Abstract
Introduction:
Dental erosion is defined as the loss of tooth structure due to chemical process that does not involve bacteria. The management of such a condition calls for a comprehensive approach to identifying the cause and treating it.
Aim:
The aim of this study is to comparatively evaluate the role of grape seed extract (GSE) and cranberry extract (CE) in preventing dental erosion using optical emission spectrometry.
Materials and Methods:
Prepared enamel specimens were subjected to the erosive challenge using HCl for 10 s, followed by immersion in experimental natural groups and control fluoride group for 30 s and artificial saliva for 60 min. This cycle was repeated three times. The amounts of calcium and phosphorous present in the acid solution after 1st, 2nd, and 3rd erosive challenges were determined for each group using induced coupled plasma-optical emission spectrometry.
Results:
The cumulative calcium and phosphorous release after the 1st, 2nd, and 3rd erosive challenges were found to be the least in SnF2 group, followed by GSE group and then in CE group.
Conclusion:
The protective of GSE and CE was inferior to the gold standard control group of stannous fluoride role, against enamel erosion. GSE showed better remineralizing effect; however, there was no statistically significant difference between the two groups.
Keywords: Cranberry, enamel erosion, grape seed extract, phytotherapy
INTRODUCTION
Dental erosion is defined as the loss of tooth structure due to chemical process that does not involve bacteria. Erosive lesions are caused mainly due to exposure of the dental hard tissues to acid, which cause demineralization of the inorganic and dissolution of the organic matrix leading to progressive softening of tooth structure.
Dental erosion is caused by acid attacks, either from extrinsic sources such as consumption of acidic beverages, occupational acid exposure such as wine tasters, and workers from battery industries or intrinsic sources of reflux of gastric acid into the oral cavity in conditions such as gastroesophageal reflux disease, anorexia nervosa, and bulimia.[1] Clinically, it appears as smooth, shiny glazed surfaces mainly on the buccal or labial surfaces in case of extrinsic acid exposure, and on the lingual or palatal surfaces in case of the acid is from intrinsic sources. Occlusal erosion is characterized by rounded cusps or concavities. It is therefore very important to protect enamel and dentin from demineralization that results from these acid attacks, to prevent irreversible loss of tooth structure.[2]
The effect of acid attacks on enamel and dentin varies; enamel consists of 90% of mineral in the form of hydroxyapatite crystals which readily dissolves at a pH of <4.5. Dentin contains 47% inorganic hydroxyapatite, 33% organic matrix made up of collagen, and 20% water. Erosive demineralization pattern in dentin shows a completely demineralized outer organic matrix, followed by a partially demineralized organic layer because the presence of degraded collagen matrix hampers the ionic diffusion and prevents further mineral loss.[3]
The management of such a condition calls for a comprehensive approach to identifying the cause and treating it. Few of the recommended measures for reducing acid exposure would be to reduce the intake of acid beverages, drinking them quickly and in a cooled state, and consumption of acidic drinks with a high content of calcium, phosphate, and fluoride.[4]
Remineralizing agents such as fluorides, Casein phosphopeptide - Amorphous calcium phosphate (CCP-ACP), xylitol, and bioactive glass can be used to reduce demineralization and enhance remineralization.[5]
More recently, plant extracts such as green tea have proven to have remineralizing property due to the presence of polyphenols which inhibit the collagen degradation due to the presence of matrix metalloproteinases inhibitors.[6] Grape seed extract (GSE) and cranberry are other natural products containing polyphenols; however, their role in preventing dental erosion is speculative. Therefore, the aim of this study is to comparatively evaluate the role of GSE and cranberry extract (CE) in preventing dental erosion using optical emission spectrometry. The null hypothesis was that there is no difference in enamel erosion-protective effect among GSE and CE.
MATERIALS AND METHODS
This in vitro study was conducted after getting approval from the Institutional Scientific Review Board. Enamel is the outermost layer of the tooth, and it is characterized by aprismatic structure and high mineral content. The initial stages of dental erosion affect this outermost layer causing dissolution and demineralization of enamel crystals leading to permanent loss of tooth structure and dentin hypersensitivity.[7] To standardize the specimen preparation and to simulate the clinical conditions, this study investigated the role of two natural products, GSE, and CE in preventing enamel erosion.
