Abstract
Importance:
Current cancer screening guidelines recommend cessation of cervical cancer screening at age 65 for most women. To examine residual risk among elderly women, we compared cervical cancer incidence rates in Massachusetts from 2004–2015 among women aged <65 vs. ≥65.
Methods:
The Massachusetts Cancer Registry (MCR) was used to identify all women diagnosed with cervical cancer between 1/1/2004–12/31/2015. Cancer incidence was calculated based on age of diagnosis (<65 vs. ≥65).
Results:
In Massachusetts, 2,418 incident cases of cervical cancer were diagnosed from 2004–2014, of which 571 (23.6%) were diagnosed among women ages 65 and older. When compared with women diagnosed under age 65, women diagnosed at age ≥65 were more likely to be diagnosed with Stage II or higher (71.8% vs. 43.8%, p < 0.001). Cervical cancer incidence rates decreased annually for women <65 from 2004–2015. Among women aged ≥65, cancer incidence rates decreased by 3.9% annually from 2004–2013 (p=0.0009), but 2013–2015 showed an increasing trend (APC + 14.1%, p=0.12).
Conclusions and Relevance:
Women 65 and over account for one quarter of cervical cancer diagnoses in Massachusetts, and present with higher stage disease than younger women. Upcoming planned revisions in screening and prevention guidelines should address the continued risk of cervical cancer for older women.
Precis:
From 2004–2015, women age >65 represented 23.6% of cervical cancers and had later stage disease.
Introduction
Current cervical cancer screening guidelines advise average risk women to stop screening at age 65 if they have had adequate prior testing with normal results. However, these recommendations were based largely on modeling studies, and more recent data have questioned the appropriateness of current guidelines for screening cessation.1,2 Recent data show that, after controlling for hysterectomy, cervical cancer incidence may not decline until at least age 85,3 that older women participating in screening are diagnosed at earlier stages than unscreened older women,4 and that profound racial disparities exist in cervical cancer diagnoses over age 65.5 Given concerns about persistent cervical cancer risk in older women, we compared the cervical cancer incidence rate and stage of cancer presentation among women aged <65 compared women aged ≥65 in Massachusetts, a state with mandated universal healthcare coverage since 2006.
Methods
Data on cases of invasive cervical cancer (International Classification of Diseases, ICD-O code C53) from 2004–2015 were obtained from the Massachusetts Cancer Registry (MCR). The MCR has been collecting data on incident cancers since 1982 and has been estimated to have over 95% complete case ascertainment.6 This analysis was conducted at Massachusetts Department of Public Health under the public health surveillance authority. The population of women with cervical cancer was divided by age (<65 vs. ≥65 years) at diagnosis, and incidence data were examined by race/ethnicity, birth country, region of residence, type of insurance, stage at diagnosis, and histology. We dichotomized at Stage II or higher because Stage I is both more curable and more likely to be diagnosed by screening, whereas Stage II and higher has poorer prognosis and is more likely to be diagnosed based on symptoms. Chi square tests and Fisher’s exact tests were calculated for these characteristics. Incidence rates were age-specific rates for each age group per 100,000 female person-years. We used Joinpoint regression to assess trends, to calculate the annual percent change (APC), and to perform hypothesis tests. Multiple APC values are shown when the regression indicated a statistically significant breakpoint. Additional difference-in-differences regression models were run to determine whether observed changes in the APC for women aged ≥65 from 2013–2015 were significant compared to 2004–2012 were significantly different from that for <65 women.7
