973-029X.185933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozlowska AK, Topchyan P, Kaur K, et al. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J Cancer. 2017;8(4):537–554. doi: 10.7150/jca.15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaforio JJ, Ortega E, Algarra I, Serrano MJ, Alvarez de Cienfuegos G. NK cells mediate increase of phagocytic activity but not of proinflammatory cytokine (interleukin-6 [IL-6], tumor necrosis factor alpha, and IL-12) production elicited in splenic macrophages by tilorone treatment of mice during acute systemic candidiasis. Clin Diagn Lab Immunol. 2002 Nov;9(6):1282–1294. doi: 10.1128/CDLI.9.6.1282-1294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott-Algara D, Vuillier F, Cayota A, Dighiero G. Natural killer (NK) cell activity during HIV infection: a decrease in NK activity is observed at the clonal level and is not restored after in vitro long-term culture of NK cells. Clin Exp Immunol. 1992 Nov;90(2):181–187. doi: 10.1111/j.1365-2249.1992.tb07925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal FE, Premeaux TA, Abdel-Mohsen M, Ndhlovu LC. Role of Natural Killer Cells in HIV-Associated Malignancies. Front Immunol. 2017;8:315. doi: 10.3389/fimmu.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005 Nov 15;106(10):3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Reeves RK. Functional perturbation of classical natural killer and innate lymphoid cells in the oral mucosa during SIV infection. Front Immunol. 2012;3:417. doi: 10.3389/fimmu.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006 Jan;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 14.Adib-Conquy M, Scott-Algara D, Cavaillon JM, Souza-Fonseca-Guimaraes F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol. 2014 Mar;92(3):256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz A, Stegmann KA, Riley EM. Activation of natural killer cells during microbial infections. Front Immunol. 2011;2:88. doi: 10.3389/fimmu.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorski KS, Waller EL, Bjornton-Severson J, et al. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol. 2006 Jul;18(7):1115–1126. doi: 10.1093/intimm/dxl046. [DOI] [PubMed] [Google Scholar]
- 18.Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J Virol. 2006 May;80(9):4286–4291. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hida S, Nagi-Miura N, Adachi Y, Ohno N. Beta-glucan derived from zymosan acts as an adjuvant for collagen-induced arthritis. Microbiol Immunol. 2006;50(6):453–461. doi: 10.1111/j.1348-0421.2006.tb03814.x. [DOI] [PubMed] [Google Scholar]
- 20.Bussey KA, Reimer E, Todt H, et al. The gammaherpesviruses Kaposi’s sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response. J Virol. 2014 Aug;88(16):9245–9259. doi: 10.1128/JVI.00841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West JA, Gregory SM, Sivaraman V, Su L, Damania B. Activation of plasmacytoid dendritic cells by Kaposi’s sarcoma-associated herpesvirus. J Virol. 2011 Jan;85(2):895–904. doi: 10.1128/JVI.01007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]