Specimen preparation
Twenty-four sound human incisor teeth that were extracted for periodontal or orthodontic reasons were included in this study. Teeth with caries, fractures, surface cracks, and enamel hypoplasia were excluded from the study. Samples were thoroughly cleaned of organic debris and sterilized by autoclaving and were stored in 10% formalin until use according to the centers for disease control and prevention guidelines.[8] Calculation of sample size was done using G Power software with the significance of 95% and power of 80%. All the specimens were randomly divided into three groups (n = 8). They were sectioned to produce enamel slabs of 6 mm × 6 mm × 2 mm dimensions. The enamel slabs were embedded in acrylic resins and polished with silicon carbide discs to obtain a smooth and flat surface.
Preparation of test and control solutions
In this study, stannous fluoride solution was used as a control, as its remineralizing property is well established.
Group A: 0.63% w/w stannous fluoride solution was prepared by adding 630 mg of stannous fluoride to 100 ml of distilled water and used as a control group[9]
Group B: GSE 0.65% w/v solution of GSE was prepared by adding 650 mg or 6.5 g of ground GSE powder to 100 ml of distilled water. According to Xie et al., 2008, in his study concluded that minimal concentration of GSE required to produce remineralization was found to be 0.6%[10]
Group C: CE 0.6% w/v solution of CE was prepared by adding 600 mg of ground CE powder in 100 ml of distilled water. Khaimar et al. in 2017 have found that CE at a concentration of 600 mg produced the maximum zone of inhibition against Streptococcus mutans.[11]
Preparation of artificial saliva
Artificial saliva containing buffering capacity was prepared by mixing 0.213 g/L of CaCl22H2O; 0.738 g/L of KH2PO4; 1.114 g/L of KCl; 0.381 g/L of NaCl; and 12 g/L of tris buffer for remineralization.[12]
The salivary pool was formed by collecting saliva samples from seven human volunteers, and all the enamel specimens were immersed in the salivary pool for 2 h, at 37°C under gentle agitation to allow the formation of the acquired pellicle.
Erosion-remineralization cycle
For the erosive challenge, 0.01 M HCl was used to simulate the intrinsic erosion.
One erosion-remineralization cycle consists of (1) 10 s immersion in 20 ml of HCl, (2) 60 s immersion in 20 ml of artificial saliva, (3) 30 s exposure to 20 ml of the SnF2 in group A, GSE in Group B or CE in Group C, and (4) 60 min immersion in 20 ml of artificial saliva. This cycle was repeated thrice during the same day.
Evaluation of mineral loss
After the 3rd erosive challenge, the acidic solution of each sample of the respective groups was individually collected in plastic-coated glass containers and was labeled as Group 1, 2, and 3, thereby blinding the evaluator. The net loss of calcium and phosphorus was determined for each group using induced coupled plasma-optical emission spectrometry (ICP-OES) that allows semiquantitative analysis of the elemental composition of the specimens. It works on the principle that calcium and phosphorous emit photons of specific wavelength when excited, this wavelength is converted into electrical signals using the photodetector and processed by a computer.[13]
RESULTS
The collected data were analyzed with IBM. The SPSS software (student version 7.01; SPSS Inc, Chicago, IL) statistics software 23.0 version to describe the data descriptive statistics mean and standard deviation were used. To find the significant difference in the multivariate analysis, the one-way analysis of variance with Tukey's post hoc test was used. In the above statistical tools, the P = 0.05 is considered as statistically significant level [Tables 1 and 2].
Table 1.
Mean and standard deviation of calcium and phosphorous levels released after 2nd and 3rd erosive challenge
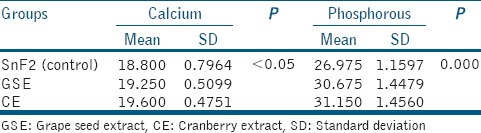
Table 2.
Post hoc Tukey
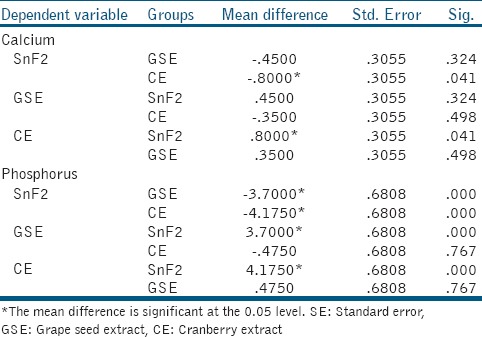
For calcium analysis, CE group showed significantly more total calcium loss when compared to control group (P = 0.041), there was no statistically significant difference between GSE and CE groups. For phosphorous analysis, both GSE and CE showed significantly greater net phosphorous loss than control group (P = 0.000 in both); however, there was a statistically significant difference between the GSE and CE. The cumulative calcium and phosphorous release after the 3rd erosive challenge were found to be the least in SnF2 group, followed by GSE group and then in CE group [Figures 1 and 2].