Results
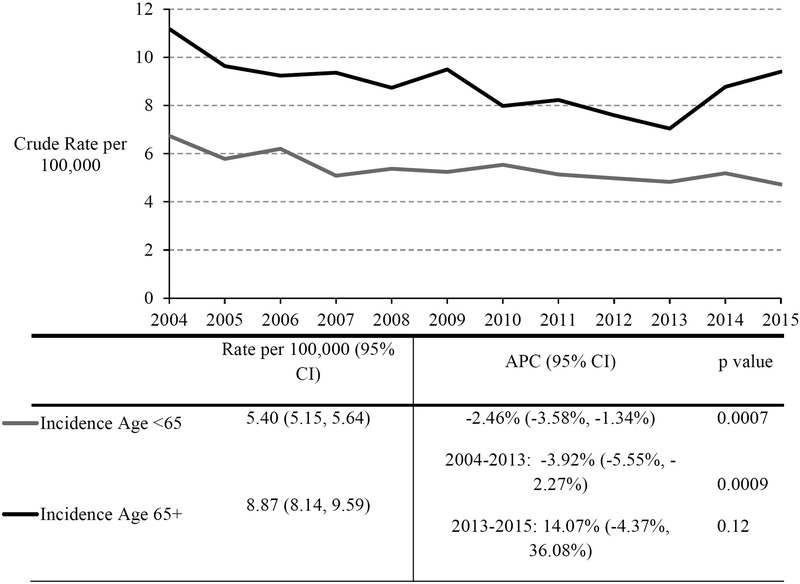
From 2004–2015, 571 (23.6%) of the 2,418 incident cases of cervical cancer diagnosed in Massachusetts occurred among women ages 65 and older. The mean ages of women diagnosed at ages <65 compared to ≥65 were 45.3 years old (SD = 10.4) and 75.5 (SD = 7.8) respectively. The age-specific incidence rate (IR) was 1.6 times higher among women age ≥65 compared to younger women (8.87 per 100,000 vs. 5.40 per 100,000; p<0.05; Figure 1). Cancer incidence rates increased after age 35, and remained stable through age 85 (Table 2). Older women were more likely to be diagnosed with cancer at stage II or higher than younger women (71.8% vs. 43.8%; p<0.0001; Table 1). Compared to women diagnosed under age 65, women age ≥65 at diagnosis were more likely to be non-Hispanic Black. No differences were noted by country of origin or county of residence within Massachusetts. As expected, older women were more likely to have Medicare insurance.
Figure 1:
Trends in the crude incidence and mortality rates per 100,000 of cervical cancer by age group in Massachusetts, 2004–2015.
APC = Annual percent change; CI = Confidence Interval; APC is for 2004–2015 unless otherwise specified; multiple APC values are shown when the regression indicated a statistically significant break point; rates are per 100,000 female person-years
Table 2:
Age-specific incidence rates for cervical cancer by age category, Massachusetts 2004–2015.
| Incidence | ||||
|---|---|---|---|---|
| Age | N. Cases | Incidence Rate (95% CI) | APC | p value |
| <35 | 322 | 1.83 (1.63, 2.03) | −4.89 | 0.024 |
| 35–44 | 547 | 9.75 (8.93, 10.57) | −0.97 | 0.44 |
| 45–54 | 546 | 8.96 (8.21, 9.71) | −1.69 | 0.13 |
| 55–64 | 432 | 8.76 (7.94, 9.59) | −3.09 | 0.025 |
| 65–74 | 291 | 9.48 (8.39, 10.57) | −1.41 | 0.28 |
| 75–84 | 188 | 8.67 (7.43, 9.90) | −2.60 | 0.09 |
| ≥85 | 92 | 7.65 (6.08, 9.21) | −2.92 | 0.28 |
Table 1:
Characteristics of women diagnosed with cervical cancer by age group in Massachusetts, 2004–2015
| Diagnosed Age <65 (N=1,847) | Diagnosed Age ≥65 (N=571) | p value | |
|---|---|---|---|
| Characteristics of Incident Cases | |||
| Age at Diagnosis, mean (SD) | 45.3 (10.4) | 75.5 (7.8) | <0.0001b |
| Race/Ethnicity, N (%) | 0.02 | ||
| Non-Hispanic, White | 1,371 (75.2) | 420 (74.2) | |
| Non-Hispanic, Black | 150 (8.2) | 68 (12.0) | |
| Non-Hispanic, Asian | 107 (5.9) | 22 (3.9) | |
| Hispanic | 196 (10.8) | 56 (9.9) | |
| Birth Country, N (%) | 0.96 | ||
| Foreign | 353 (31.6) | 138 (31.7) | |
| Native | 764 (68.4) | 297 (68.3) | |
| Massachusetts Region, N (%) | 0.80 | ||
| Western | 260 (14.1) | 79 (13.8) | |
| Central | 203 (11.0) | 74 (13.0) | |
| North Shore | 206 (11.2) | 64 (11.2) | |
| Greater Boston | 805 (43.6) | 233 (40.8) | |
| South Shore | 61 (3.3) | 21 (3.7) | |
| Southeast | 159 (8.6) | 55 (9.6) | |
| Cape and Islands | 153 (8.3) | 45 (7.9) | |
| Type of Insurance, N (%) | <0.0001 | ||
| None | 77 (4.3) | ---* | |
| Private | 959 (53.7) | 56 (10.4) | |
| Medicaid | 440 (24.6) | 72 (13.3) | |
| Medicare | 101 (5.7) | 394 (73.0) | |
| Insurance, NOS | 209 (11.7) | 16 (3.0) | |
| Stage at Diagnosis, N (%) | <0.0001 | ||
| I | 1,038 (56.2) | 161 (28.2) | |
| II | 583 (31.6) | 230 (40.3) | |
| III | 184 (10.0) | 127 (22.2) | |
| IV | 42 (2.3) | 53 (9.3) | |
| Histology, N (%) | <0.0001 | ||
| Squamous Cell Carcinoma | 1,103 (59.7) | 373 (65.3) | |