Figure 1.
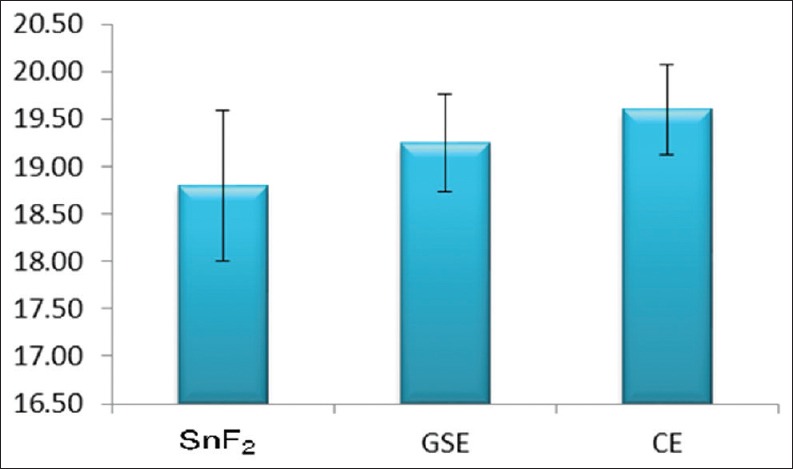
Mean of total calcium release
Figure 2.
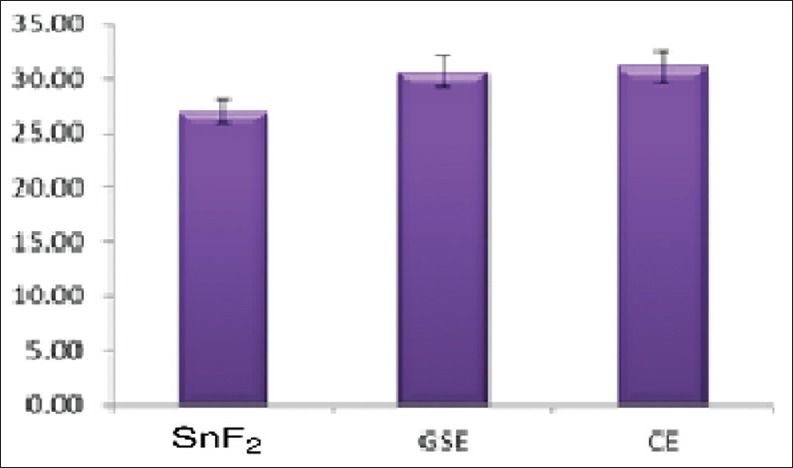
Mean of total phosphorus release
DISCUSSION
Incidences of dental erosion are high, and their prevalence is still increasing, this is an alarming issue for the clinical practitioners as erosion leads to irreversible loss of dental hard tissues. Therefore, finding ways to prevent or minimize this condition is very important and is an area of the present research. Numerous remineralizing agents have been used to prevent enamel or dentin loss due to demineralization, of which fluorides are the gold standards. In enamel, acid attacks cause dissolution of the prism cores or interprismatic areas in prismatic enamel leading to progressive softening of the surface.[14]
Fluorides act by complexing with hydroxyapatite crystals of enamel to form fluorapatite crystals, which more acid resistant. Conventional fluorides such as sodium fluoride act by the formation of calcium fluoride layer, which is assumed to act as a physical barrier that hampers the contact between the acid and the underlying enamel or to act as a mineral reservoir.[15] Ganss et al. evaluated the effectiveness of different fluoride compounds as anti-erosive agents and concluded that stannous or tin fluoride was most effective.[16] Mechanism of action of tin-containing fluoride is by complexing with calcium ions enamel crystals to form metal-rich surface precipitates which were shown to be highly acid resistant, further it may penetrate and get incorporated into the demineralized layer.[17] Tin-containing fluorides show promising results not only on enamel erosion but also on dentin erosion.[18]
Most of the commercially available oral-hygiene products contain fluoride; although they have some preventive role in dental erosion, no full protection is provided. Therefore, the development of a novel anti-erosive agent with an alternative mechanism of action would be more effective. In the present study, two natural products are as follows: GSE and CE were used to test for their remineralizing potential.
To evaluate the amount of calcium and phosphorous in the acid sample, OES was used in this study. ICP-OES is equipment used for elemental analysis of trace elements found in a sample. It acts using light emitted from an excited element and converting that light into an electrical signal, which can be read using the instrument computer and software. A sample containing several different elements will, therefore, produce light composed of wavelength specific to each of the element. By separating these wavelengths by a dispersion system, the spectrometer can determine which elements are present. The intensity of each of these wavelengths being a function of the concentration of the considered element.[19]
Results of the present study show that control SnF2 had the lowest levels of calcium and phosphorous indicating that it has the highest protective role against enamel erosion, followed by GSE and CE. However, there was no significant difference between these two natural products.
Both GSE and cranberry are phytotherapeutic agents containing polyphenols, mainly proanthocyanidins (PAs), which are condensed tannins. PA is naturally occurring plant metabolite widely available in fruits and vegetables that have studied to have numerous therapeutic values.[20] It is said to affect the remineralization process through two mechanisms as follows: first, PA may cause superficial mineral deposition over the lesion by forming insoluble complexes as studies by Kosasi et al. and Kim et al. in their research.[21,22] Second, in case of dentin erosion, the PA is dentin biomodifiers, they react with the exposed organic matrix and stabilize the collagen by inducing collagen cross-linking.[23]
The previous study by Mirkarimi et al., on the remineralizing effect of GSE on artificial caries, showed that gallic acid, a major component of GSE facilitates mineral deposition predominantly on the surface layer. Furthermore, after treating with GSE, there were scaffolding deposits on the enamel surface with cluster-like structures resembling remineralization process initiation. It was also pointed out that fluoride concentration in GSE was 0.01 ppm.[24]
Results of the present study are contradictory to the study done by Boteon et al., which tested the effect of PA-enriched extracts (10% GSE gel and 10% cranberry gel) against chlorhexidine gel and NaF gel on the inhibition of wear and degradation of demineralized organic matrix. It was concluded that GSE significantly reduced wear compared to other groups. Cranberry and chlorhexidine did not differ statistically.[25] This difference in results obtained could be due to the variation in the concentration of the agents used – 10% in the previous study as opposed to 0.6% and also the application time of 1 min as opposed to 30 s in the present study. The concentration for this study was selected in reference to previous studies done for testing the remineralizing effect and also 30 s immersion time was selected to be in consistent with a clinical recommendation for commercial mouthwashes.
CONCLUSION
Within the limitations of this study, it can be concluded that the protective of GSE and CE was inferior to the gold standard control group of stannous fluoride role, against enamel erosion. GSE showed better remineralizing effect; however, there was no statistically significant difference between the two groups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wiegand A, Attin T. Occupational dental erosion from exposure to acids: A review. Occup Med (Lond) 2007;57:169–76. doi: 10.1093/occmed/kql163. [DOI] [PubMed] [Google Scholar]
- 2.Addy M, Shellis RP. Interaction between attrition, abrasion and erosion in tooth wear. Monogr Oral Sci. 2006;20:17–31. doi: 10.1159/000093348. [DOI] [PubMed] [Google Scholar]
- 3.George B, John J, Saravanan S, Arumugham IM. Prevalence of permanent tooth loss among children and adults in a suburban area of Chennai. Indian J Dent Res. 2011;22:364. doi: 10.4103/0970-9290.84284. [DOI] [PubMed] [Google Scholar]
- 4.Magalhães AC, Wiegand A, Rios D, Honório HM, Buzalaf MA. Insights into preventive measures for dental erosion. J Appl Oral Sci. 2009;17:75–86. doi: 10.1590/S1678-77572009000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jefferies SR. Advances in remineralization for early carious lesions: A comprehensive review. Compend Contin Educ Dent. 2014;35:237–43. [PubMed] [Google Scholar]