| Adenocarcinoma | 514 (27.8) | 99 (17.3) | |
| Other Carcinomas | 184 (10.0) | 62 (10.9) | |
| Other and Unspecified Neoplasms | 46 (2.5) | 37 (6.5) | |
SD = standard deviation; N=number; NOS = not otherwise specified; p values in right column are from chi-square tests except when denoted by a for Fisher’s exact tests or b for t tests; 28 people had an unknown race/ethnicity, 863 people had missing birth country, 61 people had unknown insurance type; western = Berkshire, Franklin, Hampshire, and Hampden counties, central = Worcester county, north shore =Essex county, greater Boston = Middlesex, Norfolk, and Suffolk counties, south shore =Plymouth county, southeast =Bristol county, cape and islands = Barnstable, Dukes, and Nantucket county; ICD-O-3 codes: squamous cell carcinomas (8050–8078, 8083–8084), adenocarcinomas (8140–8141, 8190–8211, 8230–8231, 8260–8263, 8310, 8380, 8382–8384, 8440–8490, 8570–8574, 8576), other carcinomas (8560, 8010–8035, 8041, 8045, 8082, 8090, 8094, 8098, 8120, 8046, 8144, 8246, 8255, 8323, 8380), and other and unspecified neoplasms (all other codes)
Fewer than 10 women diagnosed with cervical cancer age ≥65 had no insurance.
Over time, incidence rate trends diverged for women aged <65 compared to ≥e. Cervical cancer incidence rates decreased consistently among women aged <65 by 2.5% each year (95% CI −3.6%, −1.3%; p=0.0007; Figure 1). From 2004–2012, the incidence rate among women aged ≥65, decreased by 3.9% each year (95% CI −5.5%, −2.3%; p=0.0009). However from 2013–2015, the incidence rate among women aged ≥65 showed an increasing trend, 14.1% each year (95% CI −4.4%, 36.1%; p=0.12) (Figure 1). Additional statistical models using difference-in-differences regression analyses supported a non-significant relative annual increase from 2013–2015 among older women compared to women aged <65 (+ 5.7%, p=0.62).
Discussion
Nearly a quarter of cases from cervical cancer in Massachusetts between 2004–2015 occurred among women aged ≥65. Older women were more likely to be diagnosed with cancer at stage II or higher than their <65 year-old counterparts. These disparities occurred despite mandated universal access to health insurance since 2006 in Massachusetts. Indeed, Massachusetts’ population-wide cervical cancer rates are lower than the US average (5.5 vs. 7.4/100,000), which may reflect excellent access to care overall. Current guidelines to exit screening at age 65, endorsed jointly by American Society of Colposcopy and Cervical Pathology (ASCCP), American Cancer Society, and American Society of Clinical Pathology8 and separately by American College of Obstetricians and Gynecologists9 and US Preventive Services Task Force (USPSTF)10 provide the following rationale: “In well-screened women older than the age of 65 in the United States, CIN2+ prevalence is low11,12 and cervical cancer is rare.13” However, according to the Copeland reference cited in the guidelines,12 while the rate of carcinoma in situ decreased with age, the rates of invasive cervical cancer rates rose at age 30 and did not decline through ages 80+ (Copeland et al Figure 4). Interestingly, these data are from 1985–2003 in Michigan, yet the incidence rates are are nearly identical to those seen in Massachusetts from 2004–2015, indicating that residual cervical cancer risk among elderly women has been persistent over three decades and in different US localities despite changes in screening guidelines during this time period. We do not currently know what role screening plays in residual cancer risk in women aged ≥65. Do cancers occur because screening is failing, or because women are failing to be screened?