- 6.Kato MT, Magalhães AC, Rios D, Hannas AR, Attin T, Buzalaf MA. Protective effect of green tea on dentin erosion and abrasion. J Appl Oral Sci. 2009;17:560–4. doi: 10.1590/S1678-77572009000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poggio C, Lombardini M, Dagna A, Chiesa M, Bianchi S. Protective effect on enamel demineralization of a CPP-ACP paste: An AFM in vitro study. J Dent. 2009;37:949–54. doi: 10.1016/j.jdent.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM. Guidelines for infection control in dental health-care settings-2003. MMWR Recomm Rep. 2003;52:1–61. [PubMed] [Google Scholar]
- 9.O'Toole S, Bartlett DW, Moazzez R. Efficacy of sodium and stannous fluoride mouthrinses when used before single and multiple erosive challenges. Aust Dent J. 2016;61:497–501. doi: 10.1111/adj.12418. [DOI] [PubMed] [Google Scholar]
- 10.Xie Q, Bedran-Russo AK, Wu CD. In vitro remineralization effects of grape seed extract on artificial root caries. J Dent. 2008;36:900–6. doi: 10.1016/j.jdent.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khairnar MR, Karibasappa GN, Dodamani AS, Vishwakarma P, Naik RG, Deshmukh MA. Comparative assessment of cranberry and chlorhexidine mouthwash on streptococcal colonization among dental students: A randomized parallel clinical trial. Contemp Clin Dent. 2015;6:35–9. doi: 10.4103/0976-237X.149289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira TA, Scaramucci T, Nogueira FN, Simões A, Sobral MA. Effect of mouthrinses with different active agents in the prevention of initial dental erosion. Indian J Dent Res. 2015;26:508–13. doi: 10.4103/0970-9290.172052. [DOI] [PubMed] [Google Scholar]
- 13.Hou X, Amais RS, Jones BT, Donati GL. Inductively coupled plasma optical emission spectrometry. Encyclopedia of Analytical Chemistry, Online © 2006–2016 John Wiley & Sons, Ltd. 2000 [Google Scholar]
- 14.Magalhães AC, Wiegand A, Rios D, Buzalaf MA, Lussi A. In Fluoride and the Oral Environment. Vol. 22. Karger Publishers, Basel, Switzerland: Karger Publishers; 2011. Fluoride in dental erosion; pp. 158–70. [DOI] [PubMed] [Google Scholar]
- 15.Saxegaard E, Rölla G. Fluoride acquisition on and in human enamel during topical application in vitro. Scand J Dent Res. 1988;96:523–35. doi: 10.1111/j.1600-0722.1988.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 16.Ganss C, Schlueter N, Hardt M, Schattenberg P, Klimek J. Effect of fluoride compounds on enamel erosion in vitro: A comparison of amine, sodium and stannous fluoride. Caries Res. 2008;42:2–7. doi: 10.1159/000111743. [DOI] [PubMed] [Google Scholar]
- 17.Babcock FD, King JC, Jordan TH. The reaction of stannous fluoride and hydroxyapatite. J Dent Res. 1978;57:933–8. doi: 10.1177/00220345780570092301. [DOI] [PubMed] [Google Scholar]
- 18.Ganss C, Hardt M, Lussi A, Cocks AK, Klimek J, Schlueter N. Mechanism of action of tin-containing fluoride solutions as anti-erosive agents in dentine – An in vitro tin-uptake, tissue loss, and scanning electron microscopy study. Eur J Oral Sci. 2010;118:376–84. doi: 10.1111/j.1600-0722.2010.00742.x. [DOI] [PubMed] [Google Scholar]
- 19.Ravi K, Ganapathy D, Sheeba PS. Antioxidants and cancer prevention – A review. J Pharm Res. 2018;12:35. [Google Scholar]
- 20.Zhou Z, Zhou K, Hou X, Luo H. Arc/spark optical emission spectrometry: Principles, instrumentation, and recent applications. Appl Spectrosc Rev. 2005;40:165–85. [Google Scholar]
- 21.Kosasi S, Hart LA, van Dijk H, Labadie RP. Inhibitory activity of Jatropha multifida latex on classical complement pathway activity in human serum mediated by a calcium-binding proanthocyanidin. J Ethnopharmacol. 1989;27:81–9. doi: 10.1016/0378-8741(89)90080-9. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Don S, Mainwaring DE. Effect of ion-binding on the formation of temporary viscoelastic networks of proanthocyanidin biopolymers. J Appl Polym Sci. 1997;65:1795–805. [Google Scholar]
- 23.Pierpoint WS. O-quinones formed in plant extracts. Their reactions with amino acids and peptides. Biochem J. 1969;112:609–16. doi: 10.1042/bj1120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirkarimi M, Eskandarion S, Bargrizan M, Delazar A, Kharazifard MJ. Remineralization of artificial caries in primary teeth by grape seed extract: An in vitro study. J Dent Res Dent Clin Dent Prospects. 2013;7:206–10. doi: 10.5681/joddd.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boteon AP, Kato MT, Buzalaf MA, Prakki A, Wang L, Rios D, et al. Effect of proanthocyanidin-enriched extracts on the inhibition of wear and degradation of dentin demineralized organic matrix. Arch Oral Biol. 2017;84:118–24. doi: 10.1016/j.archoralbio.2017.09.027. [DOI] [PubMed] [Google Scholar]