Based on modeling studies, current guidelines8 conclude that “for women who have been screened every 3 years prior to age 65 years, the ratio of colposcopies to years of life gained associated with further screening was large (or the years of life gained per colposcopy small) because of the small gains in life expectancy.1” Yet recent data question this assumption. Population-wide cervical cancer rates are 7 per 100,000 per year among non-Hispanic White women compared with 9.1, 9.5, and 9.7 for American Indian/Alaska Native, African-American, and Hispanic women respectively,14 with substantially higher incidence and mortality noted among elderly women of color.15 Recent data indicate residual cancer rates of 4/100,000 per year for women age ≥65, or a cumulative incidence up to 60/100,000 by age 80 years for women exiting screening at age 65 with 3 prior negative cytology tests.15 Both screening tests and colposcopies are less accurate among postmenopausal women, therefore the assumptions of modeling studies may overestimate the reassurance provided by negative testing among postmenopausal women.15 In addition, the current cohort of elderly women came of age during the sexual revolution and has a higher number of sexual partners and probable HPV exposure than prior cohorts.16 A recent study in the UK found that 21.5% of 1341 women aged ≥65 diagnosed with cervical cancer between 2007–2012 met the US exiting criteria.17 Additional research is needed to determine whether elderly women who follow current guidelines for discontinuing screening will be sufficiently protected against cervical cancer.
Furthermore, current guidelines for exiting screening are complex and may lead to unintentional discontinuation of screening among women still at risk. Guidelines state that elderly women should discontinue screening only if the clinician can document at least 3 negative Pap tests or two negative HPV tests within 10 years of stopping, with the most recent test within 5 years, and no history of cervical pre-cancer (Cervical Intraepithelial Neoplasia grades 2 or 3) within the past 20 years, no current immunosuppression or HIV infection, and no history of DES exposure.8 These guidelines are very difficult to follow in practice due to the need to obtain accurate medical record documentation over a 10–20 year period. The burden of extensive medical record review that will often require querying multiple record systems is very difficult for practicing clinicians, and raises the likelihood that screening may be discontinued among women still considered to be at risk. Patients may also incorrectly assume that any pelvic exam included cervical cancer screening,18 or fail to understand that the purpose of cytology is to prevent cervical cancer, and thus fail to present for screening.19 Cervical cancer screening rates decline after age 40, and rates in women aged ≥65 are not routinely measured.8 The extent to which screening cessation guidelines are followed is currently unknown, and our data and others indicate substantial residual risk and late stage presentation in this age group.7,20,12 Screening practices in postmenopausal women should be studied to understand the role that inadequate screening may play in cancer development. Simplification of recommendations, as well as improved public education aimed at postmenopausal women and their healthcare providers could decrease rates of underscreening in this population.
Our study has several limitations. First, as this is a retrospective analysis, we cannot accurately estimate the role of screening guidelines in age-related disparities in cancer incidence, stage, and mortality. We are also unable to adjust for hysterectomy, and based on prior data, reported rates underestimate the true risk of cervical cancer and as well as racial disparities in cancer rates among women with a cervix in Massachusetts.21 In addition, our sample size limits our power to determine the significance of the increase in incidence among older women after 2013. Finally, cervical cancer screening services in Massachusetts may be different than in other parts of the United States.
Conclusion:
Women aged ≥65 represent a growing segment of the population that bears a substantial burden from cervical cancer. Current screening guidelines for screening cessation may be undermining our ability to prevent, detect and cure cervical cancer among otherwise healthy older women. As national organizations undertake revision of national guidelines, strong consideration must be given to emerging data on the risk of cancer among older women and to changing the guidelines for screening cessation.
Key points:
Question: What was the incidence of cervical cancer in Massachusetts from 2004–2015 among women 65 or older compared to younger women?
Acknowledgments
Financial Support:
Sarah Feldman and Jennifer Haas received funding from National Cancer Institute at the National Institutes of Health (1UM1CA221940). Erin Cook has received training grant funding from the NIH grants R25 CA 98566–10, T32 CA 009001–40, and T32 ES 007069. Rebecca Perkins has received funding from the American Cancer Society (RSG-15–150-01). We acknowledge the Centers for Disease Control and Prevention for its financial support under Cooperative Agreement 1 NU58DP006271–01-00 awarded to the Massachusetts Cancer Registry, Massachusetts Department of Public Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
References:
- 1.Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for Cervical Cancer: A Decision Analysis for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011. [cited 2018 Jul 31]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK92546/ [PubMed]
- 2.Gravitt PE, Landy R, Schiffman M. How confident can we be in the current guidelines for exiting cervical screening? Prev Med 2018; [DOI] [PubMed] [Google Scholar]
- 3.White MC, Shoemaker ML, Benard VB. Cervical Cancer Screening and Incidence by Age: Unmet Needs Near and After the Stopping Age for Screening. American Journal of Preventive Medicine 2017;53(3):392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenblatt KA, Osterbur EF, Douglas JA. Case-control study of cervical cancer and gynecologic screening: A SEER-Medicare analysis. Gynecologic Oncology 2016;142(3):395–400. [DOI] [PubMed] [Google Scholar]
- 5.Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer 2017;123(6):1044–50. [DOI] [PubMed] [Google Scholar]
- 6.NAACCR. NAACCR CERTIFICATION YEAR 2017, GOLD AND SILVER. 2017; [Internet]. [cited 2017 May 4];Available from: https://www.naaccr.org/certified-registries/.
- 7.Dimick JB, Ryan AM. Methods for Evaluating Changes in Health Care Policy: The Difference-in-Differences Approach. JAMA 2014;312(22):2401. [DOI] [PubMed] [Google Scholar]
- 8.Saslow D, Solomon D, Lawson HW, et al. American cancer society, american society for colposcopy and cervical pathology, and american society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012;137(4):516–42. [DOI] [PubMed] [Google Scholar]
- 9.Practice Bulletin No. 157: Cervical Cancer Screening and Prevention. Obstetrics & Gynecology 2016;127(1):e1–20. [DOI] [PubMed] [Google Scholar]
- 10.Moyer VA. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine 2012;156(12):880. [DOI] [PubMed] [Google Scholar]
- 11.Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for Frequent Regression of Cervical Intraepithelial Neoplasia–Grade 2: Obstetrics & Gynecology 2009;113(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008;113(S10):2946–54. [DOI] [PubMed] [Google Scholar]
- 13.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002;52(6):342–62. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. Cancer Statistics Center. [Internet]. [cited 2018 Jul 31];Available from: https://cancerstatisticscenter.cancer.org/?_ga=2.20975355.1746569515.1533055502-552426982.1496697483#!/cancer-site/Cervix.
- 15.Yoo W, Kim S, Huh WK, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLOS ONE 2017;12(2):e0172548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gravitt PE, Rositch AF, Silver MI, et al. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J Infect Dis 2013;207(2):272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castañón A, Landy R, Cuzick J, Sasieni P. Cervical Screening at Age 50–64 Years and the Risk of Cervical Cancer at Age 65 Years and Older: Population-Based Case Control Study. PLoS Medicine 2014;11(1):e1001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard DL, Hostetter SS, Hunter J, Johnson N, Cooper S, Malnar G. Women’s Understanding of the Term ‘Pap Smear.’ Maternal and Child Health Journal 2015;19(7):1455–63. [DOI] [PubMed] [Google Scholar]
- 19.Kasting ML, Wilson S, Zollinger TW, Dixon BE, Stupiansky NW, Zimet GD. Differences in cervical cancer screening knowledge, practices, and beliefs: An examination of survey responses. Preventive Medicine Reports 2017;5:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diver EJ, Hinchcliff EM, Gockley AA, et al. Assessment of treatment factors and clinical outcomes in cervical cancer in older women compared to women under 65 years old. Journal of Geriatric Oncology [Internet] 2018. [cited 2018 Jul 31];Available from: http://linkinghub.elsevier.com/retrieve/pii/S1879406817302795 [DOI] [PubMed]
- 21.Stang A, Hawk H, Knowlton R, Gershman ST, Kuss O. Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995 to 2010. Annals of Epidemiology 2014;24(11):849–54. [DOI] [PubMed] [Google Scholar]